Abstract
Background
Circulating levels of pepsinogens have been used in high gastric cancer-risk Asian and European populations to triage endoscopic evaluation for more severe pathology. There are different analytic methods with uncertain correlations. We therefore compared diagnostic performance of three commonly used pepsinogen assays to detect histologically confirmed gastric atrophy.
Methods
We tested plasma samples from adult patients with (n=50) and without (n=755) moderate or severe gastric corpus atrophy, as determined histologically by consensus of three expert pathologists. A single laboratory measured pepsinogens I (PgI) and II (PgII) using commercially available assays: two ELISA assays produced by Biohit (Finland) and Vector Best (Russia), and a latex-agglutination assay from Eiken (Japan). Quantitative correlations were assessed by Spearman statistics. Receiver operating characteristic (ROC) curves vs. histological diagnosis were calculated using both the manufacturers’ and optimized cutoffs.
Results
Pepsinogen levels were highly correlated among the assays (pairwise Rhos: PgI≥0.84, PgII≥0.87; all p-values<0.01). Based on manufacturers’ cutoffs, sensitivities, specificities and areas under the ROC curve for detecting moderate to severe histological corpus atrophy by PgI/PgII were 44%/91%/0.70, 56%/84%/0.76, and 52%/90%/0.77 for Biohit, Vector Best and Eiken, respectively. Cutoffs optimized by ROC or data mining analyses did not substantially improve test-performance.
Conclusions
Commercial assays for pepsinogen have good relative agreement but are imperfect tests for clinical diagnosis of gastric atrophy.
Impact
Pepsinogen testing alone does not provide sufficient information for gastric cancer risk stratification. Future investigations should focus on other potential markers, in combination with pepsinogens.
Keywords: pepsinogen, stomach, atrophy, gastric cancer, risk stratification
INTRODUCTION
Gastric cancer is the third leading cause of cancer death worldwide [1]. Endoscopic screening may detect precancerous lesions and asymptomatic cancers for early treatment to reduce disease burden. However, it is not clear which populations have sufficiently risk to warrant this invasive procedure. Current clinical guidelines suggest pepsinogen testing for non-invasive identification of individuals at high cancer risk [2] with potential application to risk-stratification both in Asia and Europe [2,3].
Pepsinogens are pepsin pro-enzymes that may be measured in blood as indirect markers of gastric mucosal changes [4]. Two isozymogens, pepsinogen I (PgI) and pepsinogen II (PgII), are produced in different parts of the stomach [5]. PgI production is exclusively limited to acid-secreting glands of the gastric corpus (proximal stomach) whereas PgII production is widespread in diverse types of glands throughout the stomach as well as Brunner glands of the duodenum [6]. Thus, mucosal atrophy affecting the gastric corpus leads to decreased levels of PgI while PgII levels tend to be relatively stable. In particular, the presence of mucosal inflammation, also related to H. pylori infection, may increase levels of both PgI and PgII [7] and in some cases result in normal PgI values when both atrophy and inflammation are present [8]. To account for this draw-back, a decreased ratio of PgI to PgII (PgI/PgII) is considered to be the best serologic marker of gastric atrophy [2] as used for cancer screening in Japan [9] and increasingly in Europe [6].
Different methods for pepsinogen assessment are used in Eastern and Western countries. Miki and Fujishiro have expressed concerns about translating results between the assays [10]. Therefore, we aimed to compare the widely used pepsinogen assay in Japan with the most popular assay in Europe. We also evaluated another assay manufactured in Russia since this country as well as its neighbours, sharing high burden of gastric cancer, usually favor domestic products. We correlated pepsinogen measurements by these different test-systems on the same patient samples, determined sensitivity and specificity to detect gastric atrophy and explored alternative cutoff values to improve performance.
MATERIAL AND METHODS
Study population
805 consecutive patients with dyspeptic complaints referred for upper endoscopy at Digestive Diseases Centre GASTRO and Riga East University hospital, Latvia were enrolled for this study (29% males; median age 51 years, age range 18-88 years). Patients with gastric cancer were excluded. Mucosal biopsies were collected according to the Sydney protocol. Pathology evaluation was performed by consensus among three expert pathologists (SI, ILK, DJ). Grade II (moderate) or III (severe) atrophy according to the updated Sydney classification were considered atrophy for the purposes of this analysis. Since pepsinogen levels primarily indicate atrophy status of the corpus, for histological assessment we used the higher grade of either of the two biopsies from this anatomic subsite. The study was approved by the Central Medical Ethics Committee of Latvia, protocol No.01-29.1/20 on September 22, 2011. All the patients provided informed consent upon enrolment.
Laboratory methods and manufacturers’ pre-defined cutoff values
EDTA-anticoagulated plasma samples were obtained following an overnight fast, processed within 30 minutes after blood collection, and stored at −80°C until testing. Plasma samples were analysed by commercially available assays from three manufacturers: 1) a latex-agglutination test-system (PgI kit cat. no. V-IZ51, PgII kit cat. no. V-IZ53) made by Eiken Chemical Co., Tokyo, Japan [4], a widely used assay in Japan; 2) an Enzyme-Linked Immunosorbent Assay (ELISA) test made by Biohit, Plc., Helsinki, Finland (PgI ELISA kit cat. no. 601 010.01, PgII ELISA kit cat. no. 601 020.02) [6], the only test-system used in clinical practice in Europe; and 3) an ELISA test-system recently developed by Vector Best, Novosibirsk, Russia (PgI ELISA kit cat. no. D-3762, PgII ELISA kit cat. no. D-3764). Tests were conducted according to the respective manufacturers’ instructions. Briefly, measurements of sample turbidity were performed on a CS-T240 auto-chemistry analyzer (Changchun, Jilin, P.R. China). Sample absorbance was measured on a Personal Laboratory automated ELISA processor (Adaltys, S.r.l., Milano, Italy). Standard curves for PgI and PgII were used to interpolate the concentrations of unknown samples. Based on the Japanese experience and manufacturer’s recommendations, two cutoff specifications were used for the latex-agglutination assay: PgI≤70 ng/ml and PgI/PgII≤3 for “any” atrophy, and PgI≤30 ng/ml and PgI/PgII≤2 for “advanced” atrophy [4]. For the ELISA tests, the manufacturers’ determined cutoff of PgI/PgII<3 was used.
Statistical analysis
Pairwise correlations among the three different assays were assessed by Spearman statistics. Assay performance was evaluated by receiver operating curve (ROC) analysis with calculations of area under the curve (AUC) and compared by a non-parametric test [11].
Sensitivity, specificity and overall accuracy were assessed using histological diagnosis as the ‘gold standard.’ Three alternative analytical approaches were considered based on: 1) manufacturer-defined cutoffs, 2) ROC-defined cutoffs using minimal Euclidean distance, and 3) optimized cutoffs from the C4.5 algorithm decision tree method [12] with 10-fold cross-validation. In addition, sex-specific cutoffs were considered as a sensitivity analysis.
Statistical analyses were conducted with SPSS Statistics for Windows software, (version 20.0, IBM Corp., Armonk, NY). The decision tree algorithm was implemented in Weka software [13]. p<0.05 was considered statistically significant for all comparisons.
RESULTS
Fifty patients were diagnosed with atrophy in the corpus (35 grade II and 15 grade III), and 755 were classified as not having corpus atrophy. Pair-wise scatter plots of their pepsinogen measurements by the three manufacturers’ test-systems are shown in Figure 1. The measurements were highly correlated, with Spearman correlation coefficients ranging from 0.84 to 0.89 for PgI and from 0.87 to 0.90 for PgII, (all p-values≤0.01). Notably, absolute values of PgI and PgII differed substantially among the test-systems as illustrated by the deviation of the correlation line from the diagonal.
Figure 1.
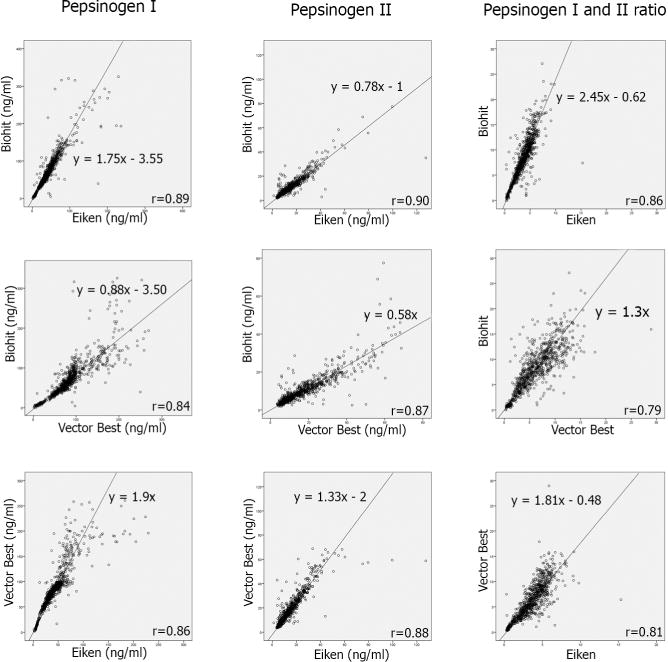
Pair-wise correlations of pepsinogen measurements by the three manufacturers’ test-systems
Means and standard deviations of PgI, PgII and PgI/PgII by atrophy status are presented in Table 1. For all 3 test-systems, PgI and PgI/PgII ratios were consistently lower in the patients with corpus atrophy.
Table 1.
Mean pepsinogen levels by presence or absence of histologically defined atrophy of the gastric corpus
| Test-system | Pepsinogen I (SD) [ng/ml] | Pepsinogen II (SD) [ng/ml] | Pg I/II Ratio (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall N=805 |
Atrophy N=50 |
No atrophy N=755 |
Overall N=805 |
Atrophy N=50 |
No atrophy N=755 |
Overall N=805 |
Atrophy N=50 |
No atrophy N=755 |
|
| Biohit | 73.9 (48.8) | 60.5 (74.0) | 74.7 (46.6) | 10.7 (8.1) | 10.3 (6.7) | 10.7 (8.2) | 8.3 (4.3) | 5.7 (5.5) | 8.4 (4.2) |
| Vector Best | 87.9 (46.3) | 59.4 (60.0) | 89.8 (44.7) | 17.8 (12.8) | 17.8 (12.4) | 17.8 (12.4) | 6.3 (3.5) | 3.9 (4.8) | 6.5 (3.4) |
| Eiken | 44.9 (30.3) | 29.7 (31.9) | 45.9 (29.9) | 14.3 (10.7) | 13.8 (8.4) | 14.4 (10.9) | 3.6 (1.8) | 2.1 (1.9) | 3.7 (1.7) |
Pg I/II – ratio between pepsinogen I and pepsinogen II
Atrophy is defined as grade II-III atrophy in the corpus
SD – Standard Deviation
Using the manufacturers’ cutoffs of PgI/PgII<3 for Biohit and Vector Best and PgI/PgII≤3 with PgI≤70 ng/ml for Eiken, 22 (44%) of the 50 patients with atrophy were correctly classified by all three test-systems while 12 cases (24%) were missed by all (Figure 2). The diagnostic performance of the three tests to detect atrophy is presented in Table 2. Under various alternative approaches, sensitivities ranged from 44-76.0%, specificities from 62.6-93.1%, and overall accuracy from 63.1-90.3%. These parameters did not systematically vary based on manufacturers’, ROC-defined or decision tree-optimized cutoffs. Similarly, sex-specific cutoffs did not substantially modify diagnostic performance (Supplemental Table).
Figure 2.
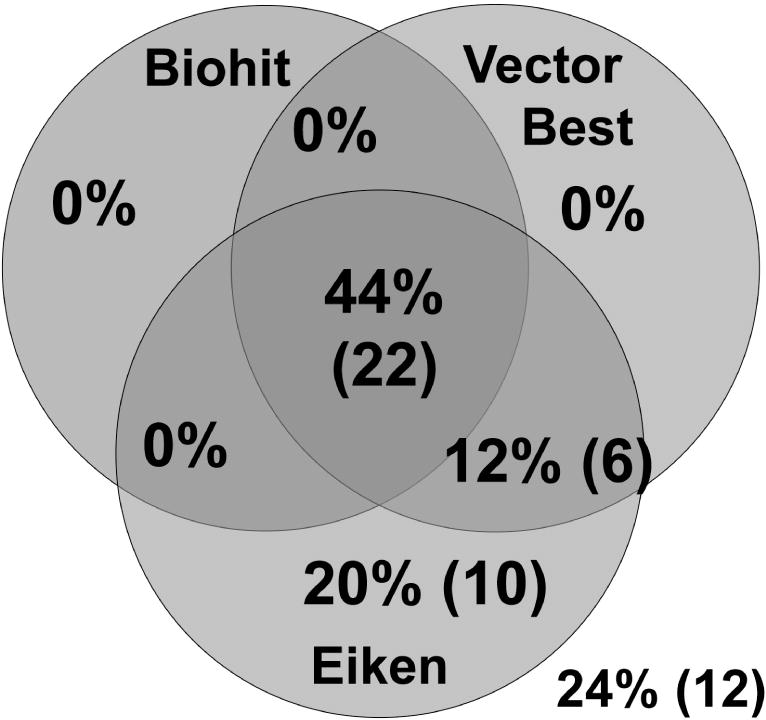
Diagnostic agreement among three pepsinogen test-systems in 50 patients with histologic diagnosis of corpus atrophy. The following cutoff values were used: PgI≤70 ng/ml and PgI/PgII≤3 for Eiken, and PgI/PgII<3 for Biohit and Vector Best
Table 2.
Performance indicators of the different pepsinogen test-systems based on varying cutoffs
| Test-system (method) | Analytical Approach | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Overall accuracya, % (95% CI) |
|---|---|---|---|---|
| Biohit (ELISA) | Manufacturer’s (PgI/PgII<3) | 44.0 (30.2-57.8) | 90.6 (88.5-92.7) | 87.7 (85.4-90.0) |
| ROC-defined cutoff (PgI/PgII<6.9) | 70.0 (57.3-82.7) | 62.6 (59.2-66.1) | 63.1 (59.8-66.4) | |
| DTC-optimized (PgI<23 and PgI/PgII<6) | 48.0 (34.2-61.9) | 93.1 (91.3-94.9) | 90.3 (88.3-92.4) | |
| Vector Best (ELISA) | Manufacturer’s (PgI/PgII<3) | 56.0 (42.2-69.8) | 83.7 (81.1-86.3) | 82.0 (79.3-84.6) |
| ROC-defined cutoff (PgI/PgII<4.1) | 70.0 (57.3-82.7) | 71.5 (68.3-74.7) | 71.4 (68.3-74.6) | |
| DTC-optimized (PgI<42.9) | 52.0 (38.2-65.9) | 91.8 (89.8-93.8) | 89.3 (87.2-91.5) | |
| Eiken (Latex-agglutination) | Manufacturer’s (PgI≤70 ng/ml and PgI/PgII≤3) | 76.0 (64.2-87.8) | 68.9 (65.6-72.2) | 69.3 (66.1-72.5) |
| Manufacturer’s for strong positivity (PgI≤30 ng/ml and PgI/PgII≤2) | 52.0 (38.2-65.9) | 89.8 (87.6-92.0) | 87.5 (85.2-89.7) | |
| ROC-defined cutoff (PgI/PgII<2.7) | 70.0 (57.3-82.7) | 71.9 (68.7-75.1) | 71.8 (68.7-74.9) | |
| DTC-optimized (PgI<50 and PgI/PgII<2.3) | 58.0 (44.3-71.7) | 83.7 (81.1-86.3) | 82.1 (79.5-84.7) |
Sensitivity x Prevalence + Specificity (1 - Prevalence); Prevalence of atrophy=6.2% (50/805)
Abbreviations: CI – Confidence Interval; DTC – Decision tree classifier; ELISA -enzyme linked immunosorbent assay; ROC – Receiver Operating Characteristic; PgI – Pepsinogen I; PgII – Pepsinogen II
ROC analyses of PgI/PgII are presented in Figure 3. The AUCs were 0.70 (95% CI, 0.60-0.79) for Biohit, 0.76 (95% CI, 0.69-0.84) for Vector Best and 0.77 (95% CI, 0.69-0.85) for Eiken. Comparing among the three AUCs, differences were statistically significant for Biohit vs. Vector Best (p=0.024) and Biohit vs. Eiken (p=0.003) but not for Vector Best vs. Eiken (p=0.575).
Figure 3.
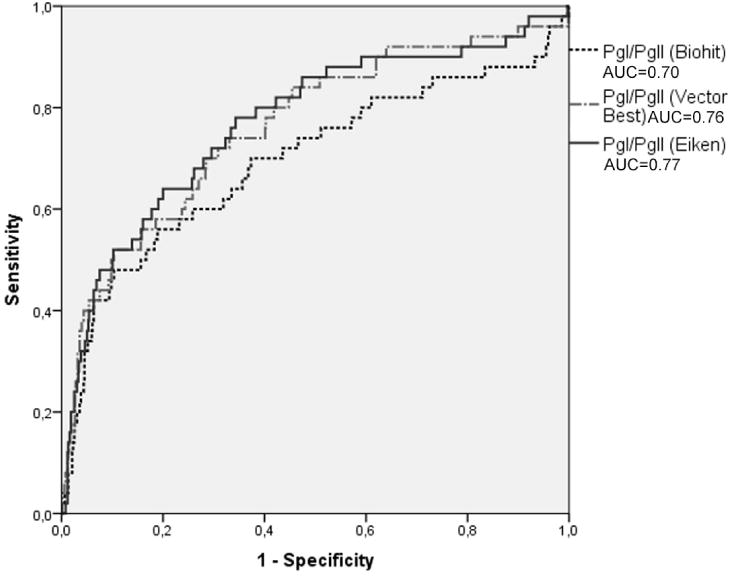
Receiver operating characteristics of PgI/PgII to detect corpus atrophy
DISCUSSION
The first pepsinogen radioimmunoassay was developed by Samloff et al. in the early 1980s [14] and soon suggested as a potential biomarker of gastric cancer [15]. Pepsinogen testing has been utilized for screening purposes in Japan for decades [9,16] and more recently in Europe [6,17]. Current test-systems are based on latex-enhanced turbidimetric immunoassay or ELISA technologies. Decreases in either PgI levels or PgI/PgII ratio by latex-agglutination test are commonly used for diagnosis of atrophy in Japan, while a decrease in PgI/PgII ratio by ELISA is the accepted criterion in Europe.
Our study has compared diagnostic performance of three widely used pepsinogen test-systems and, to the best of our knowledge, represents their first head-to-head comparison on symptomatic individuals with histological assessment of corpus atrophy.
Notable differences in absolute values for either PgI or PgII were found, however there was good relative agreement among the three test-systems. Our findings are similar to the comparison between Eiken and Biohit assays among asymptomatic individuals reported by Miki and Fujishiro [10]. One of the explanations for these differences could be the different laboratory principles (i.e. ELISA and latex-agglutination). At the same time, also the agreement between the two ELISA methods was imperfect, in particular with high pepsinogen values.
Previous evaluations of pepsinogens’ diagnostic performance have been based on various assays, cutoff values, population characteristics and definition of atrophy, with inconsistent findings. As early as 2004, a meta-analysis by Dinis-Ribeiro et al. [4] included studies with sensitivity ranging from 6% to 99% and specificity ranging from 64% to 100%. A 2015 meta-analysis by Huang et al. [18] calculated a pooled 69% (95% CI, 52-83%) sensitivity, 84% (95% CI, 68-93%) specificity and ROC AUC of 0.83 (95% CI, 0.80-0.86) for PgI/PgII. Our results are generally comparable.
Diagnostic performance of pepsinogen testing is particularly dependent upon cutoff values [19]. Most of the studies performed in Asia addressing the potential to predict gastric cancer have used uniform cutoff values of PgI≤70 and PgI/PgII≤3.0 [20]. We tried several approaches to identify better cutoffs, including ROC-based selection and data mining decision tree methods, without substantial effects on test performance. Adjustments for age, sex, height, body weight, body surface area, smoking and drinking habit have been explored without meaningful improvement [21]. Further advances in blood-based diagnosis of atrophy may require application of additional tests, and circulating levels of trefoil factors [22] and ghrelin [23] have each been proposed as alternative markers for atrophy. In addition, cancer-specific biomarkers, including panels of micro-RNAs [24] and cancer-specific autoantibodies [25] have been suggested. Combining pepsinogens with one or more of these (or other) markers, as previously evaluated for trefoil factor 3 [22], could potentially improve the performance of non-invasive testing for gastric cancer risk.
In summary, our data demonstrated good correlations of PgI, PgII and PgI/PgII measurements among three test-systems, with all methods performing equivalently. Importantly, there were substantial differences in absolute values, which must be properly considered in comparisons between methods and populations. Adjusting the cutoff values does not substantially improve sensitivity and specificity as compared to definitive histopathological diagnosis. The clinical performance of pepsinogen blood levels to detect gastric corpus atrophy is not sufficient to recommend this approach for wide implementation in cancer risk-screening.
Supplementary Material
Acknowledgments
The authors acknowledge Pentti Sipponen, the former employee of HUSLAB, Helsinki, Finland for valuable recommendations and comments on the study design.
We acknowledge the three test manufacturers for their generous support and advice. We are also thank Riga East University Hospital/University of Latvia Biobank for sample procurement and storage.
Grant Support
This study was supported in part from the Project No. 4, State Program in Research of Latvia - BIOMEDICINE 2014-2017 and Project from University of Latvia: “Actual clinical and fundamental research in biomedicine and farmacy”.
Footnotes
Authors’ Contributions
Conception and design: M.Leja
Development of methodology: M.Leja, D.Rudzite, I.Tolmanis
Acquisition of data: S.Isajevs, I. Liepniece-Karele, D.Janciauskas, D.Rudzite, I.Kikuste, A.Vanags, I.Kojalo
Analysis and interpretation of data (e.g., statistical analysis, biostatistics,
computational analysis): I.Kojalo, I.Polaka, V.Folkmanis, A.Kirsners, M.C.Camargo, C.Rabkin
Writing, review, and/or revision of the manuscript: M.Leja, M.C.Camargo, C.Rabkin review: all Administrative, technical, or material support (i.e., reporting or organizing
data, constructing databases): A.Kirsners, I.Tolmanis
Study supervision: M.C.Camargo, C.Rabkin
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; 2013. (IARC CancerBase No. 11 [Internet]). Available from: http://globocan.iarc.fr, accessed on 03/12/2016. [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 3.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of gastroenterology and hepatology. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]; Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, Kurihara M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen. 2004;11:141–147. doi: 10.1258/0969141041732184. [DOI] [PubMed] [Google Scholar]
- 5.Samloff IM. Pepsinogens, pepsins, and pepsin inhibitors. Gastroenterology. 1971;60:586–604. [PubMed] [Google Scholar]
- 6.Agreus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol. 2012;47:136–147. doi: 10.3109/00365521.2011.645501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Mario F, Cavallaro LG, Moussa AM, et al. Usefulness of serum pepsinogens in Helicobacter pylori chronic gastritis: relationship with inflammation, activity, and density of the bacterium. Dig Dis Sci. 2006;51:1791–1795. doi: 10.1007/s10620-006-9206-1. [DOI] [PubMed] [Google Scholar]; Leja M, Lapina S, Polaka I, et al. Pepsinogen testing for evaluation of the success of Helicobacter pylori eradication at 4 weeks after completion of therapy. Medicina (Kaunas) 2014;50:8–13. doi: 10.1016/j.medici.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Serum pepsinogen concentrations as a measure of gastric acid secretion in Helicobacter pylori-negative and -positive Japanese subjects. J Gastroenterol. 2005;40:938–944. doi: 10.1007/s00535-005-1677-x. [DOI] [PubMed] [Google Scholar]
- 9.Miki K. Gastric cancer0 screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 10.Miki K, Fujishiro M. Cautious comparison between East and West is necessary in terms of the serum pepsinogen test. Dig Endosc. 2009;21:134–135. doi: 10.1111/j.1443-1661.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 12.Quinlan JR. Machine Learning. Vol. 16. Morgan Kaufmann Publishers, Inc; 1994. C4.5: Programs for Machine Learning; pp. 235–240. 1993. [Google Scholar]
- 13.Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten IH. The WEKA Data Mining Software: An Update. SIGKDD Explor Newsl. 2009;11:10–18. [Google Scholar]
- 14.Samloff IM, Liebman WM. Radioimmunoassay of group I pepsinogens in serum. Gastroenterology. 1974;66:494–502. [PubMed] [Google Scholar]
- 15.Nomura AM, Stemmermann GN, Samloff IM. Serum pepsinogen I as a predictor of stomach cancer. Ann Intern Med. 1980;93:537–540. doi: 10.7326/0003-4819-93-4-537. [DOI] [PubMed] [Google Scholar]
- 16.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - “ABC method”. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:405–414. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, Vieira AS, Bento MJ, Lomba-Viana H. Serum pepsinogen test for early detection of gastric cancer in a European country. European journal of gastroenterology & hepatology. 2012;24:37–41. doi: 10.1097/MEG.0b013e32834d0a0a. [DOI] [PubMed] [Google Scholar]; di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523–530. doi: 10.1016/j.dld.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Huang YK, Yu JC, Kang WM, et al. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0142080. doi: 10.1371/journal.pone.0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. International journal of cancer. Journal international du cancer. 2007;121:2782–2786. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]; Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev. 2006;15:1083–1094. doi: 10.1158/1055-9965.EPI-05-0931. [DOI] [PubMed] [Google Scholar]
- 20.Terasawa T, Nishida H, Kato K, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014;9:e109783. doi: 10.1371/journal.pone.0109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307–319. doi: 10.5009/gnl.2010.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim HY, Kim N, Kang JM, et al. Clinical meaning of pepsinogen test and Helicobacter pylori serology in the health check-up population in Korea. European journal of gastroenterology & hepatology. 2009;21:606–612. doi: 10.1097/MEG.0b013e3283086757. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Zhang X, Lu H, et al. Serum trefoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: A monocentric cohort study in China. BMC Gastroenterol. 2014;14:74. doi: 10.1186/1471-230X-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Checchi S, Montanaro A, Pasqui L, et al. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J Clin Endocrinol Metab. 2007;92:4346–4351. doi: 10.1210/jc.2007-0988. [DOI] [PubMed] [Google Scholar]
- 24.Bornschein J, Leja M, Kupcinskas J, et al. Molecular diagnostics in gastric cancer. Frontiers in bioscience. 2014;19:312–338. doi: 10.2741/4210. [DOI] [PubMed] [Google Scholar]
- 25.Zayakin P, Ancans G, Silina K, et al. Tumor-associated autoantibody signature for the early detection of gastric cancer. Int J Cancer. 2013;132:137–147. doi: 10.1002/ijc.27667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


