Abstract
Introduction
Circulating tumor cells (CTCs) correlate with worse prognosis in patients with metastatic breast cancer, but there are little data on CTCs in operable patients. We hypothesized that primary tumor characteristics would predict the likelihood of identifying CTCs in patients with operable breast cancer.
Methods
Clinical and pathological data from 92 patients with operable breast cancer were collected. The CellSearch system was used to detect CTCs in 30 ml of peripheral blood. CTCs were defined as nucleated cells lacking CD45 but expressing cytokeratins 8, 18, or 19. Univariate analysis was performed to determine if the presence of any primary tumor characteristic was predictive of CTCs. As a secondary objective we evaluated if nodal or bone marrow status was predictive of CTCs.
Results
Thirty-eight percent of patients (35/92) had evidence of CTCs, with a median number of 1.0 CTC per CTC positive patient (range 1–22). HER2 status was the sole primary tumor characteristic that reliably predicted the presence of CTCs (P = 0.01, risk ratio = 3.66). No significant association was found between the presence of CTCs and tumor size (T), estrogen receptor (ER) status, progesterone receptor (PR) status, grade, histologic type, degree of nodal involvement (N), lymphovascular invasion (LVI) or Ki-67 proliferation. Bone marrow micrometastases were found in 17/64 (26.6%) of the patients but did not correlate with the presence of CTCs.
Conclusion
HER2 status was the only primary tumor characteristic that correlated with the presence of CTCs. Long-term follow-up will be required to determine the significance of CTCs in operable breast cancer.
Keywords: Circulating tumor cells, Breast cancer, Micrometastases, Minimal residual disease, CTC
Introduction
The presence of bone marrow micrometastases (BMM) in operable breast cancer patients is associated with adverse prognosis. In a prospective, multi-centered pooled analysis of BMM in Stage I–III breast cancer, the identification of cytokeratin positive BMM by immunocytochemistry (ICC) was an independent predictor of poor outcome on multivariate analysis [1]. While this evidence is quite compelling, routine bone marrow aspiration is not as yet standard practice in most breast cancer centers. This is likely because of perceived difficulties incorporating bone marrow aspiration into routine clinical practice and concerns related to subjecting patients to an additional invasive procedure.
Given these potential issues with the incorporation of bone marrow aspiration for standard clinical practice, it would be preferable if the same prognostic information could be yielded from peripheral blood (PB) rather than bone marrow (BM). However, the evidence supporting the prognostic value of circulating tumor cells (CTCs) in PB is not as conclusive as the evidence for the prognostic value of BMM [2]. The significance of CTCs in the peripheral blood as a prognostic factor in Stage I–III breast cancer is as yet unknown, but is an active area of research investigation [2–5]. Some groups have found that CTCs in operable breast cancer patients are associated with worse overall and recurrence free survival [6–8]. In contrast, some groups have reported that the presence of CTCs is not convincingly associated with worse survival in operable breast cancer [4, 9, 10]. Potential issues when interpreting the CTC literature include differing assays used to detect CTCs, potentially inadequate follow-up duration, small numbers of patients studied and in some cases analyzing operable and metastatic patients together rather than separately. Methods to isolate CTCs vary considerably and include immunomagnetic enrichment [11], flow-cytometry [12], Ficoll density centrifugation [4] and microfluidics chips [13]. Additional well designed studies are required to determine the significance of CTCs in operable breast cancer patients.
The isolation of CTCs is complex because these cells are rare events—less than 1 epithelial cell per million leukocytes in 1 ml of PB [11]. In the limited number of studies reported thus far involving CTCs in operable breast cancer, differing methodologies and heterogeneous study populations has limited the impact of these findings [14].
In a prospective, multi-center study, Cristofanilli et al. demonstrated that in metastatic breast cancer patients with measurable disease, the number of CTCs in PB prior to chemotherapy was an independent predictor of survival [15]. CTCs were enumerated using the CellSearch System (Veridex Corporation, Warren NJ). The CellSearch System is a semi-automated, FDA approved assay for the detection of CTCs in metastatic breast cancer patients. Based on this large prospective series of metastatic patients, five or more CTCs per 7.5 ml of PB was determined to be the threshold associated with worse overall survival.
Inherent operator dependent and statistical considerations exist for working with such rare populations as CTCs in PB. Tibbe et al. incorporated these statistical factors into a model incorporating the probability of capturing and correctly detecting CTCs with the CellSearch System [16]. They concluded that the elimination of the errors made in the identification of CTCs isolated from 7.5 ml of blood could potentially reduce the CTC threshold associated with adverse prognosis to 1 CTC per 7.5 ml of blood. Previous studies have demonstrated that persons without breast cancer have few if any detectable CTCs present. Allard et al. reported that only 8/145 healthy individuals had 1 CTC detected per 7.5 ml of blood and none with greater than or equal to 2 CTCs per 7.5 ml of blood [17]. Similarly, Riethdorf et al. reported detecting no CTCs in seven healthy persons whose blood was subjected to the CellSearch Assay [18]. To our knowledge there are no data available on the incidence of CTCs in patients with benign breast disease.
While the threshold of 5 CTCs per 7.5 ml of blood has been shown to be prognostic for patients with metastatic breast cancer [15], it is unknown what threshold is prognostic for patients with Stage I–III breast cancer since the significance of CTCs in this population is as yet unknown. The SUCCESS trial evaluated the role of CTCs in peripheral blood at primary diagnosis and during adjuvant chemotherapy, endocrine and bisphophonate treatment (n = 3,658 pts) [19, 20]. Long-term follow-up will be required to establish the appropriate threshold of significance for number of CTCs present in the SUCCESS trial, however, their preliminary analysis used the threshold of greater than one CTC per 23 ml of peripheral blood [19, 20]. In the present study we selected a threshold of one CTC per 30 ml of peripheral blood as an exploratory threshold for the current analysis. The appropriateness of this threshold must be re-evaluated after long-term follow-up and compared to the results of studies such as the SUCCESS trial.
Approximately 25% of Stage I breast cancer patients will suffer a systemic recurrence over a follow-up period of 20 years. CTCs have been reported to be present in 25–60% of Stage I–III (operable) breast cancer by various types of CTC detection assays [3, 4, 8]. It is hypothesized that CTCs may represent the occult burden of metastatic disease responsible for these recurrences. CTCs may be prognostic markers that can be used to identify early stage breast cancer patients who would benefit from systemic therapy. They also may be used to predict response to systemic therapy, although data for this are limited [5].
To achieve a better understanding of the significance of CTCs and BMM in Stage I–III breast cancer, we have initiated a prospective, single institution study to determine the characteristics of minimal residual disease (CTCs and BMM) in primary breast cancer patients. We hypothesized that primary tumor characteristics, typically used to guide prognosis and treatment [21] would also predict the likelihood of identifying CTCs in patients with operable breast cancer.
Materials and methods
Patient eligibility
A population of consecutive Stage I–III breast cancer patients was enrolled in our Institutional Review Board approved protocols (MDACC 04-0657 and MDACC 04-0698) at the time of surgical consultation pre-operatively. Written informed consent was obtained from all enrolled patients prior to any interventions. Patients who received both adjuvant and neoadjuvant chemotherapy were eligible. Additionally, patients were not excluded based on whether they underwent treatment with any particular form of chemotherapy, hormonal therapy or did not receive any type of systemic therapy. Patients underwent systemic therapy as appropriate for their malignancy irrespective of circulating tumor cell status.
PB and BMM specimens
In a companion study (MDACC 04-0657), patients consented to bone marrow aspiration of bilateral iliac crests. The technique for bone marrow aspiration has been previously described [22]. Prior to the scheduled surgical resection of the primary tumor, bone marrow aspiration of 10 ml from each side (total 20 ml) and peripheral blood draws of three tubes of 10 ml (total 30 ml) each were obtained under general anesthesia. BM specimens were layered with 4 ml of Lymphoprep (Axis-shield PoC AS, Marstrandgata 6, Norway), and were centrifuged at 1,500 rpm for 10 min. The buffy coat was then separated, washed with PBS and centrifuged. The sediment was used to prepare 10 cytospin slides. We examined all the cells generated from 5 cc of the bone marrow spread on 10 slides from each side, which would include at least 5–10,000 cells on a slide. These slides were immunostained by avidin biotin peroxidase technique using diaminobenzidine as the chromogen. The primary antibody used was a pancytokeratin cocktail comprised of AE1/AE3 (DAKO, Carpinteria, CA) in 1:50 dilution with protease enzyme for antigen retrieval. Strong cytoplasmic and membranous staining in the cells was regarded as positive for cytokeratin. Strict cytomorphological criteria were used so as not to interpret spurious staining in hematopoeitic cells as positive staining. The cytomorphological criteria included any cell with a high nuclear to cytoplasmic ratio and a distinct cytoplasmic and/or membranous staining for pancytokeratin (including a wide range of low and high molecular weight cytokeratins). Using these stringent criteria great effort was made to avoid calling false positive spurious staining in hematopoietic cells as cytokeratin positive DTC. We did not use a negative control with each case so as to analyze all the material for detecting potential DTC’s. The presence of 1 or more cytokeratin positive cells was considered a positive result. CellSave blood collection tubes (Immunicon, Inc., Huntingdon Valley, PA) were used for the PB.
Primary tumor specimens
All breast cancers were submitted to the pathology department for routine processing including estrogen receptor (ER), progesterone receptor (PR) and HER2 analysis. All cases with 2+ and 3+ staining pattern for HER2/neu were investigated by fluorescence in situ hybridization (FISH) to confirm the presence of gene amplification. Tumor size in the neoadjuvant chemotherapy group was determined by the larger value of clinical or radiographic measurements prior to the initiation of systemic therapy.
Detection of CTCs in PB
The CellSearch system was used to detect CTCs in whole PB. Samples were subject to enrichment with anti-EpCAM coated beads. CTCs were defined as nucleated cells lacking CD45 but expressing cytokeratins 8, 18, or 19. The presence of 1 or more epithelial cells per 10 ml of blood in any of the 3 tubes collected per patient was considered a positive result. Specimens were stored at room temperature and processed within one day of surgery.
Clinical and pathological data
Primary tumor characteristics included tumor size, ER, PR, HER2, tumor grade, histologic type, lymphovascular invasion (LVI) and Ki-67. The presence of lymph node metastases and BMM were also recorded and compared to the presence of CTCs. Systemic chemotherapy including adjuvant and neoadjuvant regimens was recorded.
Statistical analysis
Univariate analysis with the Chi squared test was performed. For study variables, risk ratios were used to describe the magnitude of the effect on likelihood of detecting CTCs.
Results
Ninety-two breast cancer patients with Stage I–III operable disease were enrolled in our protocol; ninety patients have completed their surgical treatment and two have surgical therapy pending. Of the ninety patients who underwent surgery, 64 had bone marrow aspirations performed for assessment for cytokeratins; 26 patients either declined bone marrow aspiration or had an inadequate quantity of bone marrow sampled for assay performance. The median follow-up duration was 1.2 years and has not yet reached maturity for evaluation of survival outcomes. Thirty-eight percent of patients (35/92) had evidence of CTC(s). Patient and tumor characteristics are shown in Table 1. For patients with CTCs detected, the median number of CTCs present was 1.0 CTC per 30 ml of blood (range 1–22). The mean number of CTCs detected was 2.9 per CTC positive patient. The number of CTCs detected in the positive samples included 50% with 1 CTC, 22% with 2 CTCs and 22% with greater than or equal to 4 CTCs per 30 ml. Of the patients with CTCs, 15/35 (42.9%) had neoadjuvant chemotherapy. Overall, 30/92 patients (32.6%) had neoadjuvant chemotherapy. Administration of chemotherapy prior to collection of blood did not significantly affect the ability to detect CTCs (P = 0.10). CTCs were identified in 50% of patients who had neoadjuvant chemotherapy as opposed to 32.2% of patients who were chemotherapy naïve.
Table 1.
Primary tumor, nodal, circulating tumor cell and bone marrow characteristics of the study population
| Variable | Status | N | Percent |
|---|---|---|---|
| Tumor size (T) | T1 | 29 | 32 |
| T2 | 37 | 40 | |
| T3 | 9 | 10 | |
| T4 | 17 | 18 | |
| Histology | Infiltrating ductal | 68 | 75 |
| Estrogen receptor status | + | 55 | 60 |
| − | 37 | 40 | |
| Progesterone receptor status | + | 47 | 51 |
| − | 45 | 49 | |
| HER2 status | + | 13 | 14 |
| − | 79 | 86 | |
| Grade | Low | 9 | 10 |
| Intermediate | 37 | 41 | |
| High | 45 | 49 | |
| Ki-67 | + | 21 | 55 |
| − | 17 | 45 | |
| Lymphovascular invasion | + | 31 | 34 |
| − | 59 | 66 | |
| Lymph node status (N) | N0 | 43 | 47 |
| N1 | 33 | 36 | |
| N2 | 3 | 3 | |
| N3 | 13 | 14 | |
| CTCs | + | 35 | 38 |
| − | 57 | 62 | |
| Bone Marrow micrometastases | + | 17 | 27 |
| − | 47 | 73 |
Thirty five patients had CTCs detected but only 17 patients had DTCs detected
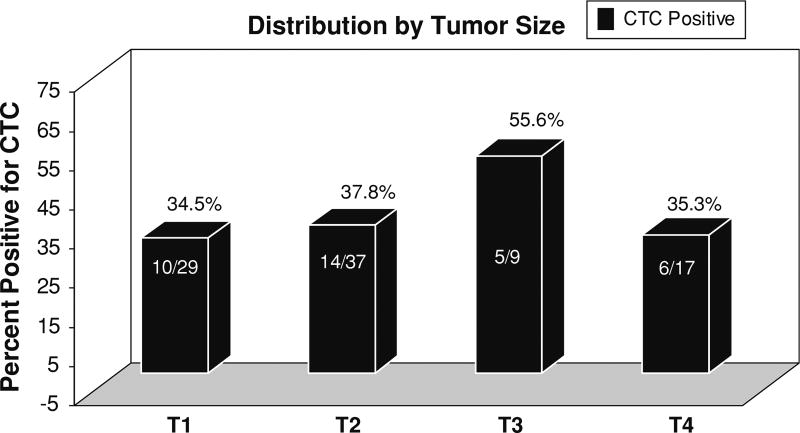
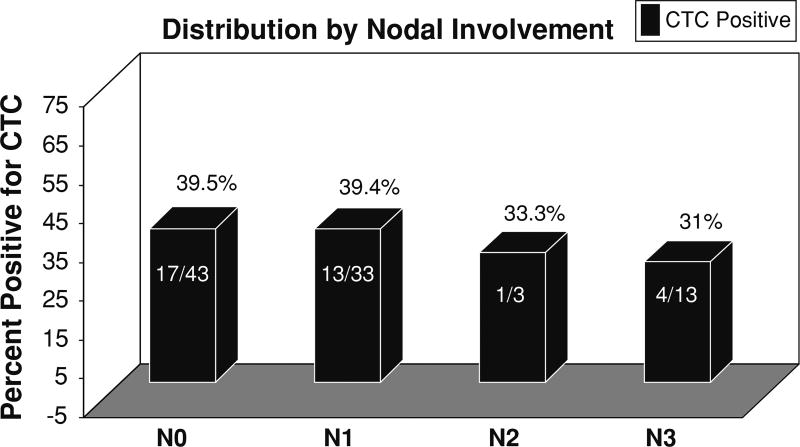
HER2 status of the primary tumor was the only characteristic that independently predicted the presence of CTCs (P = 0.01, risk ratio = 3.66) (Tables 2 and 3). There were 9 patients who had HER2 positive primary tumors and had CTCs detected. Nine of the 13 patients with HER2 positive tumors had CTCs detected. In contrast, no significant association was found between the presence of CTCs and other characteristics, including primary tumor size (Table 3). The distribution of CTC results by tumor size and nodal involvement is depicted in Figs. 1 and 2. BMM were identified in 17/64 (26.5 %) patients. Interestingly, BMM did not correlate with the presence of CTCs (P = 0.95).
Table 2.
2 × 2 Table demonstrating that HER2 predicts the presence of CTCS
| Status | Her-2 (+) | Her-2 (−) | Total |
|---|---|---|---|
| CTCs (+) | 9 | 26 | 35 |
| CTCs (−) | 4 | 53 | 57 |
| Total | 13 | 79 | 92 |
P-value = 0.01 (Fisher’s exact test), RR = 3.66, 95% CI for RR = 1.22–11.01
Table 3.
Factors analyzed for potential association with the presence of CTCs
| Characteristics | Percentage of CTCs + |
Risk ratio (RR) |
95% CI for RR |
P-value |
|---|---|---|---|---|
| Tumor size | ||||
| T1 | 34% (10/29) | 0.85 | 0.45–1.63 | 0.63 |
| T2 | 38% (14/37) | 0.99 | 0.59–1.66 | 0.97 |
| T3 | 56% (5/9) | 2.04 | 0.56–7.07 | 0.25 |
| T4 | 35% (6/17) | 0.89 | 0.36–2.19 | 0.79 |
| Infiltrating ductal histology | 38% (26/68) | 1.03 | 0.50–2.12 | 0.94 |
| Estrogen receptor positive | 35% (19/55) | 0.86 | 0.60–1.24 | 0.39 |
| Progesterone receptor positive | 36% (17/47) | 0.92 | 0.61–1.41 | 0.70 |
| HER2 positive | 69% (9/13) | 3.66 | 1.22–11.01 | 0.01 |
| High grade | 42% (19/45) | 1.23 | 0.81–1.85 | 0.34 |
| Ki-67 positive | 52% (11/21) | 1.51 | 0.86–2.66 | 0.15 |
| Lymphovascular invasion present | 39% (12/31) | 1.09 | 0.61–1.95 | 0.77 |
| Nodal status | ||||
| N0 | 40% (17/43) | 1.06 | 0.68–1.66 | 0.78 |
| N1 | 39% (13/33) | 1.06 | 0.61–1.85 | 0.84 |
| N2 | 33% (1/3) | 1.23 | 0.11–13.05 | 0.86 |
| N3 | 31% (4/13) | 0.72 | 0.24–2.17 | 0.56 |
| Bone marrow micrometastases | 41% (7/17) | 1.02 | 0.45–2.34 | 0.95 |
HER2 was the sole primary tumor characteristic that predicted the presence of CTCs
Fig. 1.
Distribution of CTC status by tumor size. Tumor size does not correlate with the presence of CTCs
Fig. 2.
Distribution of CTC status by nodal involvement. Nodal involvement does not correlate with the presence of CTCs
Discussion
In this series of operable breast cancer patients, HER2 status was the sole primary tumor characteristic that predicted the presence of CTCs. Primary tumors are routinely tested for HER2 amplification or over-expression to determine if the use of trastuzumab (Herceptin, Genentech, South San Francisco CA) is appropriate. Although HER2 positive status was previously considered an adverse prognostic feature, treatment with trastuzumab is predictive of improved survival for HER2 positive patients [23].
The significance of CTCs in operable breast cancer patients remains to be determined by large prospective studies with adequate length of follow-up. In the present study the presence of CTCs was not statistically associated with the presence of DTCs; additional accrual to our study and other studies will be required to evaluate this potential association [24]. It has been hypothesized that CTCs will be prognostic of adverse outcome in early stage breast cancer, just as they are for metastatic breast cancer. In particular, many investigators speculate that primary tumor or CTC biomarkers could predict an improved response to therapy. The HER2 status of CTCs and BMM have been the focus of several prior investigations [3, 5, 25]. The interpretation of this literature is complicated by the fact that different groups used markedly different assays to isolate CTCs/ BMM and to characterize HER2 status.
The present study utilized the CellSearch System, which is robust, reproducible, sensitive, and has been validated by large series [15, 19, 20]. Ignatiadis et al. found a higher incidence of CTCs in ER negative, triple negative and HER2 positive primary tumors [26]. Hayes et al. demonstrated that CTCs may acquire HER2 over-expression on serial examination despite the primary tumor being HER2 negative [27]. Meng et al. compared HER2 FISH status between 31 patients’ primary tumors and CTCs and found 97% concordance, although 9 of 24 HER2 negative patients’ CTCs acquired HER2 amplification during disease progression [5]. Meng et al. also reported a high concordance of co-amplification of HER2 with urokinase plasminogen activator (uPAR) when comparing CTCs to primary tumors [25]. Therefore, we hypothesize that in the majority of patients included in our study, CTCs also overexpressed HER2. In contrast to our study of CTCs in operable breast cancer patients, the study by Meng et al. included both metastatic and operable breast cancer patients [25].
Wülfing et al. reported decreased survival in 42 patients with HER2 positive CTCs assessed by ICC; interestingly, HER2 positive CTCs were detected in cases with HER2 negative primary tumors [3]. This study also included both metastatic and operable breast cancer patients. At a median follow-up of 95 months, the presence and frequency of HER2 positive CTCs correlated with significantly decreased disease-free and overall survival on univariate analysis; however, this effect was not borne out on multivariate analysis [3]. This study also evaluated the clinicopathologic features of 35 patients with operable breast cancer and correlated them with the presence of HER2 positive CTCs. They found that only intermediate/high grade rather than low grade primary tumors and ER negative status correlated with the presence of HER2 positive CTCs [3]. Therefore, unlike the present study, Wülfing et al. did not report on primary tumor characteristics that predicted the presence of CTCs [3].
Gaforio et al. looked at primary tumor characteristics that predicted the presence of CTCs in 92 breast cancer patients (Stage I–IV) [8]. The study included 25 Stage IV breast cancer patients. Detection of CTCs was stage-dependent: they found CTCs to be present in 33.3% of Stage I, 64.1% of Stage II, 63.6% of Stage III and 16%of Stage IV breast cancer patients [8]. Furthermore, they demonstrated that ER expression (P = 0.049) and lymph node positive status (P = 0.033) predicted the presence of CTCs, however, HER2 status did not predict the presence of CTCs (P = 1) [8]. Although their study reported a median follow-up duration of only 21 months, the presence of CTCs prior to starting chemotherapy was correlated with progression-free survival (P = 0.058) and overall survival (P = 0.003) [8]. It would be interesting to know if there was a survival difference associated with the presence of CTCs if the Stage IV patients were excluded from their Kaplan-Meier survival analysis, but this analysis was not included in their study. The differences between our results and those of Gaforio et al. are most likely explained by markedly different experimental techniques and their inclusion of heterogeneous populations of metastatic and operable breast cancer patients. They reported using immunohistochemistry of primary tumor samples to identify HER2 status but did not clarify what degree of IHC positive was defined as a positive result [8]; FISH results were not reported in that study. Moreover, the methodology used for the detection of CTCs consisted of density gradient separation, immunomagnetic separation and recovery by ICC is quite distinct from the CellSearch System. Unfortunately, there is no standardized approach to the enumeration of CTCs, making comparisons between studies problematic. In an effort to provide the context in which our conclusions regarding the CTC biomarker apply, we have adhered to the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria [28].
In metastatic breast cancer, the detection of CTCs has been demonstrated to be of prognostic significance independent of primary tumor characteristics, suggesting the possibility that those cells represent a population of tumorigenic tumor cells [29]. This observation further supports the need to carefully evaluate the correlation between primary tumor characteristics and CTCs in primary disease.
The ability to identify patients at higher risk of harboring CTCs would be helpful both for the purpose of establishing the prognostic value and for characterizing the biologic properties of CTCs. This study identified HER2 as the sole primary tumor characteristic that predicted the presence of CTCs. The presence of HER2 amplification is associated with aggressive tumor biology, however, the availability of trastuzumab offers a useful treatment option for HER2 positive patients [23]. Long-term follow-up will be required to determine the significance of CTCs in operable breast cancer patients. Moreover, larger datasets with determination of HER2 status of the primary tumor, CTCs and BMM would enable evaluation of the potential of CTCs and BMM for targeted therapy.
Acknowledgments
Grant support: DAMD17-03-1-0669 (A. Lucci) and NIH grant R21-DK067682 (A. Lucci).
Footnotes
Presented in part at the 2007 Society of Surgical Oncology Cancer Symposium.
Contributor Information
Julie E. Lang, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA
Kailash Mosalpuria, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA.
Massimo Cristofanilli, Department of Medical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, Houston, TX, USA.
Savitri Krishnamurthy, Department of Pathology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, Houston, TX, USA.
James Reuben, Department of Pathology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, Houston, TX, USA.
Balraj Singh, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA.
Isabelle Bedrosian, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA.
Funda Meric-Bernstam, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA.
Anthony Lucci, Department of Surgical Oncology, MD Anderson Cancer Center, Advanced Research Center for Microscopic Disease (ARC-MD), The University of Texas, 1400 Holcombe Blvd FC 12.3052, Unit 444, Houston, TX 77030, USA.
References
- 1.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 2.Lang JE, Hall CS, Singh B, et al. Significance of micrometastasis in bone marrow and blood of operable breast cancer patients: research tool or clinical application? Expert Rev Anticancer Ther. 2007;7:1463–1472. doi: 10.1586/14737140.7.10.1463. [DOI] [PubMed] [Google Scholar]
- 3.Wulfing P, Borchard J, Buerger H, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715–1720. doi: 10.1158/1078-0432.CCR-05-2087. [DOI] [PubMed] [Google Scholar]
- 4.Pierga JY, Bonneton C, Vincent-Salomon A, et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clinical Cancer Research. 2004;10:1392–1400. doi: 10.1158/1078-0432.ccr-0102-03. [DOI] [PubMed] [Google Scholar]
- 5.Meng S, Tripathy D, Shete S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA. 2004;101:9393–9398. doi: 10.1073/pnas.0402993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xenidis N, Perraki M, Kafousi M, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006;24:3756–3762. doi: 10.1200/JCO.2005.04.5948. [DOI] [PubMed] [Google Scholar]
- 7.Zach O, Kasparu H, Wagner H, et al. Prognostic value of tumour cell detection in peripheral blood of breast cancer patients. Acta Med Austriaca Suppl. 2002;59:32–34. [PubMed] [Google Scholar]
- 8.Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107:984–990. doi: 10.1002/ijc.11479. [DOI] [PubMed] [Google Scholar]
- 9.Stathopoulos EN, Sanidas E, Kafousi M, et al. Detection of CK-19 mRNA-positive cells in the peripheral blood of breast cancer patients with histologically and immunohistochemically negative axillary lymph nodes. Ann Oncol. 2005;16:240–246. doi: 10.1093/annonc/mdi043. [DOI] [PubMed] [Google Scholar]
- 10.Benoy IH, Elst H, Philips M, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94:672– 680. doi: 10.1038/sj.bjc.6602985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naume B, Borgen E, Beiske K, et al. Immunomagnetic techniques for the enrichment and detection of isolated breast carcinoma cells in bone marrow and peripheral blood. J Hematother. 1997;6:103–114. doi: 10.1089/scd.1.1997.6.103. [DOI] [PubMed] [Google Scholar]
- 12.Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Wang K, Tan W, et al. Quantitative intracellular molecular profiling using a one-dimensional flow system. Analytical chemistry. 2006;78:6246–6251. doi: 10.1021/ac060598e. [DOI] [PubMed] [Google Scholar]
- 14.Ring A, Smith IE, Dowsett M. Circulating tumour cells in breast cancer. Lancet Oncol. 2004;5:79–88. doi: 10.1016/S1470-2045(04)01381-6. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 16.Tibbe AG, Miller MC, Terstappen LW. Statistical considerations for enumeration of circulating tumor cells. Cytometry A. 2007;71:154–162. doi: 10.1002/cyto.a.20369. [DOI] [PubMed] [Google Scholar]
- 17.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 18.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 19.Rack B, Schindlbeck C, Janni W, et al. Incidence of circulating tumor cells (CTCs) in peripheral blood of breast cancer patients at primary diagnosis—a potential tool for risk stratification, ASCO Annual Meeting. J Clin Oncol (ed) (2006) 2006 ASCO Annual Meeting Proceedings Part I. 2006;24(18S: June 20 Supplement):20053. [Google Scholar]
- 20.Rack B, Schindlbeck C, Hofmann S, et al. Circulating tumor cells (CTCs) in peripheral blood of primary breast cancer patients, ASCO Annual Meeting. J Clin Oncol (2007) 2007 ASCO Annual Meeting Proceedings Part I. 2007;25(18S: June 20 Supplement):10595. [Google Scholar]
- 21.Nimeus-Malmstrom E, Ritz C, Eden P, et al. Gene expression profilers and conventional clinical markers to predict distant recurrences for premenopausal breast cancer patients after adjuvant chemotherapy. Eur J Cancer. 2006;42:2729–2737. doi: 10.1016/j.ejca.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Pantel K, Schlimok G, Braun S, et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- 23.Smith I, Procter M, D Gelber R, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 24.Alvarado M, Brissaud C, Scott J, et al. Disseminated tumor cells correlate with estrogen receptor positivity in operable breast cancer patients; San Antonio Breast Cancer Symposium.2007. [Google Scholar]
- 25.Meng S, Tripathy D, Shete S, et al. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc Natl Acad Sci USA. 2006;103:17361–17365. doi: 10.1073/pnas.0608113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ignatiadis M, Xenidis N, Perraki M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007;25:5194–5202. doi: 10.1200/JCO.2007.11.7762. [DOI] [PubMed] [Google Scholar]
- 27.Hayes DF, Walker TM, Singh B, et al. Monitoring expression of HER-2 on circulating epithelial cells in patients with advanced breast cancer. Int J Oncol. 2002;21:1111–1117. doi: 10.3892/ijo.21.5.1111. [DOI] [PubMed] [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 29.Cristofanilli M, Broglio KR, Guarneri V, et al. Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer. 2007;7:471–479. [PubMed] [Google Scholar]