Abstract
Objectives
To evaluate the reliability of normal Thyroid Stimulating Hormone (TSH) as a thyroid function test and assess the effect of Adrenocorticotropic Hormone (ACTH) on serum TSH concentration.
Design and Methods
Patients presenting to the National Institutes of Health Department of Endocrinology outpatient clinic with symptoms consistent with hypothyroidism were identified. Thyroid hormone concentrations were measured by liquid chromatography/tandem mass spectrometry and immunoassay. Patients with normal TSH concentrations were assessed for both clinical and biochemical hypothyroidism.
We evaluated the effect of ACTH stimulation (performed on patients for assessment of adrenal function) on TSH concentration.
Results
Patients with symptoms consistent with hypothyroidism but with normal TSH values in the range of 1–4 IU/mL and normal free T4 (FT4) values by immunoassay measurements were confirmed to be biochemically hypothyroid following measurements of thyroid hormones by mass spectrometry. We present case studies of two patients, a 76-year-old male and a 58-year-old female. Improvement in the male patient’s hypothyroid symptoms, including afternoon fatigue, constipation, alopecia, dry skin and high cholesterol, was documented after initiating thyroid hormone replacement.
ACTH stimulation resulted in an average decrease of 17% in TSH between time 0 and 60 minutes post stimulation.
Conclusion
Although measurement of TSH is a convenient screen for thyroid function, it is influenced by many factors which may affect its overall reliability. We believe thyroid function should be assessed by more than a single test. We recommend measurement of thyroid hormone concentrations by mass spectrometry if the patient’s clinical presentation is discordant with their TSH levels.
Keywords: Thyroid, Hypothyroidism, TSH, Thyroid hormones, Mass spectrometry
Introduction
Hypothyroidism is a disorder that affects an estimated 5%–10% of the population in the United States [1,2] and 5% of the population worldwide [2]. Individuals with hypothyroidism may also experience symptoms including fatigue, dry skin, decreased cognitive function and elevated cholesterol [3].
The clinical manifestations of hypothyroidism vary widely and are usually dependent on the age at onset, the duration, as well severity of the disease. Hence, the accurate measurement of thyroid function and the serum levels through accurate quantitation of thyroid hormones by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) is critical for the assessment, treatment and monitoring of patients with thyroid diseases as the diagnosis and management relies heavily on performing the correct laboratory tests [4].
Measurement of Thyroid Stimulating Hormone (TSH) concentrations by immunoassay is used by the majority of clinical laboratories to assess thyroid function [5]. These assays are subject to a number of interferences, including biologically inactive isoforms of TSH, heterophilic antibodies and biotin supplements [6–8]. In addition, the concentration of serum TSH is affected by a number of non-thyroid hormone factors, such as pregnancy, age, sex, exercise, individual thyroid hormone set points, the timing of levothyroxine administration, steroids, and drugs [9–17]. Additionally, TSH may also undergo seasonal and diurnal fluctuations [18]. We have conducted extensive comparisons in hypo- and hyperthyroid patients with both immunoassay and LC-MS/MS. In general, agreement is adequate for FT4, FT3 and TT3 in the hyperthyroid area but very poor in the hypothyroid area [19–21]. This is a significant clinical problem as two-thirds of patients with thyroid disease are hypothyroid [1,22], accounting for 20 million people in the USA alone. We have demonstrated a positive bias for TT3, FT3 and FT4 by IAs, particularly at low concentrations, which prevented the correct diagnosis of approximately 50% of these patients. These patients were tested to be biochemically hypothyroid by LC-MS/MS and showed hypothyroid symptoms [19,21].
We have previously reported the case study of a patient with a medical history of hypothyroidism complaining of lethargy despite treatment with T4 [21]. Her serum TSH levels were within the normal range as measured by IA, but measurements of thyroid hormones with LC-MS/MS revealed that she had low TT3 and low-normal Free T3 (FT3) [21]. Addition of T3 to this patient’s T4 regimen normalized her T3 levels, lowered her cholesterol, and resolved her symptoms, demonstrating that a normal serum TSH level does not rule out hypothyroidism.
We present two additional cases in which serum TSH measurements alone should not be the sole criteria in the diagnosis of hypothyroidism. In our clinic, several of our patients suspected of having hypothyroidism, were found to have normal serum TSH concentrations. Assessing thyroid function tests by LC-MS/MS illustrated that they did indeed suffer from biochemical hypothyroidism. One of the patients received low-dose thyroid hormones, which alleviated the patient’s hypothyroidism.
We also investigated effect of ACTH on TSH concentrations in patients undergoing ACTH stimulation to evaluate adrenal function.
Materials and Methods
Patients and samples
Patients with symptoms of decreased thyroid function were identified in the NIH outpatient clinic. Thyroid hormone measurements by mass spectrometry confirmed them to be biochemically hypothyroid.
The effect of ACTH administration on serum TSH levels was assessed in 21 patients who were undergoing adrenal function testing between 8AM and 9AM. TSH was measured before administration of ACTH and 1 hour after ACTH stimulation.
This study was approved by the National Institutes of Health institutional review board (IRB, clinical protocol number 93-CC-0094) and the Georgetown IRB (Pro0000007-01).
Cholesterol, thyroid hormone and TSH measurements
Cholesterol, TSH, and thyroid hormones were measured using the Roche Cobas 6000 (Indianapolis, IN). FT3 and FT4 were measured by ultrafiltration isotope dilution LC-MS/MS using a SCIEX Triple-Quad-6500 System (AB Sciex, Concord, ON, CA) as previously described [19]. TT3, and TT4 were measured by atmospheric pressure photoionization liquid chromatography-tandem mass spectrometry using the Agilent 6460 triple-quadrapole mass spectrometer coupled to the Agilent 1200 Infinity Series HPLC (Agilent Technologies, Santa Clara, CA, USA) as previously described [23].
Results
To date we have identified three patients who clinically presented with symptoms of hypothyroidism, yet had TSH measurements within the reference range (0.2–4 µIU/mL) (Table 1). A case presentation of the first patient identified was previously published by our group [21] and we now present two additional cases.
Table 1.
TSH and thyroid hormone levels for patients 1–3.
| Patient | TSH (0.2–4 µIU/mL) |
FT3 (2.2 – 6.2 pg/ mL) |
FT4 (1.3 – 2.4 ng/ dL) |
TT3 (87–180 ng/ dL) |
TT4 (5.1–11.3 µg/ dL) |
Diagnosis by TSH |
Diagnosis of biochemical hypothyroidism |
Clinical conditions |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.65 | 2.1 | 1.9 | 82 | 13.4 | Euthyroid | Hypothyroid | Hypothyroid |
| 2 | 2.65 | 2.1 | 1.2 | 91 | 6.4 | Euthyroid | Hypothyroid | Hypothyroid |
| 3 | 1.33 | 2.0 | 1.2 | 45 | 3.5 | Euthyroid | Hypothyroid | Hypothyroid |
TSH: Thyroid Stimulating Hormone; FT3: Free Triiodothyronine; FT4: Free Thyroxine; TT3: Free Triiodothyronine; TT4: Free Thyroxine
TSH was measured by immunoassay and free and total thyroid hormones were measured by LC/MS-MS. Reference ranges for each analyte indicated in parenthesis. Values below the reference range are shown in red. Patient 1 data from [21].
Based on TSH and FT4 measurements by IA, all patients appear to be euthyroid. However, when total and free thyroid hormone concentrations were measured by LC-MS/MS, all patients were diagnosed as having biochemical hypothyroidism.
Case Report
This is a 76-year-old male who presented with the following symptoms: fatigue by early afternoon, constipation, dry skin, and hair loss. His laboratory values by IA were normal for all thyroid tests. His TSH was 2.65 µIU/mL and his cholesterol was 250 mg/dL. The patient had low FT3 at 2.1 pg/mL (reference range 2.2 – 6.2 pg/mL) and low FT4 at 1.2 ng/mL (reference range 1.3–2.4 ng/dL) as measured by LC-MS/MS (Table 1), indicating biochemical hypothyroidism. He was started on 5 µg/d T3 and 25 µg/d T4. After 2 months on low-dose thyroid hormones, his FT4 increased to 1.8 ng/dL and FT3 increased to 3.6 pg/dL by LC-MSMS. The patient’s TSH and cholesterol dropped to 1.95 µIU/mL and 232 mg/dL, respectively. Significantly, he described having increased late afternoon energy and improved cognitive function, and he no longer suffered from constipation and dry skin. The patient also reported that his urinary flow-rate has increased by what he estimates to be roughly 3-fold since taking thyroid hormones. The patient’s hypothyroidism presented in the context of a normal TSH and was successfully treated by a low-dose thyroid hormone regimen.
The third patient is a 58-year-old female with a single kidney and nephrotic syndrome. She complained of feeling weak, dry skin, alopecia, and severe fatigue, clinically consistent with hypothyroidism. Her 24-hour urinary protein was approximately 1 g/day. Her low serum thyroxine-binding globulin (TBG, 5.6 µg/mL, reference range 13 – 39 µg/mL), the main thyroid hormone binding protein, suggests that she is losing TBG and thyroid hormones in her urine. Free and total thyroid hormones were all low as measured by mass spectrometry (Table 1), indicating that she was biochemically hypothyroid, despite her normal TSH concentration at 1.33 µIU/mL. She will shortly begin thyroid hormone therapy.
ACTH Stimulation
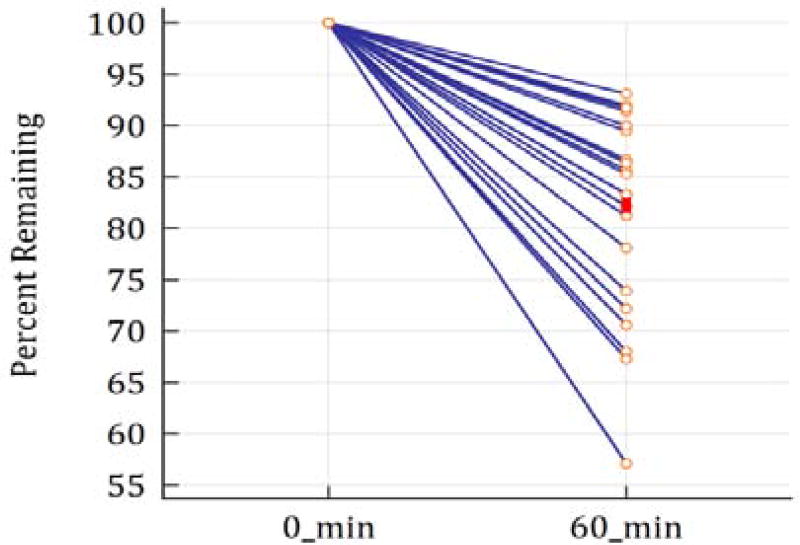
Our studies on the effect of ACTH administration on serum TSH concentration were conducted in the morning between 8AM and 9AM. Sixty minutes post ACTH simulation, TSH showed a mean decrease of 17% compared to baseline with minimum and maximum decreases of 6.9% and 42.9% (Figure 1). This effect is presumably caused by the increased steroid concentrations as a result of the ACTH stimulation. This further supports our contention that TSH measurement is interfered with by many compounds which could affect the diagnosis of hypothyroidism.
Figure 1.
ACTH stimulation lowers TSH levels. The percent decrease in TSH levels are shown relative to baseline 60 minutes post ACTH stimulation in 21 patients. The solid red dot indicates the mean TSH percentage decrease.
Discussion
Measurement of TSH by immunoassay is used by endocrinologists to screen for and diagnose thyroid disorders. Based solely on TSH levels, the three patients identified in this and a previous study [21] appeared to be euthyroid. However, the low levels of thyroid hormones as measured by LC-MS/MS are consistent with their symptoms, indicating that the patients were biochemically hypothyroid even with normal TSH concentrations. Importantly, treatment with thyroid hormones alleviated symptoms and lowered total cholesterol in the case report of the 76-year-old male presented above.
Thyroid hormone levels measured by LC-MS/MS show a higher correlation with the patients’ clinical conditions than levels measured by immunoassay, especially at the low concentrations at which clinical decisions are made [18–21]. Because many endocrinologists rely on immunoassays to assess thyroid function, the concern is raised that many patients with thyroid hormones at the lower end of the analytical measurement range are at risk of being misdiagnosed as being euthyroid. This observation may lead to new investigations into elderly patients with biochemical hypothyroidism, an important research question addressed in a comprehensive review by Deary et al [6]. Despite the need for specialized equipment, staff with extensive training, and high test-costs, LC-MS/MS could be used as a reflex test in situations where immunoassay results are incongruent with the patient’s clinical presentation. In contrast to LC-MS/MS, we have shown that IA for TT3, FT3 and FT4 have a positive bias at low concentrations resulting in misdiagnosis of these patients. A normal TSH measurement could also be associated with hypothyroidism.
The administration of glucocorticoids decreases TSH levels [6,13–17]. ACTH stimulation leads to an increase in many steroids in healthy patients [24], which is the likely cause of the decrease in TSH we observed in this study.
The suppression of TSH by corticosteroids has important implications for the diagnosis of thyroid diseases, especially in neonates. Neonatal steroid hormone levels are much higher than those in adults. For example, the reference range for pregnenolone in infants between 1 and 7 days old is 150–2000 ng/dL [25] while reference ranges in adult males and females are 23–173 ng/dL and 15–132 ng/dL, respectively [26]. The higher steroid concentrations could suppress TSH secretion in newborns, masking an underactive thyroid. Undiagnosed hypothyroidism in newborns and children results in marked deficiencies in neurocognitive development, which can be prevented by early administration of thyroid hormone [27,28]. Therefore, it may be important to measure thyroid hormone levels by mass spectrometry in infants to accurately assess their thyroid status. A rigorous study evaluating newborn screening programs with either TSH or FT4/FT3 measured by LC-MS/MS is needed.
Conclusion
Normal TSH values may not rule out patients that are hypothyroid. It is possible that a large number of hypothyroid individuals are missed using TSH as a screening tool, which is a problem for the patient, who remains hypothyroid, and to the healthcare system as a whole.
Although TSH is a useful screening tool for thyroid disorders, it is affected by many different factors. In cases where thyroid hormone levels are borderline low as measured by immunoassay, it is recommended that LC-MS/MS be used as a reflex test in order to assess the possibility of biochemical hypothyroidism.
Future studies are also needed to reassess the reliability of neonatal screening for hypothyroidism using TSH. Perhaps measurement of FT4/FT3 by LC-MS/MS would reveal that TSH is missing the diagnosis of hypothyroidism a small but important percentage of these neonates.
Abbreviations
- TSH
Thyroid stimulating hormone
- FT3
Free Triiodothyronine
- FT4
Free Thyroxine
- TT3
Free Triiodothyronine
- TT4
Free Thyroxine
- ACTH
Adrenocorticotropic Hormone
- LC-MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- TBG
Thyroxine-Binding Globulin
- HPLC
High-Performance Liquid Chromatography
References
- 1.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH. T(4) and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 3.Lithell H, Boberg J, Hellsing K, Ljunghall S, Lundqvist G, Vessby B, et al. Serum lipoprotein and apolipoprotein concentrations and tissue lipoprotein-lipase activity in overt and subclinical hypothyroidism: the effect of substitution therapy. Eur J Clin Invest. 1981;11:3–10. doi: 10.1111/j.1365-2362.1981.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 4.Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. European Society of Endocrinology. 2016;175:255–263. doi: 10.1530/EJE-16-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rifai N, Horvath AR. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 6. Vol. 1888 Elsevier; 2017. [Google Scholar]
- 6.Deary M, Buckey T, Soldin OP. TSH - Clinical aspects of its use in determining thyroid disease in the elderly. How does it impact the practice of medicine in aging? Adv Pharmacoepidemiol Drug Saf. 2012;1:9369. doi: 10.4172/2167-1052.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santhana Krishnan SG, Krishnan SGS, Pathalapati R, Kaplan L, Cobbs RK. Falsely raised TSH levels due to human anti-mouse antibody interfering with thyrotropin assay. Postgrad Med J. 2006;82:27. doi: 10.1136/pmj.2006.049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwok JS-S, Chan IH-S, Chan MH-M. Biotin interference on TSH and free thyroid hormone measurement. Pathology. 2012;44:278–280. doi: 10.1097/PAT.0b013e3283514002. [DOI] [PubMed] [Google Scholar]
- 9.Andersen S. Narrow individual variations in serum T4 and T3 in normal subjects: A clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 10.Neto RAL, de Souza Dos Santos MC, Rangel IF, Ribeiro MB, Cavalcanti-de-Albuquerque JPA, Ferreira ACF, et al. Decreased serum T3 after an exercise session is independent of glucocorticoid peak. Horm Metab Res. 2013;45:893–899. doi: 10.1055/s-0033-1351279. [DOI] [PubMed] [Google Scholar]
- 11.Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27:745–762. doi: 10.1016/j.beem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach-Huynh T-G, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94:3905–3912. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamstedt A, Jarnerot G, Kagedal B, Soderholm B. Corticosteroids and thyroid function. Different effects on plasma volume, thyroid hormones and thyroid hormone-binding proteins after oral and intravenous administration. Acta Med Scand. 1979;205:379–383. [PubMed] [Google Scholar]
- 14.Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest. American Society for Clinical Investigation. 1969;48:2096–2103. doi: 10.1172/JCI106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franchimont P, Cramilion C. The effect of danazol on anterior pituitary function. Fertil Steril. 1977;28:814–817. [PubMed] [Google Scholar]
- 16.Jensen J, Nolan G, Jubiz W. The effect of prednisone on serum thyrotropin, thyroxine and triiodothyronine concentrations in hypothyroid patients. J Endocrinol Invest. 1978;1:171–173. doi: 10.1007/BF03350367. [DOI] [PubMed] [Google Scholar]
- 17.Brabant A, Brabant G, Schuermeyer T, Ranft U, Schmidt FW, Hesch RD, et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol. 1989;121:95–100. doi: 10.1530/acta.0.1210095. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Mommen K, Hendrickx D, Peeters D, D’Hondt P, Ranjan R, et al. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol (Oxf) 1997;46:587–598. doi: 10.1046/j.1365-2265.1997.1881002.x. [DOI] [PubMed] [Google Scholar]
- 19.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011;57:122–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 20.Gounden V, Jonklaas J, Soldin SJ. A pilot study: subclinical hypothyroidism and free thyroid hormone measurement by immunoassay and mass spectrometry. Clin Chim Acta. 2014;430:121–124. doi: 10.1016/j.cca.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masika LS, Zhao Z, Soldin SJ. Is measurement of TT3 by immunoassay reliable at low concentrations? A comparison of the Roche Cobas 6000 vs. LC-MSMS. Clin Biochem. 2016;49:846–849. doi: 10.1016/j.clinbiochem.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002) Thyroid. 2007;17:1211–1223. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen LTGJ, Soldin OP, Soldin SJ. Development and validation of an isotope dilution tandem mass spectrometry method for the simultaneous quantitation of 3-iodothyronamine, thyroxine, triiodothyronine, reverse t3 and 3,3-diiodo-L-thyronine in human serum. Clin. Chem. 2011;57:A82. [Google Scholar]
- 24.Holst JP, Soldin SJ, Tractenberg RE, Guo T, Kundra P, Verbalis JG, et al. Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids. 2007;72:71–84. doi: 10.1016/j.steroids.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldin SJ, Wong EC, Brugnara C, Soldin OP. Pediatric reference intervals. 7. Washington, DC: AACC Press; 2011. [Google Scholar]
- 26.Laboratory test directory [Internet] 2017 [Google Scholar]
- 27.Klein AH, Meltzer S, Kenny FM. Improved prognosis in congenital bypothyroidism treated before age three months. J Pediatr. 1972;81:912–915. doi: 10.1016/s0022-3476(72)80542-0. [DOI] [PubMed] [Google Scholar]
- 28.Selva KA, Harper A, Downs A, Blasco PA, Lafranchi SH. Neurodevelopmental outcomes in congenital hypothyroidism: comparison of initial T4 dose and time to reach target T4 and TSH. J Pediatr. 2005;147:775–780. doi: 10.1016/j.jpeds.2005.07.024. [DOI] [PubMed] [Google Scholar]