Abstract
Marrow adipocytes, collectively termed marrow adipose tissue (MAT), reside in the bone marrow in close contact to bone cells and hematopoietic cells. Marrow adipocytes arise from the mesenchymal stem cell and share their origin with the osteoblastst. Shifts in the lineage allocation of the mesenchymal stromal cell could potentially explain the association between increased MAT and increased fracture risk in diseases such as postmenopausal osteoporosis, anorexia nervosa and diabetes. Functionally, marrow adipocytes secrete adipokines, such as adiponectin, and cytokines, such as RANK-ligand and stem cell factor. These mediators can influence both bone remodeling and hematopoiesis by promoting bone resorption and hematopoietic recovery following chemotherapy. In addition, marrow adipocytes can secrete free fatty acids, acting as a energy supply for bone and hematopoietic cells. However, this induced lipolysis is also used by neoplastic cells to promote survival and proliferation. Therefore, MAT could represent a new therapeutic target for multiple diseases from osteoporosis to leukemia, although the exaxt characteristics and role of the marrow adipocyte in health and diseases remains to be determined.
Keywords: Bone marrow adipocyte, Hematopoiesis, Bone remodeling, Mesenchymal stem cell
INTRODUCTION
Marrow adipose tissue (MAT) as the bone marrow adipocytes are collectively termed, is virtually absent at birth, expands during growth and development of the skeleton and accelerates its expansion during aging and menopause. Historically, marrow adipose tissue was considered inactive filling of the bone marrow cavity when bone mass was low or hematopoiesis impaired. But, animal and human studies during the past two decades have shown that MAT is increased in diseases such as osteoporosis, obesity, diabetes, and paradoxically, anorexia nervosa. This increase in MAT is often associated with deterioration of bone mass resulting in increased fracture risk although this association may not be causal. Therapies such as bisphosphonates, estrogen and parathyroid hormone (PTH) that improve bone quality, coincide with a decrease in MAT and this has led to a growing interest in marrow adipose tissue as a therapeutic target for osteoporosis. More recently, the association between MAT and hematopoietic diseases such as multiple myeloma and myeloid leukemia has been recognized. Bone marrow adipocytes have been shown to play an active role in the support of neoplastic cells in the bone marrow niche and are now considered a potential therapeutic target. In addition, genetic mouse models targeting the bone marrow adipocyte, have shown surprising effects on other adipose tissue depots and whole body energy metabolism.
Emerging mechanisms for the interaction between bone marrow adipocytes and bone remodeling and hematopoiesis are 1] the regulation of differentiation of the mesenchymal stromal cell, which is the progenitor of both the bone marrow adipocyte and the bone-forming cell, the osteoblast 2] the supply of free fatty acids as a source of fuel to energy-demanding processes such as bone formation, hematopoiesis and neoplastic growth 3] the secretion of soluble adipocyte-specific mediators termed ‘adipokines’ such as adiponectin and factors such as RANK ligand (RANKL) that can directly or indirectly affect bone, tumour and systemic metabolism.
In sum, marrow adiposity is considered within the realm of bone metabolism, hematopoiesis, cancer and systemic energy metabolism. It remains a possible therapeutic target for bone disease, hematopoietic diseases, bone marrow neoplasms and obesity. Nevertheless, crucial questions regarding its origin, function and secretory capacity in health and disease remain to be answered.
BONE MARROW ADIPOSITY IN HEALTH
Growth
During fetal life, the skeleton is formed through endochondral and intramembranous ossification. Longitudinal growth continues after birth, during childhood and adolescence, while acquisition of peak bone mass occurs between ages 15-30. During skeletal growth, bones not only increase in length, but also in diameter, a process known as modelling. During modelling, the marrow cavity also increases. The marrow cavity becomes filled with hematopoietic marrow during fetal life and is fully populated with hematopoietic cells at the end of the first term of pregnancy1. During fetal life, the main sites of hematopoiesis are firstly the yolk sac, followed by the liver and spleen during second and third trimester, when finally the hemtopoietic bone marrow becomes the main site of hematopoiesis2. Shortly before birth, the replacement of hematopoietic marrow by adipose marrow is initiated. The earliest conversion of hematopoietic to adipose marrow was studied in an infant autopsy study in 1964 which showed that, irrespective of term of birth, the conversion already started shortly before birth, accelerated following birth and was complete at 12 months of age in the proximal phalanx3. However, in the femur and tibia the appearance of adipose marrow starts later, around the age of 7 and is complete by the age of 18. In the ribs and vertebrae, macroscopic fat is not detected at all until old age4,5. These studies establish a pattern of peripheral to central replacement of hematopoietic marrow by adipose marrow in the skeleton; from the appendicular to the axial skeleton and from the diaphysis and distal metaphysis to the proximal metaphysis (figure 1). The same patterns of hematopoietic and adipose marrow development were observed using magnetic resonance imaging (MRI) scans in cross-sectional studies in infancy and adolescence6,7. These studies showed that indeed marrow adiposity increases with age in 4- to 10-year old girls and is accompanied by an increase in total body fat and total bone mineral content in the femur8. Although total bone mineral content is positively associated with adipose marrow during childhood, other studies reported a negative association between adipose marrow and femoral cortical bone9 and pelvic bone mineral density10.
Figure 1.
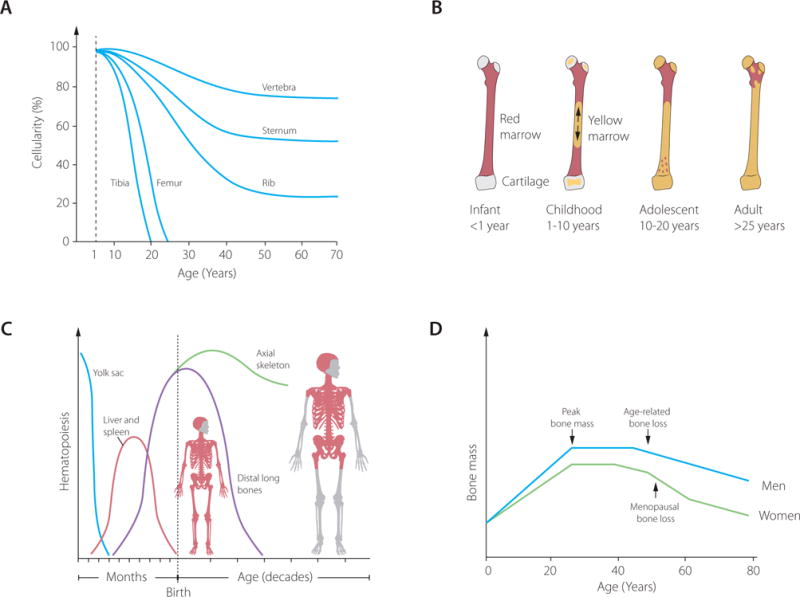
Changes in bone marrow hematopoietic cellularity per bone (A), conversion of hematopoietic to adipose marrow in the femur (B) development of hematopoiesis (C) and bone mass (D) during human life.
Aging
In healthy adults, marrow adiposity continues to increase with aging. Cross-sectional studies have shown that, depending on the MRI method used, marrow adiposity in the lumbar spine is 20–30% around the age of 20 and increases approximately 7% per 10 years to ±50% at age 5011-15. During these years, men have higher marrow adiposity than women, but this difference reverses later in life16. From 50 years of age, women have accelerated marrow adiposity accumulation resulting in >70% of the marrow volume at age 80 years compared to 60% in men. In the femur, marrow adiposity also increases with age but the marrow adiposity is higher at younger age starting at 60% in the diaphysis and 80% in the femur head at age 20–30, increasing to 80% and 90% respectively at age 50–6017. After reaching peak bone mass between the age of 15–30 years, bone mass declines with aging. In men, this decline is gradual whereas in women the decline accelerates perimenopausally and continues postmenopause.
Menopause
The accelerated marrow adiposity accumulation in women over 50 years of age coincides with menopause. Menopause is characterized by ovarian follicle depletion leading to decreased estrogen and progesterone and increased luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production18. Clinically, menopause is associated with bone loss and fat gain19,20. The increase in marrow adiposity in women around age 50 is hypothesized to be due to the hormonal changes of menopause although marrow adiposity during menopause has not been investigated longitudinally. However, two weeks of estrogen supplementation in postmenopausal women decreases marrow adiposity by 5% demonstrating that estrogen is indeed a regulator of marrow adiposity21. Although menopause induces irreversible hormonal changes, during the follicular and luteal phase of the menstrual cycle concentrations of gonadal and gonadotropic hormones fluctuate on a smaller scale. Interestingly, marrow adiposity increases during the follicular phase and decreases during the luteal phase although the magnitude of the change is indeed much smaller compared to menopause21.
In conclusion, marrow adipose tissue appears in the bone marrow cavity shortly before birth and expands during growth of the skeleton in childhood and adolescence in a distal-to-proximal fashion, replacing the hematopoietic marrow almost completely in the long bones by the age of 30 when reaching peak bone mass. During aging, vertebral marrow adipose tissue continues to increase and bone mass decreases. Men have higher marrow adiposity than women, but this difference reverses after menopause, implying that marrow adiposity is, at least partially, hormonally controlled. Further studies are needed to elucidate the exact role of marrow adiposity in growth and development and its regulation during the different stages of life.
EVALUATION OF MARROW ADIPOSITY
To measure bone marrow adiposity in humans, several methods have been developed (Figure 2). Historically, bone biopsies were performed to assess bone parameters and these biopsies included the marrow space. Bone biopsies are performed at the iliac crest bone. Marrow adipocytes are shown as holes in the biopsy, since the actual adipocytes are destroyed during the processing of the bone biopsies and therefore these are termed adipocyte ‘ghosts’. Marrow adipocytes can be quantified using imaging software manually or automatically. The parameters determined are the adipocyte total volume, individual volume (adipocyte size) and number.
Figure 2.
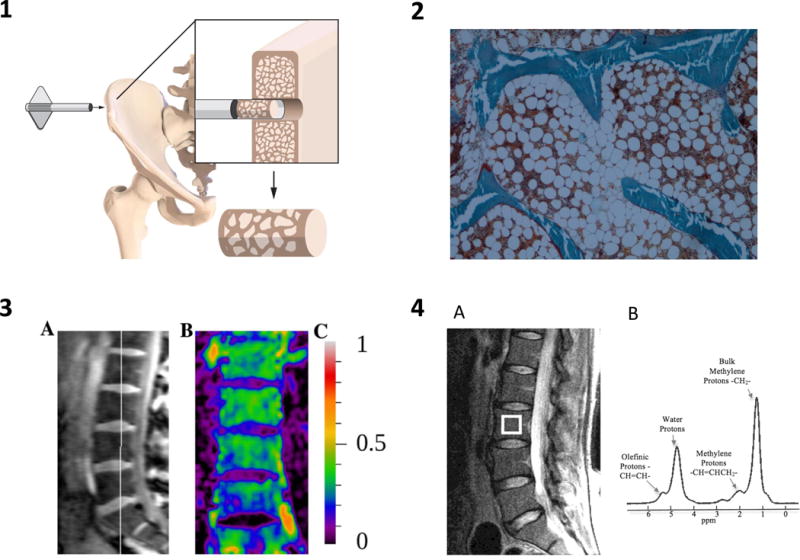
Methods of MAT evaluation
1. Iliac crest bone biopsy or marrow aspiration procedure.
2. Bone biopsy of the iliac crest (Goldner trichrome staining) showing trabecular bone (green), hematopoietic marrow (red) and the adipocyte ghosts (white). Image courtesy of Dr. N. Bravenboer.
3. Magnetic Resonance Imaging (MRI) Quantitative Chemical Shift Imaging (QCSI) of the lumbar vertebrae (L1-4). A shows the MRI image of the lumbar vertebrae with the white line indicating the QCSI localizer, B shows the fat signal fraction image, with (C) color coding: from black (no fat), to white (all fat). Image courtesy of Dr. E.M. Akkerman33.
4. Magnetic Resonance Spectroscopy (MRS). A shows the MRI image of the lumbar vertebrae with the white box indicating the site of measurement B is an axample spectrum showing four peaks: olefinic, double bond -CH=CH- protons at 5.31 ppm, water protons at 4.65 ppm, the CH2methylene protons α- to a double bond (-CH=CHCH2-), at 2.03 ppm, and the bulk CH2methylene protons at 1.3 ppm. Image from Li JMRI 2011132.
Since bone biopsies are invasive and therefore not readily available, MRI methods have been developed. MRI using Quantitative Chemical Shift Imaging (QCSI) separates the water and the fat signals of the bone marrow and computes a fat fraction. MRI is mainly performed of the lumbar vertebrae in humans. Another MRI method, Magnetic Resonance Spectroscopy (MRS) determines the fat fraction and the fatty acid composition of the bone marrow by separating the signals from the different lipids.
Although these methods are able to determine the amount of bone marrow adipocytes and the lipid composition, it does not allow functional studies. Therefore, investigators are now exploring bone marrow aspiration to recover marrow adipocytes for culture and gene expression analysis ex vivo.
THE BONE MARROW ADIPOCYTE
Overview of adipocytes
Adipocytes are present in many tissues within the body and can be divided into several types based on their characteristics and their anatomical depots22. Three types of adipocytes are now widely recognized; white, brown and beige adipocytes. White adipocytes are the classic large, lipid droplet-rich cells, storing and releasing fatty acids. The white adipocytes make up the majority of the subcutaneous and visceral adipose tissue depots. Brown adipocytes are small, mitochondria-rich cells, capable of uncoupling energy by burning lipid and releasing heat. Although brown adipose tissue (BAT) was long considered to exist only in the neonatal period, it is now clear that BAT persists into adulthood in the interscapular region. Certain stimuli, such as cold exposure and adrenergic signaling, can induce some characteristics of brown adipocytes in white adipose depots and these are termed beige or bright adipocytes. These cells have thermogenic properties, although it is unclear whether beige adipocytes modulate body temperature in vivo. In addition to storing or burning fatty acids, adipocytes secrete mediators and hormones collectively called ‘adipokines’. The classic adipokines are leptin and adiponectin which both regulate systemic energy metabolism, but many more adipokines have been identified.
Bone marrow adipocytes
The presence of adipocytes in the bone marrow has long been recognized, but these adipocytes were often considered a passive ‘filler’ in the absence of bone and hematopoietic cells. Interest in marrow adiposity was reignited in the 1970s when the dynamic interactions with hematopoiesis became evident after studies of bone marrow transplantation revealed a temporal relationship between marrow adiposity and hematopoietic reconstitution23,24,25. Since then, many studies have investigated the association between marrow adipose tissue and clinical conditions such as osteoporosis, anorexia and obesity. But due to the location of the marrow adipocytes in the bone marrow, it has been more difficult to study its characteristics directly. Therefore, studies in animal models, especially in mice, have been pivotal in understanding the origin and function of the marrow adipocytes.
In 1996, Gimble and colleagues reported an increase in MAT in mice treated with thiazolidinediones (TZDs)26. Subsequent studies by Jilka and Lecka-Czernik described the cellular and molecular signatures of marrow adipocytes27,28. More recently, investigators have focused on inbred and mutant mice as models for describing the origin and development of bone marrow adipocytes, particularly using new lineage tracing methods29,30. In addition, some investigators have proposed that different types of marrow adipocytes exist within the marrow cavity. Drugs (e.g. the TZDs), environmental factors (e.g. radiation), and nutritional determinants (e.g. high fat diet or calorie restriction) can induce premature marrow adiposity in the long bones of mice. This inducible marrow adiposity has been termed ‘regulated’ MAT (rMAT) in contrast to the distal sites that are referred to as ‘constitutive’ (cMAT) because of their presence at the time of birth31. In mouse models regulated MAT is dynamic and reversible in contrast to constitutive MAT. Whether constitutive and regulated MAT exists in humans is not clear, although what is certain is that marrow adiposity is also inducible in both men and women.
Origin and Regulation
Marrow adipocytes arise from the mesenchymal stromal cell (MSC) in the bone marrow. Mesenchymal stromal cells can also give rise to other cell types such as osteoblasts, the bone forming cells and chondrocytes, the cartilage forming cells. However, adipocyte and osteoblast differentiation is closely related and both types of cells share some common steps during their development (Figure 3). Several transcription factors are upregulated during early pre-osteoblast (Runt-related transcription factor 2 (Runx2), homeobox protein 1 (Prx1) and osterix (Sp7)) and pre-adipocyte differentiation (platelet-derived growth factor receptor-β (PDGFRb), CCAAT/enhancer binding protein alpha (C/EBP), Zinc finger proteins (Zfp) 423 and 467 and Prx1. For a particular window of time, plasticity might exist and the adipocyte and osteoblast progenitor cells may interconvert between phenotypes. The terminal differentiation is regulated by phenotype-specific transcription factors, in case of osteoblasts collagen type 1 (col1a1), osteocalcin (ocn), and RANKL (reviewed in32). And in case of adipocytes peroxisome proliferator-activated receptors gamma (PPARg), fatty acid binding protein (FABP4), perilipin 2 (Pln2) and fatty acid synthase (FASN). Trans-differentiation in a later developmental stage has also been suggested, but not established at this moment. Finally, osteoblasts terminally differentiate into osteocytes which secrete sclerostin (SOST) and fibroblast growth factor (FGF)-2333.
Figure 3.
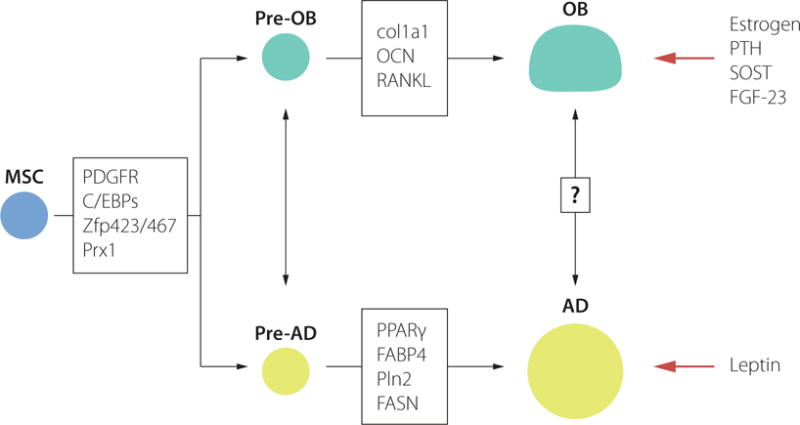
Bone marrow mesenchymal stem cell differentiation
The allocation of the mesenchymal stromal cell to either the adipocytic or the osteoblastic differentiation is affected by several factors. One of the earliest recognized factors is estrogen. Marrow adiposity increases after menopause or ovariectomy and estrogen prevents or reverses this increase. At the same time, bone loss accelerates following menopause and postmenopausal estrogen treatment increases bone mass. The effect of estrogen on marrow adipocytes is thought to be mediated through the estrogen receptor alpha (ERa) since ERa knockout mice show increased marrow adiposity with an increase in adipocyte number34 and this effect was not observed in estrogen receptor beta knockout mice. In addition, mice with deletion of the ERa in mature adipocytes (through conditional adiponectin dependent cre-recombinase), also had normal marrow adiposity indicating the effect of estrogen must be before expression of adiponectin, a marker of late adipocytic differentiation. Indeed, in vitro studies show that estrogen promotes osteoblastic and suppresses adipogenic differentiation of the mesenchymal stromal cell35,36.
Decreasing estrogen concentrations coincide with increasing FSH concentrations due to the endocrine feedback of the gonadal-pituitary axis. A recent study showed that blocking the binding of FSH to its receptor with an antibody prevented the increase in marrow adipose tissue after ovariectomy and treatment with this antibody in sham-operated mice, also decreased marrow adipose tissue37. The authors showed that both adipocytes and MSCs express the FSH receptor and that FSH receptor deficient mice have increased MSC adipogenesis, suggesting that postmenopausal elevated FSH contributes to the increased adipogenic differentiation of the MSC.
Parathyroid hormone regulates calcium concentrations by influencing osteoclast activity, but intermittent PTH (iPTH) treatment can increase bone mass38, among others through stimulation of osteoblastic differentiation of the MSC. In rats, intermittent PTH treatment prevented the increase in marrow adiposity induced by caloric restriction39 and in mice intermittent PTH treatment reduced marrow adiposity. Genetic experiments in mice showed that loss of PTH receptor expression in mesenchymal stromal cells increased marrow adiposity40 whereas overexpression of the PTH receptor in bone cells suppressed marrow adiposity41. These experiments show that PTH can influence the cell fate of the mesenchymal stromal cell to increase or decrease the development of adipocytes. The suppressing effect of PTH on adipogenesis of mesenchymal stromal cells was also shown in vitro previously42.
Phosphate deficiency leads to impaired bone mineralization and the clinical condition is known as rickets. In mice, phosphate restriction during growth results not only in impaired bone mineralization, but also a decrease in osteoblasts and an increase in bone marrow adipocytes43 although in vitro phosphate restriction did not change MSC differentiation, implying an indirect effect of phosphate availability on MSC differentiation.
Leptin is an adipokine produced by white adipocytes that regulates satiety, reproduction and energy homeostasis44. In addition, leptin could play a role in hematopoiesis45 and can also influence bone mass both directly and indirectly through the central nervous system46. These studies have also shown that both subcutaneous and hypothalamic leptin therapy decrease the obesity-induced marrow adiposity in two mouse models, the leptin receptor knockout mouse (ob/ob) and mice on a high fat diet47,48.
Osteocytes produce sclerostin which stimulates bone resorption and inhibits bone formation. Recently sclerostin was shown to induce adipogenesis in vitro. A new osteoporosis drug in phase III trials, romosozumab, is an anti-sclerostin antibody, that enhances bone mass but can also suppress marrow adipose tissue volume in mice49,50.
Bone marrow adipocyte function
1. Secretion of mediators
The first proposed role for the marrow adipocytes is the release of adipokines; peptides and fatty acids acting potentially in both a paracrine and endocrine manner. Adiponectin has been the most studied of the adipokines secreted from MAT, especially in animal models. Adiponectin is secreted primarily by adipocytes and it regulates among others insulin sensitivity and energy metabolism. Adiponectin concentration is lower in obese individuals, but it increases during energy deficit (reviewed in51). This could indicate that marrow adipocytes become an significant source of adiponectin during caloric restriction. Indeed, Cawthorn et al reported that in mice and rabbits adiponectin expression and secretion is higher in MAT compared to peripheral adipose tissue depots52. These same authors also demonstrated that mice with higher MAT due to calorie restriction had greater serum levels of adiponectin. In contrast, when a genetic manipulated mouse that had virtually no MAT (osteocalcin specific Wnt10b over-expressing mouse) underwent calorie restriction, adiponectin secretion was much lower. In the bone marrow, adiponectin has been linked to both bone metabolism and hematopoiesis. Although in vitro adiponectin promotes osteoblast differentiation, in clinical studies circulating adiponectin concentrations are associated with lower bone mass, possibly as a marker of increased marrow adiposity (reviewed in53). Furthermore, increased adiponectin production by bone marrow adipocytes following myeloablative chemotherapy, promoted hematopoietic recovery in mice54.
Recently another study in mice showed that the production of stem cell factor (SCF) by bone marrow adipocytes following myeloablative chemotherapy also promoted hematopoietic recovery and a mouse model with impaired adipogenesis (A-ZIP/F1 mouse) indeed had impaired hematopoietic regeneration55.
In addition, Mattiucci et al showed that human bone marrow adipocytes secrete CXCL12 which is important for hematopoietic stem cell (HSC) maintenance in the bone marrow and that coculture of human bone marrow adipocytes can maintain HSCs in long term culture, suggesting that bone marrow adipocytes could play a role in steady state HSC maintenance56.
Another cytokine produced by MAT is IL-6, an inflammatory protein that may suppress osteoblast activity. IL-6 was identified by Duque et al by comparing cytokines produced by marrow adipocytes with subcutaneous adipocytes. They found 53 proteins from the marrow adipocytes that were upregulated with aging and that could negatively impact neighboring osteoblasts leading to age-related bone loss57. Similarly this same group reported that palmitate, a saturated fatty acid, is released from adipocytes and can suppress osteoblast function58. Moreover, treatment of mice with a fatty acid synthetase inhibitor prevented that osteoblast toxicity59.
Another cytokine that might be released by adipocytes is RANKL. Increased MAT is often associated with decreased bone mass due to uncoupling of bone remodeling with a shift towards more bone resorption. In a study exploring the role of PTH in regulating marrow adiposity, Fan et al found that mice lacking the PTH receptor in MSCs had more marrow adipocytes and that these adipocytes were adjacent to sites of active bone resorption40. Additional studies from that group revealed that marrow adipocytes express high levels of RANKL in the absence of the PTH receptor, and the protein is secreted into the marrow space potentially influencing bone resorption, as well as making its way into the systemic circulation. RANKL has also been shown to systemically impair hepatic glucose utilization and high levels of RANKL may be associated with glucose intolerance60, although it is unclear whether the source of that RANKL is the marrow adipocyte. Intriguingly, peripheral adipocytes do not express significant amounts of RANKL.
Lecka-Czernik and colleagues showed that marrow adipocytes secrete growth factors including IGFBP-2 and Wnt 10b that enhance osteoblast differentiation and may also have endocrine properties in a mouse model of adipocyte beighing61.
Recently, Kousteni and colleagues62 reported that lipocalin 2, a protein which is made by adipocytes and osteoblasts, improves insulin sensitivity and reduces apetite through activation of the melanocortin 4 receptor in the hypothalamus. In that study, marrow adiposity was not reported, and expression of lipocalin 2 was significantly higher in bone cells than marrow adipocytes. However, it is conceivable that during states of increased MAT, lipocalin-2 is secreted by both cell types and targets whole body metabolism through changes in appetite.
2. Secretion of fatty acids as an energy source
A second hypothesis is that marrow adipocytes provide a fuel source for neighboring osteoblasts and hematopoietic stem cells by releasing lipids (lipolysis). It is striking that marrow fat is increased during periods when the osteoblast or hematopoietic stem cell are under extreme duress, including injury models, diet induced changes, calorie restriction and aging. Evidence to support that tenet has been difficult to prove in part because ex vivo model systems are challenging. An indirect measure of lipolysis can be the shrinkage of the adipocyte, since the size of an adipocyte is mainly determined by its lipid content. Preliminary studies have suggested that both estrogen and PTH treatment can reduce marrow adipocyte size in women with osteoporosis63 and in mice on a calorie restricted diet (personal communication, C.J. Rosen). These data could implicate a role for estrogen and parathyroid hormone in mediating local lipolysis in addition to mesenchymal stromal cell fate. Whether this process is active during states of impaired bone cell function remains to be determined. Very recently though, Shafat et al showed in a series of in vitro experiments that adipocytes can actively transfer lipids (free fatty acids (FFAs)) to hematopoietic stem cells, and even more efficiently so to acute myeloid leukemia (AML) blasts which enhanced the survival and proliferation of the AML blasts. In addition, when these investigators blocked the lipolysis in a murine leukemia model, this prolonged the survival of the mice64. Tabe et al also report that bone marrow adipocytes promote the survival of AML cells in vitro by stimulating fatty acid oxidation using a genetic analysis65. Previous studies have suggested that fatty acid oxidation through bone marrow adipocyte lipolysis could also play an important role in growth and survival of prostate cancer bone metastases66,67.
In summary, these studies collectively show that the marrow adipocyte is heterogeneous both in terms of its function and its origin. Similarities between marrow adipocytes and peripheral adipocytes are the expression of the insulin receptor, the leptin receptor, and all the markers of mature adipocytes. Similarly, marrow adipocytes can store and release fatty acids and adipokines and cytokines. However, marrow adipocytes differ from peripheral adipocytes in several important ways as well both in function and origin. Functionally, marrow adipocytes can secrete RANKL which stimulates bone resorption40 and adiponectin and stem cell factor which enhances hematopoiesis54,55. As for the origin, some marrow adipocyte progenitors express Osterix (sp7), an early osteoblast transcription factor29,68 and others stain positively for Nestin, an early marker of neural progenitors69 and not all the marrow adipocyte progenitors trace with platelet derived growth factor receptor alfa (PDGFRa) unlike visceral or subcutaneous adipocytes (Horowitz, personal communication); yet virtually all the marrow adipocytes trace with the leptin receptor70. In addition, a direct comparison of the gene profiles of human adipose tissue derived and bone marrow derived adipocytes showed different transcriptomes, although the functional relevance of these differences has to be determined56.
Furthermore, it is clear that marrow adipocytes secrete factors influencing processes within the bone marrow; bone remodeling and hematopoiesis, but they also secrete factors which are released into the circulation, potentially acting outside of the bone marrow. Bone remodeling and hematopoiesis are energy consuming processes which are tightly regulated to maintain bone mass and blood cell counts. Integration of information on enery availability and energy consumption is important to maintain whole body homeostasis. Previous studies in mice have shown that bone remodeling and whole body homeostasis are balanced through the combined actions of bone specific peptides and systemic hormones (e.g. osteocalcin, and insulin, reviewed in71), but marrow adipocytes could very well play a role in this balance as well and the secretion of adipokines could be the mechanism.
BONE MARROW ADIPOSITY IN DISEASE
Skeletal disease: osteoporosis
In healthy subjects an inverse relation between marrow adiposity and bone mineral density exists, independent of age, sex and total body fat14,16,72-75. In addition, a wealth of studies showed that marrow adiposity is higher in osteopenic compared to healthy, and in osteoporotic compared to osteopenic women and men. Marrow adiposity ranged from 45 to 60% in healthy subjects around age 60, was 5% higher in osteopenia and again 5% higher in osteoporosis15,76-81. Previously, biopsy and postmortem studies also showed 10% higher marrow adiposity in osteoporotic patients compared to healthy subjects23,25,82. Moreover, several studies showed that marrow adiposity is higher in patients with vertebral fractures and in patients with signs of vertebral weakness76,83-85. The lipid constituents of marrow adiposity may also differ between healthy and osteoporotic patients in respect to the degree of saturated vs unsaturated fatty acids, although more studies are needed to assess the importance of this feature.
Bisphosphonates
Two randomized, double-blind, placebo-controlled trials investigated the effect of bisphosphonate therapy on marrow adiposity. A single injection of 5 mg zoledronic acid decreased marrow adiposity from 65% to 57% after a year compared to a 3% increase in the placebo group in 50 women per group as assessed by MRI in the lumbar vertebra86. Daily administration of 5 mg of risedronate during 3 years decreased marrow adiposity by 20% compared to a 15% increase in the placebo group in 28 women as assessed by paired bone biopsies87. These studies show that bisphosphonates indeed decrease marrow adiposity in humans. Studies in rats showed the same effect88,89 and in vitro bisphosphonates indeed suppress adipogenesis of mesenchymal stem cells90,91, however a direct effect on the marrow adipocyte or an indirect effect via the osteoclast have not been investigated at the moment.
Estrogen
Estrogen supplementation decreases marrow adiposity in postmenopausal women. Daily administration of transdermal estradiol during one year prevented the increase in marrow adiposity as observed in the placebo-treated group of postmenopausal women with osteoporosis. Paired bone biopsies showed that during one year, both adipocyte size and number increased by 20% in untreated women and estradiol prevented the increase in number and even decreased adipocyte size63. The same effect was observed in a short-term study of postmenopausal healthy women treated with oral estradiol for just two weeks. MRI scans of the lumbar vertebrae showed that the fat fraction decreased 5% during 2-weeks supplementation and increased again to baseline values in 2-weeks after withdrawal of treatment21. Also in animals, ovariectomy increases marrow adiposity92-94 and estrogen supplementation reverses this histological manifestation.
Parathyroid hormone
Parathyroid hormone is the most important calcium-regulating hormone in the body and is secreted by the parathyroid glands. PTH increases serum calcium concentrations by releasing calcium from the bone by stimulating bone resorption. Therefore continuous PTH oversecretion due to a parathyroid adenoma (primary hyperparathyroidism) causes bone loss leading to osteoporosis. However, intermittent treatment with PTH in contrast stimulates bone formation and iPTH is currently the only registered anabolic treatment for osteoporosis. Besides an increase in bone mass, iPTH treatment is also associated with a decrease in marrow adiposity in premenopausal osteoporotic women (as assessed by MRI)95 and in osteoporotic men (as assessed by histomorphometry)40.
Animal and in vitro studies show the same effect of PTH on marrow adiposity39,40,41,42.
Energy imbalance: anorexia nervosa, obesity, and diabetes
Anorexia Nervosa
In 1949, Cheatum reported that bone marrow adiposity was a marker of malnutrition in deer96. That seminal observation was confirmed in other studies of wildlife starvation but more importantly, it set the stage for the subsequent analysis of women with anorexia nervosa. Several case reports and case series reported changes in biopsied bone marrow in anorexia patients, ranging from no changes to hypoplasia to gelatinous transformation97-99. More recently, MRI assessments showed that marrow adiposity is increased in anorectic compared to healthy age-matched controls and that the relative contribution of MAT as a percentage of total body fat almost triples52,100. Marrow adiposity in recovered anorectic patients however, is comparable to healthy age-matched controls101, implying a reversible effect. No difference in marrow fatty acid composition was observed in anorectic compared to control subjects102. Interestingly, anorexia patients with amenorrhoea exhibited even higher marrow adiposity than anorexia patients with normal menstrual cycles100, which might very well be due to the additive effect of estrogen withdrawal on marrow adiposity. Bone loss and increased incidence of fracture are well-established hallmarks of anorexia, and indeed the inverse association between bone mineral density and marrow adiposity was confirmed in these studies of anorexia patients.
Rodent models of caloric restriction report conflicting results; in some models marrow adiposity indeed increases52,103,104 whereas in others marrow adiposity remains constant105,106. These differences might be explained by differences in sex, age and genetic background of the rodents, the degree of caloric restriction or the amount of weight loss achieved.
Obesity
Obesity is a major health issue leading to adipose tissue inflammation with adverse effects on systemic metabolism. Bone density is increased in obesity, which might be due to the higher load on the bones, but bone quality is impaired leading to increased fracture risk. Increased marrow adiposity could contribute to the impaired bone quality and increased fracture risk in obesity. Indeed, Cohen et al showed that marrow adiposity measured in bone biopsies was higher in obese compared to overweight and healthy subjects107. These findings were confirmed in pre- and postmenopausal women and in young and older men using MRI108-111. In these studies, marrow adiposity was also inversely related to bone mass and measures of skeletal fragility and increased marrow adiposity was associated with a decrease in bone formation histologically. In contrast to these results, a recent study did not detect a difference in marrow adiposity in healthy-weight versus obese men and women112. Also in rodents, high fat diet induced obesity increases marrow adiposity, independent of sex and genetic background113-116.
Treatment options for obesity are either lifestyle interventions (diet and exercise) or bariatric surgery and in all these the effect on marrow adiposity has been studied.
Diet
Three studies investigated the effect of a diet intervention on marrow adiposity. The first study included 55 premenopausal women and 12 men and subjected these to 12 weeks of low calorie diet resulting in 8 and 12 kilograms of weight loss respectively, accompanied by decreases in fat mass. Marrow adiposity decreased by 3.5%. Six months after the intervention, 24 subjects had >30% weight regain and marrow adiposity returned to the baseline levels117. The second study initiated a 12 week low calorie diet in 10 women resulting in 5 kilograms of weight loss and a decrease in subcutaneous and visceral fat depots. However, marrow adiposity did not change118. The third study initiated a 4 week low calorie diet in 20 women resulting in 7 kilograms of weight loss, a decrease in subcutaneous and visceral fat, a dramatic derease in liver fat of 40% and beneficial changes in serum lipid profiles. But again, marrow adiposity did not change119.
Bariatric surgery
Three studies reported the effect of bariatric surgery on marrow adiposity. Bredella included 18 women and 3 men, mean BMI 40, of whom 11 underwent roux-en-Y gastric bypass and 10 sleeve gastrectomy. Weight loss was 30 and 25 kilograms respectively and >30% decrease in subcutaneous and visceral fat 12 months following surgery. Marrow adiposity did not change following roux-en-Y gastric bypass and increased slightly following sleeve gastrectomy, bone mineral density decreases in both groups were as described before120. Ivaska included 42 women and 4 men (19 diagnosed with diabetes), mean BMI 42, of whom 21 underwent roux-en-Y gastric bypass and 25 sleeve gastrectomy. Weight loss was 25 and 20 kilograms respectively 6 months following surgery and 13 of 19 diabetic patients reached remission of diabetes. Again, marrow adiposity did not change following surgery121. Finally, Schafer included 11 women, 6 with diabetes and mean BMI 40, all of which underwent Roux-en-Y gastric bypass. Weight loss was 25 kilograms 6 months following surgery and >30% decrease in fat mass and 5 of 6 diabetic patients reached remission of diabetes. Overall, marrow adiposity decreased 3.5%, however this effect was due to a 7.5% decrease in marrow adiposity in the diabetic patients, in the non-diabetic patients marrow adiposity increased 1%122. Recently Schafer et al published the extension of their data indeed showing that marrow adiposity decreases following weight loss after bariatric surgery in diabetic patients, but not in non-diabetic obese patients. The decrease in HbA1c and in marrow adiposity were significantly correlated. In addition, this study shows that bone loss following bariatric surgery is associated with increases in marrow adiposity123.
Exercise
Exercise reduces body fat and body weight, and several studies have shown that exercise can also decrease marrow fat. In female athletes tibial marrow adiposity estimated by CAT (computerized axial tomography)-scanning was lower than in active control women124. In young male wrestlers, spine marrow adiposity assessed by MRI was also lower compared to healthy controls125. Two studies investigated the effect of an exercise intervention on marrow adiposity. In preschool children 10 weeks of school-based physical activity of 20 minutes 3 days a week reduced femoral marrow adiposity compared to an increase in age and sex matched control group126. In older men (60 years) resistance training 3 days a week during 18 months reduced femoral marrow adiposity compared to controls in a randomized trial127. In contrast, bed rest for prolonged periods of time increases marrow adiposity and this could be prevented by exercise128,129. Studies in animals also show that exercise prevents the increase in MATduring high-fat diets115.
Diabetes
Diabetes, both type 1 (DMT1) and type 2 (DMT2), is associated with increased fracture risk and marrow adiposity is considered a potential contributing factor in both types of diabetes (recently extensively reviewed in130).
Type 1
Diabetes type 1 is characterized by insulin deficiency due to auto-immune mediated beta cell dysfunction. Patients are in general diagnosed at younger age and are treated with lifelong insulin therapy. Two studies investigated marrow adiposity in type 1 diabetes. Slade detected no difference between type 1 diabetes patients (HbA1c 7.5%) and age, sex and weight matched healthy controls in vertebral marrow adiposity (men T1DM 54% healthy 57%, women T1DM 55% healthy 52%) and marrow adiposity was not related to HbA1c. However marrow adiposity was positively correlated to serum lipids131. Abdalrahaman recruited 30 young women with T1DM (HbA1c 9.8%) and age, sex and weight matched controls and did not find a difference in vertebral marrow adiposity (31% in T1DM and 26% in healthy controls)132.
Type 2
Diabetes type 2 is characterized by insulin resistance, adipose tissue inflammation and deranged metabolic profiles. Patients are generally overweight and are diagnosed later in life, although this is changing with the rising obesity rates in children. The cornerstone of treatment is metformin, but many patients eventually need insulin therapy. Several studies investigated marrow adiposity in type 2 diabetes with conflicting results. Sheu reported higher vertebral marrow adiposity in 38 older diabetic participants in the Osteoporotic Fractures in Men (MrOS) study compared to the 118 participants without diabetes (59 vs 55%)133. Araujo did not find a difference in vertebral marrow adiposity in 28 type 2 diabetes patients (15 women, 13 men) compared to healthy controls (14 women, 10 men) or obese subjects (16 women, 10 men) (mean age 50 years, marrow adiposity 37% (T2DM) vs 36% (healthy) vs 32% (obese), but again marrow adiposity was positively related to HbA1c112. Baum as well did not find a difference in 13 postmenopausal women with T2DM (HbA1c 7.6%) compared to 13 age, sex and weight matched controls in vertebral marrow adiposity (69% versus 67%), however lipid unsaturation was lower in diabetic subjects134. Patsch compared vertebral marrow adiposity in four groups of 17 postmenopausal women per group; diabetic and nondiabetic with and without fragility fractures, and did not find an association with vertebral marrow adiposity (control 72%, T2DM 71%, fracture 65%, fracture and T2DM 69%), but again lipid unsaturation was negatively associated with diabetes and fracture135.
Interventions
Three interventional studies reported marrow adiposity in type 2 diabetes. Schafer showed that diabetic patients had 10% higher marrow adiposity before bariatric surgery, which decreased 7.5% 6 months postsurgery, resulting in the same marrow adiposity as the non-diabetic subjects122, however in the extension of that study published more recently, diabetic subjects had the same marrow adiposity as non-diabetic subjects before surgery, although diabetic subjects lost marrow fat following surgery whereas non-diabetic subjects did not123. In addition this study showed that the improvement in diabetes was associated with the decline in marrow fat. Ivaska reported 10% higher marrow adiposity in diabetes patients who reached remission following bariatric surgery, compared to the diabetes patients who remained diabetic121. Vogt showed that after 6 weeks of low calorie diet in 19 women and 10 men with type 2 diabetes, 13 kilograms of weight loss was accompanied by a 5% decrease in vertebral marrow adiposity (58% before and 53% afterwards), decrease in liver fat and serum lipids136.
Studies in mouse models of diabetes show consistently increased marrow adiposity137,138 in hyperglycaemic mice. This discrepancy between mice and humans can be explained in several ways. Firstly, human studies are underpowered to show a difference; most of the studies do show higher marrow adiposity in diabetes, but the group sizes might be too small to detect a significant difference. Secondly, hyperglycaemic mice remain hyperglycaemic, whereas humans are treated to reach normoglycaemia. Therefore the level of hyperglycaemia or the relative insulin deficiency can impact marrow adiposity. In addition, metformin is the first choice in therapy for type 2 diabetes and almost all diabetic patients are treated with metformin. Recently, the mechanism of its antidiabetic effect by reducing hepatic gluconeogenesis was elucidated139, but interestingly, a recent study reports a suppressive effect of metformin on adipogenesis of bone marrow mesenchymal stem cells140. Therefore metformin therapy can have a favorable effect on marrow adiposity in diabetic patients. Thirdly, mice have in general lower levels of marrow adiposity and this is usually assessed in the tibia and femur, whereas human marrow adiposity in mostly measured in the vertebrae. In addition, mice share the same genetic background mostly and indeed marrow adiposity is strain-specific, whereas humans have heterogeneous genetic backgrounds.
Neoplastic disease
Since it became clear that obesity increases cancer risk141, many studies have been conducted investigating the link between adiposity and tumour growth. These studies mainly focused on white adipose tissue depots and showed that adipocyte hypertrophy can promote neoplastic disease (reviewed in142). Several solid tumour types have a preference to metastasize to adipocyte-rich environments and the adipocytes are thought to represent an important reason for this preference. Omental adipocytes for example have been shown to provide energy via direct lipid transfer to ovarian cancer metastases and to promote tumour growth via secretion of mediators143; mammary adipocytes have been shown to release fatty acids to promote growth and stimulate invasion of breast cancer cells144; and adipocytes surrounding the prostate have been shown to promote the migration of prostate cancer cells into the surrounding tissues145. Since the bone marrow also frequently hosts cancer cells, both deriving from the bone marrow and metastasizing from different organs, some researchers have focused on bone marrow adipocytes and the interaction with neoplastic cells.
Hematological cancers
Hematological malignancies induce changes in the bone marrow; it has long been known that marrow adiposity is increased in aplastic anemia and decreased in leukemia146. This was thought to be due to the absence or accumulation of hematological cancer cells, but this has been revisisted recently. In fact, the marrow adipocytes are now thought to play an active role in the pathophysiology of hematological neoplastic disease (extensively reviewed for multiple myeloma in147). In vitro experiments have shown that human bone marrow adipocytes actively promote leukemic growth through transfer of lipids and secretion of mediators64,65. In addition, pharmacological blocking of the lipid transfer between the bone marrow adipocyte and the leukemic blast through inhibition of FABP4, the fatty acid binding protein necessary for transport of the lipids, reduced the tumor survival in vitro and increased survival of leukemic mice in vivo64 (Shafat). In addition, blocking carnitine palmitoyl transferase (CPT)1a which is necessary for the entry of the fatty acids into the mitochondria, decreased the survival of human leukemia cells in vitro148, implying that indeed fatty acids are an important source of energy for these cells. Although these animal and in vitro data are promising, no human clinical studies have been published yet.
Solid tumours
Breast cancer and prostate cancer are two solid tumours that preferentially metastasize to the bone marrow. Bone marrow adipocytes could be involved in the attraction and growth of these tumour types in the bone marrow. Previous in vitro studies provided some evidence that bone marrow adipocytes promote tumor growth of prostate cancer metastases through the secretion of fatty acids66 and HIF (hypoxia inducible factor)-1a activation67. A study in mice showed that melanoma cells induce lipolysis in the bone marrow adipocytes and that bone marrow adipocytes secrete factors promoting melanoma cell proliferation149. Supporting these findings in mice, a recent clinical study in humans showed that marrow adiposity was higher in breast cancer patients compared to age, sex and weight matched healthy controls (56 patients in both groups, 73% versus 60% marrow adiposity)150, suggesting that breast cancer is associated with higher bone marrow adiposity, although these patients did not have metastasized disease.
Therapy
Neoplastic disease is treated with either chemotherapy, radiation therapy or both. Both therapies induce increased bone marrow adiposity and both therapies share bone marrow toxicity leading to decreased hematopoiesis as a frequent complication. Bolan showed that 6 months of treatment with chemo- or radiotherapy increased marrow adiposity from 45% to 63% in gynaecological cancer patients151. Indeed Schraml also found higher marrow adiposity, although not significant, in cancer patients (66%) previously treated with chemotherapy compared to untreated cancer patients (59%)152. And Mostoufi observed a vertebral marrow adiposity of 57% versus 27% in childhood cancer patients treated with an allogenic hematopoietic stem cell transplantation (which includes chemotherapy and total body irradiation) compared to age-, sex and weight matched healthy controls153. In mice, marrow adiposity is increased as well following chemo- or radiotherapy. However, the effect of the increased marrow adiposity on hematopoietic regeneration is complex according to new studies. The first study to report on bone marrow adiposity and hematopoietic reconstitution following radiation showed that the increase in marrow adiposity is related to the decrease in hematopoiesis following therapy. In addition, prevention of marrow adipogenesis pharmacologically or genetically, promoted hematopoietic repopulation of the bone marrow154,155. However, more recently another study showed that indeed in the caudal vertebrae of the mouse, hematopoietic regeneration was better in genetically modified mice lacking bone marrow fat, but in the long bones (the femur and tibiae) hematopoietic regeneration was markedly impaired without adipocytes. This study also showed that the bone marrow adipocytes are an important source of stem cell factor, an important factor for hematopoietic stem cell survival, therefore these authors hypothesize that the increase in adipogenesis in the bone marrow following chemo- or radiotherapy, is promoting hematopoietic reconstitution and therefore could be beneficial55. In humans, hematopoiesis is concentrated in the axial skeleton and not in the appendicular (long bones), therefore it remains to be determined what the role of the bone marrow adipocyte is in human hematopoietic reconstitution.
Another possible role of the bone marrow adipocytes could be to protect the neoplastic cells in the bone marrow from the effects of chemotherapy. One study showed that leukemic cells in the gonadal adipose tissue of mice were more resistant to conventional chemotherapy regimens than leukemic cells in other locations156, possibly through the induction of fatty acid metabolism. Whether bone marrow adipocytes could also induce therapy-resistance in neoplastic remains to be determined.
CLINICAL IMPLICATIONS
In conclusion, bone marrow adipocytes appear shortly after birth and steadily increase during growth and aging. Bone marrow adipocytes reside in the bone marrow together with bone cells and hematopoietic cells and mounting evidence suggests that the adipocytes are actively involved in both bone remodeling and hematopoiesis. The adipocytes can provide fuel to the energy consuming processes of bone remodeling and hematopoiesis through the release of fatty acids which could be an important energy source in the hypoxic bone marrow environment. In addition bone marrow adipocytes secrete mediators which can have local paracrine, but perhaps also systemic, endocrine effects. The exact role of the bone marrow adipocyte in local and systemic energy metabolism will become clear from future studies.
Weight gain and weight loss are both associated with increased marrow adiposity. It remains to be determined whether these two conditions induce the same marrow adipocytes to increase, or that perhaps different adipocyte depots are present in the bone marrow as suggested in mice studies for example, ie constitutive versus regulated. A possible modifier of the relation between weight and marrow adiposity could be inflammatory status and insulin resistance, since some studies clearly show stronger associations with HbA1c than fat mass. Further studies are needed to resolve this issue and to understand the relation between peripheral and bone marrow adipose tissue depots. Some diseases are associated with increased marrow adiposity and it remains to be determined whether this is a physiological response, ie the increase in bone marrow adipocytes support hematopoietic reconstitution following chemotherapy by providing stimulating factors and fatty acids, or a pathological mechanism, ie the increase in bone marrow adipocytes provide fuel for neoplastic cells outgrowing their metabolic demands. This will be an important question to be answered, especially when considering the bone marrow adipocyte as a potential therapeutic target. Inhibiting bone marrow adipogenesis or blocking fatty acid metabolism could have detrimental effects if indeed the bone marrow adipocytes are rescueing metabolically stressed bone or hematopoietic cells. However, it could also be a very effective therapy possibly with few systemic side-effects if indeed the marrow adipocytes are supporting the pathological response.
In addition, marrow adiposity can now be measured non-invasively with MRI and this could add to the diagnostic or prognostic process for example in skeletal disease. Increased marrow adiposity has been shown to be an independent predictor of fracture and lipid saturation of the marrow adipose tissue also could be an important addition.
Over the last decades the bone marrow adipocyte has evolved from passive filler to key player in the bone marrow niche and many investigators are now exploring the characteristics, function and origin of the bone marrow adipocyte. These studies will provide more insight into its role in health and disease and the possibility for new treatments.
Figure 4.
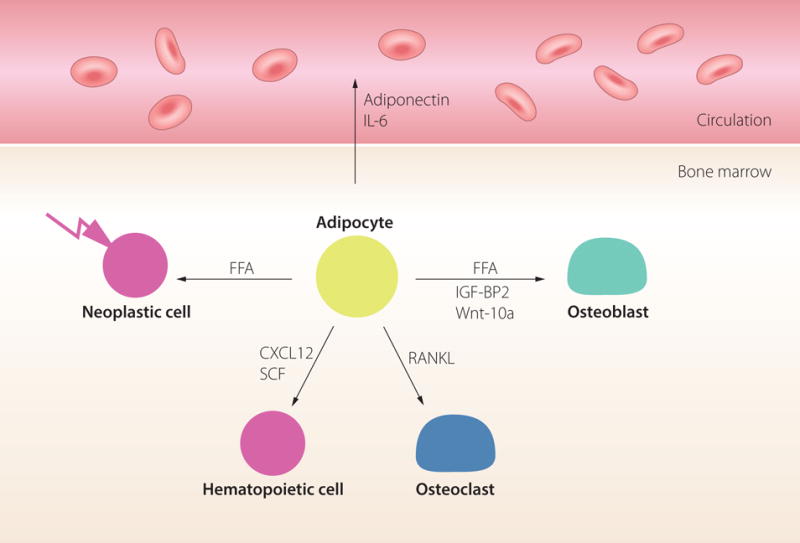
Function of the marrow adipocyte
TABLE 1.
Effects of interventions on marrow adipose tissue and bone mass, fracture risk and fat mass
| MAT | Bone Mass | Fracture Risk | Fat Mass | |
|---|---|---|---|---|
| Bisphosphonate (86, 87) | ↓ | ↑ | ↓ | — |
| Estrogen (21,63) | ↓ | ↑ | ↓ | ↓ |
| Parathyroid hormone (95, 40) | ↓ | ↑ | ↓ | |
| Diet (117, 118, 119) | ↓/— | ↓ | ||
| Bariatric surgery (120,121, 122) | ↓/—* | ↓ | ↓ | |
| Exercise (124, 125, 126, 127) | ↓ | ↑ | ↓ | ↓ |
| Metformin (140) | ↓ | |||
| Chemo-/radiotherapy (151,152, 153) | ↑ |
depending on HbA1c
references between brackets
TABLE 2.
Possible Clinical Implications of MAT
|
Acknowledgments
AGV is supported by the European Society of Endocrinology (Short-Term Fellowship 2017 and International Endocrine Scholars Programme 2017) and the Catharine van Tussenbroek Fund and CJR is supported by the NIH (R24: DK 092759, DK 112374, AR 071739).
ABBREVIATIONS
- MAT
marrow adipose tissue
- PTH
parathyroid hormone
- RANKL
RANK ligand
- MRI
magnetic resonance imaging
- LH
luteinizing hormone
- FSH
follicle stimulating hormone
- QCSI
Quantitative Chemical Shift Imaging
- MRS
Magnetic Resonance Spectroscopy
- BAT
brown adipose tissue
- TZD
thiazolidinediones
- MSC
mesenchymal stromal cell
- RUNX2
Runt-related transcription factor 2
- PRX1
homeobox protein 1
- Sp7
osterix
- PDGFR
platelet-derived growth factor receptor-β (b),
- C/EBP
CCAAT/enhancer binding protein alpha
- Zfp
Zinc finger proteins
- Col1a1
osteoblasts collagen type 1
- Ocn
osteocalcin
- PPARg
peroxisome proliferator-activated receptors gamma
- FABP
fatty acid binding protein
- Pln
perilipin
- FASN
fatty acid synthase
- SOST
sclerostin
- FGF
fibroblast growth factor
- ER
estrogen receptor
- iPTH
intermittent PTH treatment
- SCF
stem cell factor
- HSC
hematopoietic stem cell
- FFA
free fatty acids
- AML
acute myeloid leukemia
- PDGFRa
platelet derived growth factor receptor alfa
- CAT
computerized axial tomography
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- CPT
carnitine palmitoyl transferase
- HIF
hypoxia inducible factor
Footnotes
DR. ANNEGREET VELDHUIS-VLUG (Orcid ID : 0000-0003-0277-2533)
Conflicts of Interest Statement
The authors declare no conflicts of interest.
Contributions
AGV and CJR contributed equally to the literature search, discussion, and writing of this manuscript.
References
- 1.Kalpaktsoglou PK. The development of haemopoiesis in foetuses, newborns and young infants. University of Athens; 1960. [Google Scholar]
- 2.Mikkola HKA, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 3.Emery JL, Follett GF. REGRESSION OF BONE-MARROW HAEMOPOIESIS FROM THE TERMINAL DIGITS IN THE FOETUS AND INFANT. Br J Haematol. 1964;10:485–9. doi: 10.1111/j.1365-2141.1964.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Piney A. The Anatomy Of The Bone Marrow: With Special Reference To The Distribution Of The Red Marrow. The British Medical Journal. 1922;2:792–5. [Google Scholar]
- 5.Custer RP, Ahlfeldt FE. Studies on the structure and function of bone marrow. II. Variations in cellularity in various bones with advancing years of life and their relative response to stimuli. The Journal of Laboratory and Clinical Medicine. 1932;17:960–2. [Google Scholar]
- 6.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–9. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 7.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175:219–23. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 8.Newton L, Hanks J, Davis M, Casazza K. The relationships among total body fat, bone mineral content and bone marrow adipose tissue in early-pubertal girls. BoneKEy Rep. 2013;2 doi: 10.1038/bonekey.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Zong K, Gao Z, et al. Magnetic Resonance Imaging–Measured Bone Marrow Adipose Tissue Area Is Inversely Related to Cortical Bone Area in Children and Adolescents Aged 5–18 Years. Journal of Clinical Densitometry. 2015;18:203–8. doi: 10.1016/j.jocd.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen W, Velasquez G, Chen J, et al. Comparison of the Relationship Between Bone Marrow Adipose Tissue and Volumetric Bone Mineral Density in Children and Adults. Journal of Clinical Densitometry. 2014;17:163–9. doi: 10.1016/j.jocd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bisschop E, Luypaert R, Louis O, Osteaux M. Fat fraction of lumbar bone marrow using in vivo proton nuclear magnetic resonance spectroscopy. Bone. 1993;14:133–6. doi: 10.1016/8756-3282(93)90239-7. [DOI] [PubMed] [Google Scholar]
- 12.Schellinger D, Lin CS, Fertikh D, et al. Normal Lumbar Vertebrae: Anatomic, Age, and Sex Variance in Subjects at Proton MR SpectroscopyΓÇöInitial Experience1. Radiology. 2000;215:910–6. doi: 10.1148/radiology.215.3.r00jn42910. [DOI] [PubMed] [Google Scholar]
- 13.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. Journal of Magnetic Resonance Imaging. 2001;13:263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: A preliminary study at 3.0Tesla. Journal of Magnetic Resonance Imaging. 2007;26:787–93. doi: 10.1002/jmri.21072. [DOI] [PubMed] [Google Scholar]
- 15.Ergen FB, Gulal G, Yildiz AE, Celik A, Karakaya J, Aydingoz U. Fat fraction estimation of the vertebrae in females using the T2*-IDEAL technique in detection of reduced bone mineralization level: comparison with bone mineral densitometry. J Comput Assist Tomogr. 2014;38:320–4. doi: 10.1097/RCT.0b013e3182aa4d9d. [DOI] [PubMed] [Google Scholar]
- 16.Griffith JF, Yeung DKW, Ma HT, Leung JCS, Kwok TCY, Leung PC. Bone marrow fat content in the elderly: A reversal of sex difference seen in younger subjects. Journal of Magnetic Resonance Imaging. 2012;36:225–30. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 17.Pansini V, Monnet A, Salleron J, Hardouin P, Cortet B, Cotten A. 3 Tesla (1) H MR spectroscopy of hip bone marrow in a healthy population, assessment of normal fat content values and influence of age and sex. Journal of magnetic resonance imaging : JMRI. 2014;39:369–76. doi: 10.1002/jmri.24176. [DOI] [PubMed] [Google Scholar]
- 18.Broekmans FJ, Soules MR, Fauser BC. Ovarian Aging: Mechanisms and Clinical Consequences. Endocrine Reviews. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 19.Black DM, Rosen CJ. Postmenopausal Osteoporosis. The New England journal of medicine. 2016;374:2096–7. doi: 10.1056/NEJMc1602599. [DOI] [PubMed] [Google Scholar]
- 20.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 21.Limonard EJ, Veldhuis-Vlug AG, van Dussen L, et al. Short-Term Effect of Estrogen on Human Bone Marrow Fat. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:2058–66. doi: 10.1002/jbmr.2557. [DOI] [PubMed] [Google Scholar]
- 22.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkhardt R, Kettner G, Bohm W, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–64. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Tavassoli M, Crosby WH. Bone marrow histogenesis: a comparison of fatty and red marrow. Science. 1970;169:291–3. doi: 10.1126/science.169.3942.291. [DOI] [PubMed] [Google Scholar]
- 25.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Gimble JM, Robinson CE, Wu X, et al. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–94. [PubMed] [Google Scholar]
- 27.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tisue characteristics, which are attenuated with aging and diabetes. Bone Interactions Between Bone, Adipose Tissue and Metabolism. 2012:546–52. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–84. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Strecker S, Wang L, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–84. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nature communications. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono N, Kronenberg HM. Mesenchymal progenitor cells for the osteogenic lineage. Current molecular biology reports. 2015;1:95–100. doi: 10.1007/s40610-015-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dallas SL, Prideaux M, Bonewald LF. The Osteocyte: An Endocrine Cell ΓǪ and More. Endocrine Reviews. 2013;34:658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wend K, Wend P, Drew BG, Hevener AL, Miranda-Carboni GA, Krum SA. ERalpha regulates lipid metabolism in bone through ATGL and perilipin. J Cell Biochem. 2013;114:1306–14. doi: 10.1002/jcb.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okazaki R, Inoue D, Shibata M, et al. Estrogen Promotes Early Osteoblast Differentiation and Inhibits Adipocyte Differentiation in Mouse Bone Marrow Stromal Cell Lines that Express Estrogen Receptor (ER) +¦ or +¦. Endocrinology: The Endocrine Society. 2002:2349–56. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J-W, Gao Z-L, Mei H, Li Y-L, Wang Y. Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci. 2011;341 doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546:107–12. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. Journal of endocrinological investigation. 2011;34:801–10. doi: 10.3275/7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner RT, Iwaniec UT. Low dose parathyroid hormone maintains normal bone formation in adult male rats during rapid weight loss. Bone. 2011;48:726–32. doi: 10.1016/j.bone.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Hanai JI, Le PT, et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;25:661–72. doi: 10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvi LM, Sims NA, Hunzelman JL, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–86. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishizuya T, Yokose S, Hori M, et al. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–70. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko FC, Martins JS, Reddy P, et al. Acute Phosphate Restriction Impairs Bone Formation and Increases Marrow Adipose Tissue in Growing Mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:2204–14. doi: 10.1002/jbmr.2891. [DOI] [PubMed] [Google Scholar]
- 44.Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–93. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Current biology : CB. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 46.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64:105–13. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 48.Lindenmaier LB, Philbrick KA, Branscum AJ, Kalra SP, Turner RT, Iwaniec UT. Hypothalamic Leptin Gene Therapy Reduces Bone Marrow Adiposity in ob/ob Mice Fed Regular and High-Fat Diets. Frontiers in Endocrinology. 2016;7 doi: 10.3389/fendo.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandra A, Lin T, Young T, et al. Suppression of Sclerostin Alleviates Radiation-Induced Bone Loss by Protecting Bone-Forming Cells and Their Progenitors Through Distinct Mechanisms. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32:360–72. doi: 10.1002/jbmr.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulzele K, Lai F, Dedic C, et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32:373–84. doi: 10.1002/jbmr.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Current opinion in lipidology. 2017;28:347–54. doi: 10.1097/MOL.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 52.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naot D, Musson DS, Cornish J. The Activity of Adiponectin in Bone. Calcified Tissue International. 2017;100:486–99. doi: 10.1007/s00223-016-0216-5. [DOI] [PubMed] [Google Scholar]
- 54.Masamoto Y, Arai S, Sato T, et al. Adiponectin Enhances Quiescence Exit of Murine Hematopoietic Stem Cells and Hematopoietic Recovery Through mTORC1 Potentiation. Stem Cells. 2017;35:1835–48. doi: 10.1002/stem.2640. [DOI] [PubMed] [Google Scholar]
- 55.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattiucci D, Maurizi G, Izzi V, et al. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2017 doi: 10.1002/jcp.26037. [DOI] [PubMed] [Google Scholar]
- 57.Gasparrini M, Rivas D, Elbaz A, Duque G. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Experimental gerontology. 2009;44:613–8. doi: 10.1016/j.exger.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–16. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 59.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. Journal of Cellular and Molecular Medicine. 2010;14:982–91. doi: 10.1111/j.1582-4934.2009.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiechl S, Wittmann J, Giaccari A, et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–63. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 61.Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible Brown Adipose Tissue, or Beige Fat, Is Anabolic for the Skeleton. Endocrinology. 2013;154:2687–701. doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosialou I, Shikhel S, Liu JM, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–90. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Osteoporos Int Osteoporosis International. Springer-Verlag; 2008. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women; pp. 1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafat MS, Oellerich T, Mohr S, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–32. doi: 10.1182/blood-2016-08-734798. [DOI] [PubMed] [Google Scholar]
- 65.Tabe Y, Yamamoto S, Saitoh K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer research. 2017;77:1453–64. doi: 10.1158/0008-5472.CAN-16-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herroon M, Rajagurubandara E, Hardaway AL, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013 Nov;4(11) doi: 10.18632/oncotarget.1482. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diedrich JD, Rajagurubandara E, Herroon MK, Mahapatra G, Huttemann M, Podgorski I. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget. 2016;7:64854–77. doi: 10.18632/oncotarget.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Developmental cell. 2014;29:340–9. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell. 2016;18:782–96. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 71.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93:2281–6. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 75.Shen W, Scherzer R, Gantz M, et al. The Journal of Clinical Endocrinology & Metabolism. The Endocrine Society; 2012. Relationship between MRI-Measured Bone Marrow Adipose Tissue and Hip and Spine Bone Mineral Density in African-American and Caucasian Participants: The CARDIA Study; pp. 1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–38. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 77.Yeung DKW, Griffith JF, Antonio GE, Lee FKH, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. Journal of Magnetic Resonance Imaging. 2005;22:279–85. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 78.Griffith JF, Yeung DKW, Antonio GE, et al. Vertebral Marrow Fat Content and Diffusion and Perfusion Indexes in Women with Varying Bone Density: MR Evaluation1. Radiology. 2006;241:831–8. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 79.Griffith JF, Yeung DK, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 80.Tang GY, Lv ZW, Tang RB, et al. Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clinical Radiology. 2010;65:377–81. doi: 10.1016/j.crad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Li GW, Xu Z, Chen QW, et al. Quantitative evaluation of vertebral marrow adipose tissue in postmenopausal female using MRI chemical shift-based waterΓÇôfat separation. Clinical Radiology. 2014;69:254–62. doi: 10.1016/j.crad.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2 doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 83.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. American Journal of Roentgenology. American Roentgen Ray Society; 2004. Bone Marrow Fat and Bone Mineral Density on Proton MR Spectroscopy and Dual-Energy X-Ray Absorptiometry: Their Ratio as a New Indicator of Bone Weakening; pp. 1761–5. [DOI] [PubMed] [Google Scholar]
- 84.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR American journal of neuroradiology. 2001;22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98:2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Luo X, Yan F, et al. Effect of zoledronic acid on vertebral marrow adiposity in postmenopausal osteoporosis assessed by MR spectroscopy. Skeletal Radiology. 2015;44:1499–505. doi: 10.1007/s00256-015-2200-y. [DOI] [PubMed] [Google Scholar]
- 87.Duque G, Li W, Adams M, Xu S, Phipps R. Osteoporos Int Osteoporosis International. Springer-Verlag; 2011. Effects of risedronate on bone marrow adipocytes in postmenopausal women; pp. 1547–53. [DOI] [PubMed] [Google Scholar]
- 88.Wang G, Zhu Z, Lei C, et al. Low-dose risedronate sodium protects bone cells after abrupt oestrogen withdrawal. The Journal of international medical research. 2012;40:1761–74. doi: 10.1177/030006051204000515. [DOI] [PubMed] [Google Scholar]
- 89.Li GW, Xu Z, Chang SX, et al. Influence of early zoledronic acid administration on bone marrow fat in ovariectomized rats. Endocrinology. 2014;155:4731–8. doi: 10.1210/en.2014-1359. [DOI] [PubMed] [Google Scholar]
- 90.Duque G, Rivas D. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1603–11. doi: 10.1359/jbmr.070701. [DOI] [PubMed] [Google Scholar]
- 91.Jin J, Wang L, Wang XK, et al. Risedronate inhibits bone marrow mesenchymal stem cell adipogenesis and switches RANKL/OPG ratio to impair osteoclast differentiation. The Journal of surgical research. 2013;180:e21–9. doi: 10.1016/j.jss.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Martin RB, Chow BD, Lucas PA. Bone marrow fat content in relation to bone remodeling and serum chemistry in intact and ovariectomized dogs. Calcif Tissue Int. 1990;46:189–94. doi: 10.1007/BF02555043. [DOI] [PubMed] [Google Scholar]
- 93.Sottile V, Seuwen K, Kneissel M. Enhanced Marrow Adipogenesis and Bone Resorption in Estrogen-Deprived Rats Treated with the PPARgamma Agonist BRL49653 (Rosiglitazone) Calcified Tissue International. 2004;75:329–37. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 94.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology. 2009;10:747–55. doi: 10.1007/s10522-009-9221-7. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y, Luo X, Xie X, et al. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric. 2016;19:285–91. doi: 10.3109/13697137.2015.1126576. [DOI] [PubMed] [Google Scholar]
- 96.E LC. Bone marrow as an index of malnutrition in deer. NY State Conservationist. 1949;3:4. [Google Scholar]
- 97.Abella E, Feliu E, Granada I, et al. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. American journal of clinical pathology. 2002;118:582–8. doi: 10.1309/2Y7X-YDXK-006B-XLT2. [DOI] [PubMed] [Google Scholar]
- 98.Nonaka D, Tanaka M, Takaki K, Umeno M, Okamura T, Taketa H. Gelatinous bone marrow transformation complicated by self-induced malnutrition. Acta haematologica. 1998;100:88–90. doi: 10.1159/000040872. [DOI] [PubMed] [Google Scholar]
- 99.Bailly D, Lambin I, Garzon G, Parquet PJ. Bone marrow hypoplasia in anorexia nervosa: a case report. The International journal of eating disorders. 1994;16:97–100. doi: 10.1002/1098-108x(199407)16:1<97::aid-eat2260160112>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 100.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fazeli PK, Bredella MA, Freedman L, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bredella MA, Fazeli PK, Daley SM, et al. Marrow fat composition in anorexia nervosa. Bone. 2014;66:199–204. doi: 10.1016/j.bone.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:2078–88. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baek K, Bloomfield SA. Blocking +¦-adrenergic signaling attenuates reductions in circulating leptin, cancellous bone mass, and marrow adiposity seen with dietary energy restriction. Journal of Applied Physiology. 2012;113:1792–801. doi: 10.1152/japplphysiol.00187.2012. [DOI] [PubMed] [Google Scholar]
- 105.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–8. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 106.Zgheib S, Mequinion M, Lucas S, et al. Long-term physiological alterations and recovery in a mouse model of separation associated with time-restricted feeding: a tool to study anorexia nervosa related consequences. PLoS One. 2014;9:e103775. doi: 10.1371/journal.pone.0103775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98:2562–72. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bredella MA, Gill CM, Gerweck AV, et al. Ectopic and Serum Lipid Levels Are Positively Associated with Bone Marrow Fat in Obesity. Radiology. 2013 doi: 10.1148/radiol.13130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring, Md) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–22. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bredella MA, Gerweck AV, Barber LA, et al. Effects of growth hormone administration for 6 months on bone turnover and bone marrow fat in obese premenopausal women. Bone. 2014;62:29–35. doi: 10.1016/j.bone.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Araujo IM, Salmon CE, Nahas AK, Nogueira-Barbosa MH, Elias J, Jr, de Paula FJ. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. European journal of endocrinology/European Federation of Endocrine Societies. 2017;176:21–30. doi: 10.1530/EJE-16-0448. [DOI] [PubMed] [Google Scholar]
- 113.Doucette CR, Horowitz MC, Berry R, et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J Cell Physiol. 2015;230:2032–7. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adler BJ, Green DE, Pagnotti GM, Chan ME, Rubin CT. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One. 2014;9:e90639. doi: 10.1371/journal.pone.0090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 117.Bosy-Westphal A, Later W, Schautz B, et al. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring, Md) 2011;19:1503–10. doi: 10.1038/oby.2011.40. [DOI] [PubMed] [Google Scholar]
- 118.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-Measured Bone Marrow Adipose Tissue: Changes During Weight Loss and Its Relationship with DXA-Measured Bone Mineral. The FASEB Journal. 2007;21:A1057. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cordes C, Dieckmeyer M, Ott B, et al. MR-detected changes in liver fat, abdominal fat, and vertebral bone marrow fat after a four-week calorie restriction in obese women. Journal of Magnetic Resonance Imaging. 2015;42:1272–80. doi: 10.1002/jmri.24908. [DOI] [PubMed] [Google Scholar]
- 120.Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. doi: 10.1016/j.bone.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 122.Schafer AL, Li X, Schwartz AV, et al. Changes in vertebral bone marrow fat and bone mass after gastric bypass surgery: A pilot study. Bone. 2015;74:140–5. doi: 10.1016/j.bone.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim TY, Schwartz AV, Li X, et al. Bone Marrow Fat Changes After Gastric Bypass Surgery Are Associated With Loss of Bone Mass. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 doi: 10.1002/jbmr.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rantalainen T, Nikander R, Heinonen A, Cervinka T, Siev+ñnen H, Daly RM. Differential Effects of Exercise on Tibial Shaft Marrow Density in Young Female Athletes. Journal of Clinical Endocrinology & Metabolism. 2013;98:2037–44. doi: 10.1210/jc.2012-3748. [DOI] [PubMed] [Google Scholar]
- 125.Hu M, Sheng J, Kang Z, Zou L, Guo J, Sun P. Magnetic resonance imaging and dual energy X-ray absorptiometry of the lumbar spine in professional wrestlers and untrained men. The Journal of sports medicine and physical fitness. 2014;54:505–10. [PubMed] [Google Scholar]
- 126.Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: A pilot study. Bone. 2012;50:23–7. doi: 10.1016/j.bone.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Daly R, Ebeling P, Kukuljan S, Harvey S, C N. Effects of Exercise on Appendicular Bone Marrow Fat and Its Relationship to Changes in Bone Density, Structure and Strength in Older Men. Journal of Bone and Mineral Research. 2009;24 [Google Scholar]
- 128.Trudel G, Payne M, M+ñdler B, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. Journal of Applied Physiology. 2009;107:540–8. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 129.Trudel G, Coletta E, Cameron I, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. Journal of Applied Physiology. 2012;112:1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 130.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–19. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 131.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. Journal of diabetes and its complications. 2012;26:1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Abdalrahaman N, McComb C, Foster JE, et al. Deficits in Trabecular Bone Microarchitecture in Young Women With Type 1 Diabetes Mellitus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:1386–93. doi: 10.1002/jbmr.2465. [DOI] [PubMed] [Google Scholar]
- 133.Sheu Y, Amati F, Schwartz AV, et al. Vertebral bone marrow fat, bone mineral density and diabetes: The Osteoporotic Fractures in Men (MrOS) study. Bone. 2017;97:299–305. doi: 10.1016/j.bone.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? Journal of magnetic resonance imaging : JMRI. 2012;35:117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. Journal of Bone and Mineral Research. 2013:n/a–n/a. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vogt LJ, Steveling A, Meffert PJ, et al. Magnetic Resonance Imaging of Changes in Abdominal Compartments in Obese Diabetics during a Low-Calorie Weight-Loss Program. PLoS One. 2016;11:e0153595. doi: 10.1371/journal.pone.0153595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 138.Devlin MJ, Van Vliet M, Motyl K, et al. Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology. 2014;155:3806–16. doi: 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen SC, Brooks R, Houskeeper J, et al. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57–68. doi: 10.1016/j.mce.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
- 142.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et biophysica acta. 2013;1831:1533–41. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang YY, Attane C, Milhas D, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI insight. 2017;2:e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laurent V, Guerard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nature communications. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosen BR, Fleming DM, Kushner DC, et al. Radiology. Radiological Society of North America; 1988. Hematologic bone marrow disorders: quantitative chemical shift MR imaging; pp. 799–804. [DOI] [PubMed] [Google Scholar]
- 147.Falank C, Fairfield H, Reagan MR. Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Frontiers in Endocrinology. 2016;7 doi: 10.3389/fendo.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ricciardi MR, Mirabilii S, Allegretti M, et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood. 2015;126:1925–9. doi: 10.1182/blood-2014-12-617498. [DOI] [PubMed] [Google Scholar]
- 149.Wang J, Chen GL, Cao S, et al. Adipogenic niches for melanoma cell colonization and growth in bone marrow. Lab Invest. 2017;97:737–45. doi: 10.1038/labinvest.2017.14. [DOI] [PubMed] [Google Scholar]
- 150.Li G, Xu Z, Zhuang A, et al. Magnetic Resonance Spectroscopy-Detected Change in Marrow Adiposity Is Strongly Correlated to Postmenopausal Breast Cancer Risk. Clinical breast cancer. 2017 doi: 10.1016/j.clbc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 151.Bolan PJ, Arentsen L, Sueblinvong T, et al. WaterΓÇôfat MRI for assessing changes in bone marrow composition due to radiation and chemotherapy in gynecologic cancer patients. Journal of Magnetic Resonance Imaging. 2013;38:1578–84. doi: 10.1002/jmri.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schraml C, Schmid M, Gatidis S, et al. Multiparametric analysis of bone marrow in cancer patients using simultaneous PET/MR imaging: Correlation of fat fraction, diffusivity, metabolic activity, and anthropometric data. Journal of magnetic resonance imaging : JMRI. 2015;42:1048–56. doi: 10.1002/jmri.24865. [DOI] [PubMed] [Google Scholar]
- 153.Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:1657–66. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu RJ, Wu MQ, Li ZJ, Zhang Y, Liu KY. Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. International journal of hematology. 2013;97:58–72. doi: 10.1007/s12185-012-1233-4. [DOI] [PubMed] [Google Scholar]
- 156.Ye H, Adane B, Khan N, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016;19:23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


