Abstract
Helicobacter pylori is a prevalent cause of gastrointestinal infections. Recently, several studies have shown a relationship between H. pylori infection and a variety of extradigestive manifestations. The aim of this study was to review the literature regarding the prevalence of this infection in cases of central serous chorioretinopathy (CSR). We reviewed the EMBASE, Cochrane Library, and Google Scholar search engines; hand-searched many journals; and searched the cited references in published articles for relevant studies. We assessed 81 studies for eligibility. Finally, nine articles that met the inclusion criteria were included. The relationship between H. pylori infection (as the etiologic factor) and chorioretinal involvement was assessed by the effect size with 95% confidence interval (CI). Both fixed- and random-effects models showed that the prevalence of H. pylori infection in patients with CSR was significantly higher than in the control group (2.5-fold and 2.7-fold higher, respectively; P < 0.01). The results were not significantly different between the two models. Treatment of H. pylori infection should be considered in patients with CSR. However, additional randomized controlled clinical trials are required to determine the possible role of H. pylori eradication in the prognosis and treatment of patients with CSR.
Key Words: Prevalence, Helicobacter pylori, Central Serous Chorioretinopathy, Systematic Review, Gastrointestinal Infections
INTRODUCTION
Helicobacter pylori is a helix-shaped, Gram-negative microphilic bacterium that inhabits various regions of the stomach and duodenum. It is one of the most common causes of gastric infections worldwide [1] and the etiological basis of many diseases such as gastritis, gastric ulcer, gastric cancer, and lymphoma. Notably, H. pylori is suspected as a possible cause of early-onset focal obstructive arterial diseases [2]. In the past decade, several studies have investigated the possible relationship between H. pylori infection and various other complications and manifestations, such as Raynaud’s phenomenon, cardiovascular diseases, pulmonary diseases, urticaria, and rosacea [3, 4]. Recently, the relationship between H. pylori infection and eye diseases, including blepharitis, uveitis, dry eye syndrome, glaucoma, and central serous chorioretinopathy (CSR), has been a matter of interest [5]. However, it is unclear how H. pylori might affect the eye [5, 6]. CSR is an idiopathic condition that is characterized by the development of a well-circumscribed serous detachment of the neurosensory retina (neuroepithelium), resulting from altered barrier and deficient pumping function at the level of the retinal pigment epithelium (RPE) in the macular region [7]. Studies have estimated the annual incidence rate of CSR, which is 10/100,000 in men (six times more common than in women) [8]. Many individuals with CSR may be asymptomatic or may suffer from visual disturbances, such as a sudden-onset decrease of vision (blurred vision), color vision deficiency, micropsia, metamorphopsia, or central scotoma [8].
A decrease in visual acuity (VA) can be detected with a Snellen visual acuity chart; color vision changes can be measured via standard testing (for example, using pseudoisochromatic plates such as the Ishihara plate, or by panel tests, such as the Lanthony 15-Hue Desaturated Test); central scotoma may be identified on macular perimetry (microperimetry) or by using the Amsler grid test [9]. Retinal lesions can be observed on optical coherence tomography (OCT) images. Occasionally, angiographic studies (fluorescein angiography, indocyanine green angiography, or three-dimensional angiography) are required for further investigation [10]. Fortunately, CSR exhibits a benign natural course and symptoms improve over a period of 3–4 months [11]. Because of the spontaneous resolution of the neurosensory retinal detachment (RD), the prognosis of patients with CSR is often favorable; however, a small percentage of patients can progress to chronic or progressive disease with severe visual loss for more than 6 months; this is defined as diffuse decompensation of the RPE [11]. Men become more susceptible in the third to fifth decades of life, and are more likely to be affected if they exhibit a Type A personality. Other known predisposing factors include corticosteroid therapy or psychotropic medications, emotional strain, organ transplant, pregnancy, and connective tissue diseases, such as systemic lupus erythematosus [8].
The pathophysiology of CSR remains unknown, and no effective treatment is available. Recently, immune-dependent mechanisms have been proposed. A possible immune mechanism is based on the molecular mimicry between H. pylori-expressed antigens and homologous host proteins (e.g., vascular endothelial proteins) [12]. Many other studies have investigated the causal relationship between H. pylori infection and CSR [2, 13-15]. In a prospective pilot study of 16 patients with CSR and diffuse retinal pigment epitheliopathy (DRE), the prevalence of H. pylori infection was significantly higher in patients with CSR than in the general population [2]. In another study, the prevalence was also significantly higher in patients with CSR than in a control population from the same country [16]. Subsequent investigations confirmed that H. pylori is an important etiologic factor in CSR [12-21]. Nevertheless, the prevalence of H. pylori infection in patients with CSR remains controversial, as some studies have reported that the prevalence of this infection is not higher in patients with CSR than in controls [22, 23]. The aim of this study was to systematically review the literature regarding the prevalence of H. pylori infection in CSR to more clearly describe the relationship between H. pylori infection and chorioretinal involvement in CSR. All retrospective studies that investigated H. pylori infection as an etiologic factor in CSR were analyzed.
MATERIALS AND METHODS
This review study was conducted in accordance with the guidelines for systematic reviews in health care [24]. An extensive article search was conducted using the MEDLINE, EMBASE, and Cochrane Library databases, with a search period through March 2016 and no language restriction. Retrospective observational studies regarding the prevalence of H. pylori infection in patients with CSR were screened using a search strategy that was based on the following Medical Subject Heading (MeSH) terms:
“(((central serous Chorioretinopath*) OR (Chorioretinopath* AND central serous) OR (serous Chorioretinopath* AND central) OR (central serous Retinopath*) OR (Retinopath* AND central serous) OR (serous Retinopath* AND central) OR cscr OR CSR OR CSC)) AND (((campylobacter pylori) OR (rdxa protein AND h. pylori) OR (LPS AND h. pylori) OR (helicobacter pylori lipopolysaccharide) OR (antibacterial cecropin-like h. pylori peptide hp (2-20)) OR (vacuolating cytotoxin AND h. pylori) OR (hvlt protein AND helicobacter pylori) OR (vacuolating toxin AND helicobacter pylori) OR (helicobacter pylori vacuolating toxin) OR (hydrogenase nickel incorporation protein hypa AND h pylori) OR (hypa protein AND helicobacter pylori) OR (outer membrane protein homb AND h pylori) OR (tumor necrosis factor-alpha-inducing protein AND helicobacter pylori) OR (hp0596 protein AND h pylori) OR (tnf-alpha-inducing protein AND helicobacter pylori) OR (clone p32 protein AND h pylori) OR (asd enzyme AND h pylori) OR (ylxh protein AND h pylori)))”.
The search strategy for the MEDLINE, EMBASE, and Cochrane Library databases was similar to that utilized for the PubMed database. In the next step, we performed a comprehensive literature search using the Google Scholar search engine (scholar.google.com). Additionally, a manual search was conducted in the following journals: American Journal of Ophthalmology (www.ajo.com), Retina Journal (www.journals.lww.com/retinajournal), British Journal of Ophthalmology (bjo.bmj.com), and European Journal of Ophthalmology (www.eur-j-ophthalmol.com).
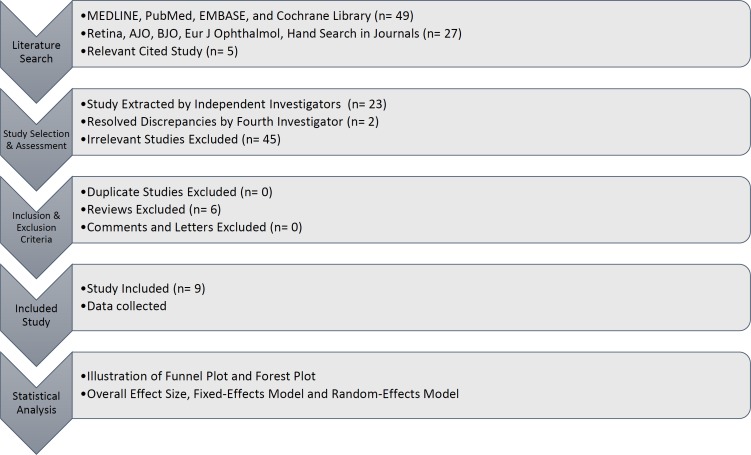
Finally, the cited references in the retrieved studies were manually reviewed for additional relevant results. After the structured search, we assessed 81 studies for eligibility, and their full-text versions were retrieved. All papers were reviewed by three independent investigators to avoid potential bias or errors. Essential data extracted by researchers, and literature with an agreement rate above 80%, were included. Discrepancies were resolved by discussion or referral to a fourth investigator if necessary (Fig 1).
Figure 1.
This figure shows the methodology of the study, in a stepwise fashion.
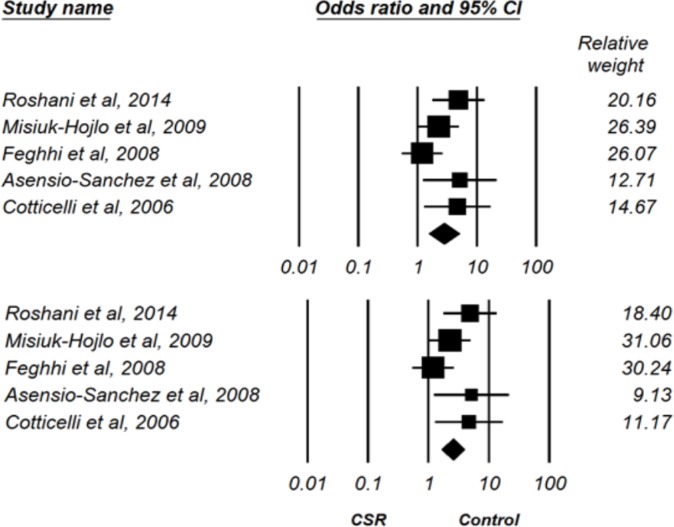
Of the included studies, two did not have a control group and two did not provide sufficient data for analysis. Therefore, the data of five studies were ultimately entered into Comprehensive Meta-Analysis (CMA.2) software for analysis. To assess the overall effect size, we used both fixed- and random-effects models (Fig 2). According to these models, the prevalence of H. pylori infection was 2.5-fold and 2.7-fold higher, respectively, in patients with CSR than in the control group (fixed-effects model: OR = 2.52, 95% CI 1.64–3.89; random-effects model: OR = 2.77, 95% CI 1.53–5.02) (Fig 2).
Figure 2.
Prevalence of Helicobacter Pylori Infection in Central Serous Chorioretinopathy (CSR) Group versus Control Group based on Random-Effects Model (up) and Fixed-Effects Model (down).
In this study, the final agreement rate among the reviewers was 90%. The following information was obtained from all studies and entered in an extraction table: the first author’s surname, the publication year, the city and country, the number of subjects, and the odds ratio (OR) with P-value (for studies that contained a control group). Data were analyzed by CMA.2 software. The relationship between H. pylori infection (as an etiologic factor) and chorioretinal involvement was assessed by the effect size with 95% confidence interval (CI). To estimate the overall effect size, we used fixed- and random-effects models. In addition, by utilization of a funnel plot, these two models were evaluated to identify any potential publication bias.
RESULTS
Of the 81 studies assessed for eligibility, a total of nine retrospective studies met the criteria for entry and were included in the analysis. The studies examining the prevalence of H. pylori infection in CSR included a total of 309 patients with CSR and 295 cases in control groups, or in a historical control population (as in two studies). The extracted data are listed in Table 1.
Table 1.
Extracted Data from All Included Studies
| Authors | Year | City, country | Prevalence of H. pylori infection in patients with CSR | Prevalence of H. pylori infection in the control group | Odds ratio (P-value) |
|---|---|---|---|---|---|
| Roshani et al. [21] | 2014 | Tehran, Iran | 30/35 (85.7%) | 76/138 (55.1%) | 4.895 (0.001) |
| Warrow et al. [23] | 2012 | New York, USA | 3/18 (16.7%) | ---- | ---- |
| Misiuk-Hojlo et al. [13] | 2009 | Wroclaw, Poland | 37/55 (67.0%) | 26/55 (47.0%) | 2.29 (<0.05) |
| Kmera-Muszynska et al. [18] | 2008 | Warszawie, Poland | 11/14 (78.5%) | ----- | ---- |
| Feghhi et al. [22] | 2008 | Ahvaz, Iran | 37/54 (68.5%) | 38/59 (65.0%) | 1.20 (0.64) |
| Asensio-Sanchez et al. [14] | 2008 | Valladolid, Spain | 11/16 (68.75%) | 6/20 (30.0%) | 5.13 (<0.05) |
| Cotticelli et al. [15] | 2006 | Napoli, Italy | 18/23 (78.2%) | 10/23 (43.5%) | 4.6 (<0.03) |
| Ahnoux-Zabsonre et al. [16] | 2004 | Côte d’Ivoire, France | 31/78 (39.7%) | Overall population of 58,419,710 inhabitants of France in 1999 (25.4%) | ---- |
| Mauget-Faysse et al. [2] | 2002 | Lyon, France | 9/16 (56.3%) | Historical control population (27.5%) | ---- |
H. pylori, Helicobacter pylori; CSR, central serous chorioretinopathy
The prevalence of H. pylori infection was significantly greater in patients with CSR than in the control group (P < 0.01) and there was no difference between the two models (Table 2).
Table 2.
The Results of Systematic Review and Meta-analysis
| Model | Number of Studies | Point estimate | Lower limit | Upper limit | P-value |
|---|---|---|---|---|---|
| Fixed | 5 | 2.528 | 1.643 | 3.890 | < 0.001 |
| Random | 5 | 2.778 | 1.535 | 5.027 | 0.001 |
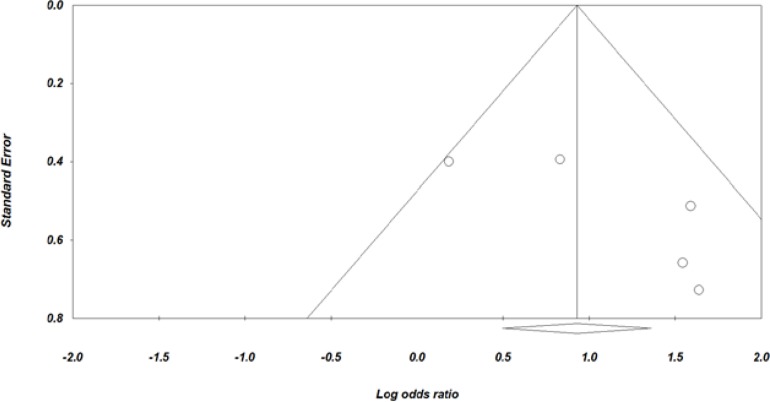
Furthermore, funnel plot analysis revealed that there was no publication bias (Fig 3).
Figure 3.
Funnel Plot of Standard Error against Log Odds Ratio.
DISCUSSION
This review of the literature was conducted to elucidate the relationship between H. pylori infection and CSR. Data from 302 patients with CSR were included in this study. A total of nine papers were selected to study the possible relationship between H. pylori infection and CSR, and five papers were included in the final analysis. The results of fixed- and random-effects models showed that the prevalence of H. pylori infection in patients with CSR is 2.5 and 2.7 times higher, respectively, compared with the control group. Similarly, in a prospective pilot study of 16 patients with CSR and DRE, the prevalence of H. pylori infection was significantly higher in patients with CSR than in the general population [2]. In another study, the prevalence was also significantly higher in patients with CSR than in a control population from the same country [16]. These results have been confirmed by many other studies [2, 13-16, 18, 22]. For example, in a prospective study involving 35 CSR cases and 135 controls, Roshani et al. (2014) demonstrated that the prevalence of H. pylori infection was significantly higher in patients with CSR than in controls. They reported that the symptoms of patients with CSR improved after successful H. pylori eradication using standard therapy, and suggested additional randomized controlled clinical trials were needed to confirm the usefulness of H. pylori eradication as a therapeutic option for patients with CSR who exhibit an H. pylori infection [21].
In contrast, a prospective study by Warrow et al. (2012) reported a much lower prevalence of H. pylori infection in patients with CSR, compared with previous, predominantly retrospective studies (prevalence range: 44%–78%). This disparity may have arisen because H. pylori testing in the study by Warrow et al. was performed during periods of CSR quiescence in four of the negative subjects [23]. Ahnoux-Zabsonre et al. (2004) reported that recurrent CSR was always associated with the presence of H. pylori infection, whereas improvement in both symptoms (blurred vision and decreased visual acuity) and signs (fundoscopic findings) was associated with successful H. pylori eradication [16]. In a study by Mauget-Faysse et al. (2002), 16 patients with long-lasting CSR (> 6 months) or DRE were tested for gastric H. pylori infection. The authors reported that 56.3% of patients were positive for H. pylori, which was significantly higher than the rate of the historical control population (27.5%) [2]. The same study also reported that the prevalence of H. pylori infection was significantly higher in patients with CSR and DRE (39.7%), compared with the general French population (25.4%) [16]. The authors indicated that these results could provide a basis for further studies regarding the etiopathogenesis of chronic CSR or DRE, which could provide evidence to support effective therapy, i.e., H. pylori eradication [2]. Kmera-Muszinska et al. (2008) suggested a role for H. pylori infection in the etiopathogenesis of CSR, based on a survey of 14 patients with histopathologically confirmed H. pylori infection [18]. The association between H. pylori infection and atherosclerosis has been documented in previous studies of cagA-positive strains [25]. This study provided new clues regarding the pathophysiology of CSR and its relationship with H. pylori infection. It has been suggested that antibodies against the CagA antigen may cross-react with vascular endothelial antigens, thus promoting vascular wall damage and atherosclerosis development [25, 26]. Endothelial cell dysfunction via the proposed mechanism could explain the pathophysiology of CSR in H. pylori-infected patients. However, further studies are needed to investigate this and other possible autoimmune mechanisms. One of the limitations of this study was the small number of included papers and the lack of a control group in two of these papers. In conclusion, this review confirmed that the prevalence of H. pylori infection is significantly higher in patients with CSR. Treatment of H. pylori infection should be considered in patients with CSR. However, this recommendation merits further investigation. Additional clinical trials are required to clearly discern the role of H. pylori eradication in the prognosis and treatment of patients with CSR. If this relationship is confirmed, a new therapeutic protocol for CSR could be introduced in the future.
DISCLOSURE
This study was approved by the review board ethics committee of the training hospital and Tabriz University of Medical Sciences, Tabriz, Iran. No funding or sponsorship was received for this study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
References
- 1.Hosseini H, Ghaffariyeh A, Nikandish R. Noxious compounds in exhaled air, a potential cause for ocular manifestations of H pylori gastrointestinal infection. Med Hypotheses. 2007;68(1):91–3. doi: 10.1016/j.mehy.2006.06.029. doi: 10.1016/j.mehy.2006.06.029 pmid: 16919889. [DOI] [PubMed] [Google Scholar]
- 2.Mauget-Faysse M, Kodjikian L, Quaranta M, Ben Ezra D, Trepsat C, Mion F, et al. [Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy Results of the first prospective pilot study] J Fr Ophtalmol. 2002;25(10):1021–5. pmid: 12527825. [PubMed] [Google Scholar]
- 3.Sherman PM, Lin FY. Extradigestive manifestation of Helicobacter pylori infection in children and adolescents. Can J Gastroenterol. 2005;19(7):421–4. doi: 10.1155/2005/971974. doi: 10.1155/2005/971974 pmid: 16010304. [DOI] [PubMed] [Google Scholar]
- 4.Zbinden R doi: 10.1007/s15010-005-7205-3 pmid: 15827869. Expanding the spectrum of Helicobacter pylori-associated diseases. Infection. 2005;33(2):49. doi: 10.1007/s15010-005-7205-3. [DOI] [PubMed] [Google Scholar]
- 5.Sacca SC, Vagge A, Pulliero A, Izzotti A. Helicobacter pylori infection and eye diseases: a systematic review. Medicine (Baltimore) 2014;93(28) doi: 10.1097/MD.0000000000000216. doi: 10.1097/MD.0000000000000216 pmid: 25526440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardhan N, Chaudhary KP, Sharma V, Sharma SK, Sharma B. Helicobacter Pylori Positive Dyspepsia in Relation to Ocular Manifestations and Primary Open Angle Glaucoma. Offic Sci J Delhi Ophthalmol Soc. 2014;24(4):237–40. [Google Scholar]
- 7.Ojima Y, Tsujikawa A, Hangai M, Nakanishi H, Inoue R, Sakamoto A, et al. Retinal sensitivity measured with the micro perimeter 1 after resolution of central serous chorioretinopathy. Am J Ophthalmol. 2008;146(1):77–84. doi: 10.1016/j.ajo.2008.02.016. doi: 10.1016/j.ajo.2008.02.016 pmid: 18405876. [DOI] [PubMed] [Google Scholar]
- 8.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–14. doi: 10.1111/j.1442-9071.2012.02848.x. doi: 10.1111/j.1442-9071.2012.02848.x pmid: 22788735. [DOI] [PubMed] [Google Scholar]
- 9.Albert DM, Jakobiec FA. Principles and practice of ophthalmology. Philadelphia, USA: Saunders; 1994. [Google Scholar]
- 10.Shah SP, Desai CK, Desai MK, Dikshit RK. Steroid-induced central serous retinopathy. Indian J Pharmacol. 2011;43(5):607–8. doi: 10.4103/0253-7613.84985. doi: 10.4103/0253-7613.84985 pmid: 22022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk EH, Dijkman G, Biermasz NR, van Haalen FM, Pereira AM, Boon CJ. Chronic central serous chorioretinopathy as a presenting symptom of Cushing syndrome. Eur J Ophthalmol. 2016;26(5):442–8. doi: 10.5301/ejo.5000790. doi: 10.5301/ejo.5000790 pmid: 27135093. [DOI] [PubMed] [Google Scholar]
- 12.Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63(3):524–7. doi: 10.1016/j.mehy.2004.02.020. doi: 10.1016/j.mehy.2004.02.020 pmid: 15288381. [DOI] [PubMed] [Google Scholar]
- 13.Misiuk-Hojlo M, Michalowska M, Turno-Krecicka A. Helicobacter pylori--a risk factor for the developement of the central serous chorioretinopathy. Klin Oczna. 2009;111(1-3):30–2. pmid: 19517842. [PubMed] [Google Scholar]
- 14.Asensio-Sanchez VM, Rodriguez-Delgado B, Garcia-Herrero E, Cabo-Vaquera V, Garcia-Loygorri C. [Central serous chorioretinopathy as an extradigestive manifestation of Helicobacter pylori gastric infection] Arch Soc Esp Oftalmol. 2008;83(3):177–82. pmid: 18311677. [PubMed] [Google Scholar]
- 15.Cotticelli L, Borrelli M, D'Alessio AC, Menzione M, Villani A, Piccolo G, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16(2):274–8. doi: 10.1177/112067210601600213. pmid: 16703546. [DOI] [PubMed] [Google Scholar]
- 16.Ahnoux-Zabsonre A, Quaranta M, Mauget-Faysse M. [Prevalence of Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy: a complementary study] J Fr Ophtalmol. 2004;27(10):1129–33. doi: 10.1016/s0181-5512(04)96281-x. doi: MDOI-JFO-12-2004-27-10-0181-5512-101019-ART06 [pii] pmid: 15687922. [DOI] [PubMed] [Google Scholar]
- 17.Nenciu A, Stefan C, Melinte D, Postaliu D. [Central serous chorioretinopathy--clinical aspects and course] Oftalmologia. 2006;50(3):123–7. pmid: 17144519. [PubMed] [Google Scholar]
- 18.Kmera-Muszynska M, Wojcicka I, Muszynski J. [Is Helicohacter pylori infection associated with CRS occurrence?] Klin Oczna. 2008;110(7-9):273–6. pmid: 19112860. [PubMed] [Google Scholar]
- 19.Misiuk-Hojlo M, Michalowska M, Zolynska M. [The role of Helicobacter pylori in etiopathogenesis of central serous choroidopathy] Klin Oczna. 2007;109(10-12):479–81. pmid: 18488401. [PubMed] [Google Scholar]
- 20.Mansuetta CC, Mason JO 3rd, Swanner J, Feist RM, White MF Jr, Thomley ML, et al. An association between central serous chorioretinopathy and gastroesophageal reflux disease. Am J Ophthalmol. 2004;137(6):1096–100. doi: 10.1016/j.ajo.2004.01.054. doi: 10.1016/j.ajo.2004.01.054 pmid: 15183795. [DOI] [PubMed] [Google Scholar]
- 21.Roshani M, Davoodi NA, Seyyedmajidi MR, Zojaji H, Sherafat SJ, Hashemi M, et al. Association of Helicobacter pylori with central serous chorioretinopathy in Iranian patients. Gastroenterol Hepatol Bed Bench. 2014;7(1):63–7. pmid: 25436099. [PMC free article] [PubMed] [Google Scholar]
- 22.Feghhi M, Hajiani E, Khataminia G. Incidence of Helicobacter pylori in central serous chorioretinopathy a case control study. Jundishapur J Microb. 2008;1(1):15–9. [Google Scholar]
- 23.Warrow D, Mukkamala K, Rosen RB. Relationship between Helicobacter Pylori and Central Serous Chorioretinopathy. Invest Ophthalmol Vis Sci. 2012;53(14):5227–7. [Google Scholar]
- 24.Egger M, Smith GD. Principles of and Procedures for Systematic Reviews. 2nd ed. London, UK: BMJ Books; 2001. pp. 23–42. [Google Scholar]
- 25.Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, et al. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106(4):430–4. doi: 10.1161/01.cir.0000024100.90140.19. pmid: 12135941. [DOI] [PubMed] [Google Scholar]
- 26.Jahani Sherafat S, Tajeddin E, Reza Seyyed Majidi M, Vaziri F, Alebouyeh M, Mohammad Alizadeh AH, et al. Lack of association between Helicobacter pylori infection and biliary tract diseases. Pol J Microbiol. 2012;61(4):319–22. pmid: 23484417. [PubMed] [Google Scholar]