Abstract
We aimed to compare the results of pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling, an alternative therapeutic strategy, with those of medical treatment for chronic macular edema. We conducted a review of the literature on the microscopic, anatomical, and functional reasons for performing PPV with ILM peeling in patients with diabetic macular edema (DME). We searched the PubMed database for articles published between 2000 and 2017. We used the medical subject heading “vitrectomy diabetic macular edema” and the keywords “diabetic macular edema”, “internal limiting membrane peeling”, “pars plana vitrectomy”, “diabetic retinopathy”, and “optical coherence tomography”. Analysis of the literature revealed that cytokines, vascular endothelial growth factor, reactive oxygen species (ROS), and advanced glycation end-products (AGEs) play a unique role in DME. The vitreous cavity serves as a physiological reservoir for all inflammatory molecules. AGE receptors are localized at the footplates of Müller cells and the external limiting membrane (ELM). The footplates of Müller cells are in contact with the ILM, which suggests that they might be responsible for the structural damage (i.e., thickening) observed in the ILM of patients with DME. Therefore, PPV could allow a reduction of cytokines and pro-inflammatory molecules from the vitreous cavity. ILM peeling could eliminate not only the physical traction of a thickened structure, but also the natural reservoir of AGEs, ROS, and inflammatory molecules. PPV with ILM peeling is a surgical option that should be considered when treating patients with chronic DME.
Key Words: Diabetic Retinopathy, Vitrectomy, Diabetic Macular Edema, Optical Coherence Tomography, Internal Limiting Membrane
INTRODUCTION
Diabetic macular edema (DME) is one of the most common complications of diabetic retinopathy (DR) and is a frequent cause of sudden impairment of visual acuity (VA). The incidence of DME ranges from 14% to 29% in patients with DR. According to the Wisconsin Epidemiologic Study of Diabetic Retinopathy, the estimated prevalence of DME after 20 years following a diagnosis of diabetes mellitus (DM) is 12%–29% in patients with DM type 1 and 2 [1]. DME represents a severe threat to public health. The World Health Organization (WHO) Global Report of Diabetes 2016 indicates that Mediterranean East, South East Asia, and the American continent are the most affected regions in the world [2]. The cost of the disease varies according to the country. However, according to data from countries in the European Union, the annual cost is EURO 777 to EURO 7153, depending on the need for different treatments, such as laser or anti-vascular endothelial growth factor (VEGF) drugs [3].
The aim of this study was to review scientific evidence for considering pars plana vitrectomy (PPV) as a treatment for DME.
MATERIALS AND METHODS
We conducted a review of the literature about the microscopic, anatomical, and functional reasons supporting the use of PPV with internal limiting membrane (ILM) peeling as a treatment option for DME. We searched the PubMed database for articles published between 2000 and 2017. We used the medical subject heading “pars plana vitrectomy diabetic macular edema” and the keywords “diabetic macular edema,” “internal limiting membrane peeling,” “pars plana vitrectomy,” “diabetic retinopathy,” and “optical coherence tomography.” As inclusion criteria, we used clinical trials, review, systematic review, case reports, clinical studies, and multicenter studies as filters. Exclusion criteria were repeated papers, incomplete information, or missing data. If no statistical analysis of data was reported, we focused on molecular analysis and clinical evidence reported in the surgical management of DME.
RESULTS
A searched was performed in PubMed database, and 190 papers were found with PPV and DME. After filters and key words were applied, 67 papers were retained; however, many of these were discarded because of incomplete data, confusing evidence, or repeated information. We finally selected 22 publications for this review, which are summarized in Table 1.
Table 1.
Summary of the Most Relevant Data Regarding Pars Plana Vitrectomy and Diabetic Macular Edema from 2000 to 2017
| Year | Author | Type of Study | Main Outcome |
|---|---|---|---|
| 2001 | Augustin et al. | Clinical Trial | Oxidative metabolites are able to modulate growth activity via VEGF activity directly |
| 2002 | Kumagai et al. | Clinical Trial | ILM peeling accelerates the absorption of edema in more severe DME without change in VA |
| 2005 | Holeamp et al. | Case Series | Vitrectomy increases intraocular oxygen tension, which may lead to nuclear cataract formation |
| 2006 | Stefánsson et al. | Review | Both laser and vitrectomy treatment help to increase inner retina oxygenation |
| 2009 | Bhagat et al. | Review | Combined surgical and medical treatment is the best option to manage DME |
| 2009 | Sakamoto et al. | Retrospective | Postoperative photoreceptor status of the fovea is closely related to the final VA |
| 2010 | DRCR.Net et al. | Clinical Trial | Vitrectomy performed for DME and vitreomacular traction; retinal thickening was reduced in most eyes |
| 2010 | Otani et al. | Case Series | OCT showed that the integrity of the external limiting membrane and inner and outer segments of the photoreceptors were more strongly correlated with VA |
| 2011 | Deissler et al. | Experimental | VEGF 165 is mainly responsible for changes in cellular permeability in retinal endothelial cells |
| 2011 | Yanyali et al. | Case Series | The integrity of the ELM and IS/OS lines was positively correlated with final visual acuity after PPV |
| 2012 | Tamura et al. | Case Series | The ILM is thickened and numerous types of inflammatory cells are attached |
| 2012 | Chhablani et al. | Retrospective | Preoperative evaluation of the ELM predicts vision improvement more accurately than the IS/OS junction |
| 2013 | Koskela et al. | CaseControl | Elevated IL-6 and IL-8 levels in vitreous, but not in plasma, are evidence favouring local over systemic inflammation in PDR |
| 2013 | Yamada et al. | Clinical Trial | Glycemic control may be important for retinal thickness after ocular surgery |
| 2013 | Nizawa et al. | CaseControl | PPV either with or without preoperative treatments can significantly improve the BCVA and reduce the central macular thickness MT in patients with diffuse DME |
| 2014 | Romano et al. | Retrospective | DME with intraretinal cysts larger than 390 µm should not be treated with vitrectomy with ILM peeling, because this may induce subfoveal atrophy |
| 2015 | Kumagai et al. | Clinical Trial | PPV with or without peeling improves long term VA of DME |
| 2015 | Bonnin et al. | CaseControl | Vitrectomized eyes are not different in terms of anatomical and visual outcomes in patients with or without tractional DME |
| 2016 | Ichiyama et al. | Clinical Trial | Vitrectomy can be a useful treatment option for diffuse DME, particularly for eyes with subretinal fluid |
| 2017 | Gonzalez-Salinas et al. | CaseControl | Different VEGF polymorphisms could be related to grades of proliferative DR |
| 2017 | Jackson et al. | Systematic Review | Vitrectomy produces structural and functional improvements in select eyes with DME, but the visual gains are not significantly better than with laser or observation. |
Abbreviations: Pars plana vitrectomy (PPV); internal limiting membrane (ILM); reactive oxygen species (ROS); advanced glycation end-product (AGE); external limiting membrane (ELM); diabetic macular edema (DME); diabetic retinopathy (DR); visual acuity (VA); diabetes mellitus (DM); World Health Organization (WHO), vascular endothelial growth factor (VEGF); intercellular adhesion molecule-1 (ICAM-1); vascular cell adhesion molecule-1 (VCAM-1); vascular endothelial growth factor receptor (VEGFR); fluorescein angiography (FA); optical coherence tomography (OCT); posterior hyaloidal traction (PHT); optical coherence tomography angiography (OCTA); best-corrected visual acuity (BCVA); glial fibrillary acid protein (GFAP); inner segments/outer segments (IS/OS)
There is a close association between DME and diabetic metabolic control. A higher level of glycosylated hemoglobin, high systolic blood pressure, and borderline proteinuria are all risk factors for DME [3]. The pathophysiology of this condition is complex, and some aspects are still being debated. The oxidative stress caused by multiple inflammatory cytokines is believed to be a key factor for vascular damage [4]. Chronic hyperglycemia causes oxidative stress by inducing the formation of advanced glycation end-products (AGEs) and non-enzymatic glycosylation of proteins. AGEs can cross-link the amino-termini of proteins, lipids, and DNA, leading to modification of their tertiary structure and function. They can also activate intercellular adhesion molecule-1 (ICAM-1), causing damage to cellular membranes and endothelia by increasing transcription factors, such as nuclear factor kappa B [5]. In addition, alteration in the expression of adhesion molecules and release of pro-inflammatory cytokines induce the upregulation of other proteins. such as adhesion molecule-1 (E-selectin) and vascular cell adhesion molecule-1 (VCAM-1), which in conjunction with ICAM-1, induce leukostasis and further cell damage [6]. The formation of reactive oxygen species (ROS) has also been implicated in the pathophysiology of DME. ROS cause oxidative damage to the lipid bilayer of cell membranes by lipid peroxidation [7]. Damage to specific cells, such as perivascular pericytes, induces microvessel occlusion and microaneurysm formation. The resulting hemodynamic dysfunction leads to abnormal autoregulation of the retinal blood flow and an imbalance in retinal metabolism homeostasis. The damage exerted on capillary-vessel basement membranes by ROS and AGEs induces an increase in the deposition of extracellular matrix components, which in turn further exacerbate hemodynamic dysfunction, thus impairing autoregulation [8-10].
VEGF is a dimeric glycoprotein that has a fundamental role in pathological angiogenesis. Its concentration in the vitreous is increased exponentially in patients with DR [11]. VEGF-A has six major isoforms (121, 145, 165, 183, 189, and 206) and three main membrane receptors (VEGFR-1, 2, and 3) [5]. In DME, VEGF can induce vasodilation of the retinal vessels, enhance vascular permeability, and activate endothelial cells [6]. VEGF also phosphorylates claudin-1, a protein that is present in the tight junctions of retinal endothelial cells. The protein is then downregulated and delocalized, leading to increased vascular permeability and retinal edema [12, 13].
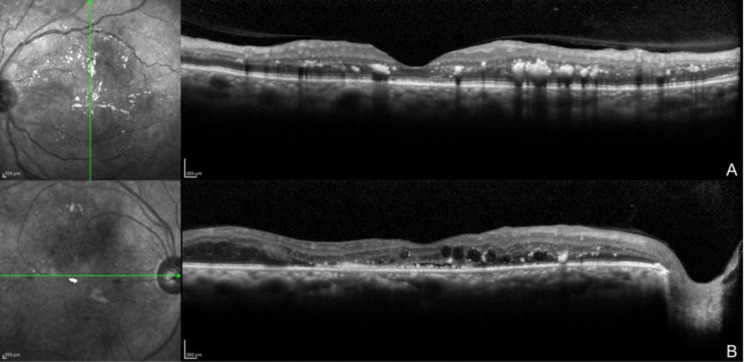
There is evidence that RD depends not only on the usual diet or glycemic levels, but also on a genetic background that promotes development of diabetes and its complications, and which varies across different populations [14]. Traditionally, DME is classified as focal or diffuse based on leakage patterns on fluorescein angiography (FA). More recently, optical coherence tomography (OCT) is used to provide in-depth information about tissue structure, which is essential for elaborate therapeutic strategies in DME [15]. The classification proposed by Kim et al. in 2006 describes five well-defined patterns of DME by OCT: diffuse retinal thickening, cystoid macular edema, serous retinal detachment (RD) without posterior hyaloidal traction (PHT), PHT without tractional RD, and PHT with RD [16] (Fig 1 A and B). OCT angiography (OCTA) is a new diagnostic device that allows the theoretical identification of the blood flow through retinal blood vessels [17]. Early reports have shown areas of decreased signal intensity that correlate well with areas of intraretinal fluid accumulation on OCT. Areas of cystoid edema appear entirely devoid of flow with borders where there is no vascular flow surrounding the capillary meshwork [18].
Figure 1.
Optical coherence tomography images showing diffuse bilateral diabetic macular edema.
Multiple hard exudates (hyperreflective dots) and vitreous macular adhesion. B. Hard exudates (hyperreflective dots), cystic macular spaces (black cysts), and serous retinal detachment.
Nevertheless, the diagnostic value of this new technology is still debated, since issues such as image artifacts and hard-to-understand changes limit the clinical application of the results.
Medical Treatment for DME
Anti-angiogenic drugs that specifically target VEGF are the current standard of care for DME treatment. There are currently two approved anti-VEGF drugs (ranibizumab and aflibercept) and one open-label anti-VEGF drug (bevacizumab), with more drugs to come in the near future (conbercept, bevasiranib, razumab, xlucane, etc.). Several studies sponsored by the National Institutes of Health, such as those conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) [19], and studies sponsored by the pharmaceutical industry (RESOLVE, RESTORE, RIDE, RISE, and DAVINCI) demonstrate that, alone or in combination, anti-VEGF drugs are superior to laser, sham treatment, or intravitreal triamcinolone for the treatment of DME, with excellent security profiles [20-22]. However, it is important to highlight that patients with chronic DME will probably develop tachyphylaxis after years of intravitreal injections of anti-VEGF. Moreover, there are many cytokines as important as VEFG in DME [23]. In relapsing or chronic cases of DME, a treatment alternative is the intravitreal implant of dexamethasone. Dexamethasone is five times more powerful than triamcinolone and is more hydrophilic, which allows higher vitreous concentrations [24]. A 6-mm implant containing 700 mg of dexamethasone is placed in the eye, where the drug is slowly released into the vitreous cavity over a period of 6 months [25-27]. In clinical trials of difficult cases of DME, the implant has demonstrated to be an effective alternative, alone or in combination with anti-VEGF drugs, by improving central macular thickness, best-corrected visual acuity (BCVA), microperimetry results, and electrofunctional test results after treatment [28-31].
Surgical Treatment for DME
Inflammatory cytokines, ROS, and AGEs play unique roles in DME. In patients with DR, the vitreous cavity serves as a physiological reservoir to all of the above-mentioned molecules. As the blood–retinal barrier becomes damaged with time, more pro-inflammatory cytokines and growth factors can gain access into this anatomical space. The accumulation of AGEs can destroy the vitreoretinal interface and induce neurovascular damage [5]. Moreover, accumulation of AGEs can also be found in the posterior vitreous cortex and ILM. AGE receptors have been found in the footplates of Müller cells and the external limiting membrane (ELM), suggesting that they might be responsible for the structural damage (thickening) observed in the ILM of patients with DME. Activation of AGEs may induce non-tractional cell-mediated effects on adjacent retinal layers (from ILM to ELM, including the deep retinal capillary plexus) [5]. Finally, the accumulation of AGEs in the posterior vitreous cortex can increase the adhesion of the posterior vitreous cortex to the ILM. The consequence is a thickened and taut posterior hyaloid cortex, which can be seen on slit lamp examination as a glittering taut membrane, and by FA and OCT imaging, as a firm attachment of the posterior hyaloid to the fovea with diffuse leakage in the macular area [5]. VEGF levels are substantially increased in the vitreous cavity of patients with DR. As retinal hypoxia increases, the concentration of VEGF also increases, inducing retinal neovascularization and capillary vasodilation [32-35].
Following PPV, vitreous oxygen content improves, and VEGF concentration decreases. This can potentially decrease vascular permeability and stimulate vasoconstriction with the concomitant reduction of the blood flow, hindering DME formation [36]. In addition, the peeling of the thickened ILM eliminates physical traction and removes AGEs from the vitreoretinal interface. In some cases, there is an associated epiretinal membrane and/or vitreoretinal traction, which can also be removed. In summary, there is strong evidence supporting the removal of the vitreous, the vitreous cortex, and the ILM in selected cases of DME.
The identification of patients with DME that can possibly benefit from surgery is of uttermost importance [37-39]. A good metabolic control is essential. Patients with low levels of glycosylated hemoglobin have a greater likelihood of improved BCVA after PPV [40]. The integrity of the ELM, the myoid, and ellipsoid zones (interdigitation zone) has been mentioned in several studies as a possible predictive factor of visual improvement [37, 41]. Patients with DME and signs of retinal gliosis are associated with increased expression of glial fibrillary acid protein (GFAP) in Müller cells and astrocytes. GFAP has an essential role in cell adhesion through the cytoskeleton, surface receptors, and extracellular matrix. Surgeons must be aware of large intraretinal cysts and enlarged foveal avascular zones because, as Romano et al. demonstrated, patients with those characteristics have a higher risk of subfoveal atrophy and visual deterioration [42]. The identification of the morphological pattern of DME by OCT, as described by Ichiyama et al., could also be a prognostic factor for visual improvement. Patients with sponge-like diffuse retinal thickening are the least likely to improve BCVA significantly after PPV [43].
Clinical Evidence Supporting PPV as a Treatment Option for DME
In 2002, Kumagai et al. published the results of PPV in the treatment of DME. The study reported a 90% reduction of DME, with or without ILM peeling. They discussed that ILM removal might accelerate the resolution of the edema in more severe cases, but no statistical difference in VA was observed [44]. In another study, Kumagai et al. treated patients with non-tractional DME with PPV, dividing the population into two groups: ILM peeling and no ILM peeling. Both groups achieved a statistically significant reduction in central macular thickness during the follow-up. However, the role of ILM peeling was still unclear, since it did not affect the results conclusively [45]. The results were similar to those of the study by Bonnin et al., in which patients with tractional and non-tractional DME were treated with PPV. Both groups had improved BCVA at the end of the follow-up period, but the authors failed to demonstrate any anatomical or functional differences between the two groups [46]. Nizawa et al. explored the effect of PPV in patients with diffuse DME, with or without previous treatments (bevacizumab, triamcinolone, or micropulse laser). BCVA was improved in both groups, and there was no significant difference between them [47]. A study from the DRCR.net reported that 68% of the participants achieved at least a 50% reduction in edema; 38% (95% confidence interval [CI]: 28%–49%) had improved BCVA (more than 10 letters) and 22% had deteriorated BCVA (less than 10 letters). Complications included vitreous hemorrhages, RD, and elevated intraocular pressure (IOP) at low rates [48].
As in any other scenario, PPV for DME patients is not without risk and complications. However, the incidence is similar to any other PPV surgical event. The most frequent intraoperative complication is retinal breaks (7.08%) and iatrogenic tears (0.68%). During the follow-up period, patients can experience cataract formation (2.56%), elevation of the IOP (5.19%), epiretinal membrane development (3.27%), vitreous hemorrhages (2.43%), and neovascular glaucoma (1.06%) [49].
CONCLUSIONS
In summary, PPV with ILM peeling can be considered a treatment option for chronic DME. The vitreous cavity in patients with DME is rich in cytokines and pro-inflammatory molecules such as VEGF; decreasing their levels could reduce their effects on retinal layers and increase oxygen levels in the vitreous. ILM peeling can not only eliminate physical traction, but can also remove the natural reservoir of AGEs, ROS, and inflammatory molecules. However, PPV is not a risk-free treatment, with retinal breaks and elevation of IOP being the most frequent adverse effects. In future, multicenter clinical trials are needed to understand the microscopic changes that occur in the ILM of patients with diabetes as well as the rate of anatomical and functional success. A prospective follow-up of those patients should provide further knowledge about DME recurrence.
DISCLOSURE
No funding or sponsorship was received for this study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
References
- 1.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497–503. doi: 10.1016/j.ophtha.2008.10.016. doi: 10.1016/j.ophtha.2008.10.016 pmid: 19167079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global Report on Diabetes: World Health Organization. 2016. [[cited 2017]]. Available from: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1.
- 3.Romero-Aroca P, de la Riva-Fernandez S, Valls-Mateu A, Sagarra-Alamo R, Moreno-Ribas A, Soler N, et al. Cost of diabetic retinopathy and macular oedema in a population, an eight year follow up. BMC Ophthalmol. 2016;16 doi: 10.1186/s12886-016-0318-x. doi: 10.1186/s12886-016-0318-x pmid: 27491545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke SJ, Karlstad MD, Eder AE, Regal KM, Lu D, Burk DH, et al. Pancreatic beta-Cell production of CXCR3 ligands precedes diabetes onset. Biofactors. 2016;42(6):703–15. doi: 10.1002/biof.1304. doi: 10.1002/biof.1304 pmid: 27325565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1–32. doi: 10.1016/j.survophthal.2008.10.001. doi: 10.1016/j.survoph thal.2008.10.001 pmid: 19171208. [DOI] [PubMed] [Google Scholar]
- 6.Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49(2):108–14. doi: 10.1159/000342977. doi: 10.1159/000342977 pmid: 23257933. [DOI] [PubMed] [Google Scholar]
- 7.Mishra S, Mishra BB. Study of Lipid Peroxidation, Nitric Oxide End Product, and Trace Element Status in Type 2 Diabetes Mellitus with and without Complications. Int J Appl Basic Med Res. 2017;7(2):88–93. doi: 10.4103/2229-516X.205813. doi: 10.4103/2229-516X.205813 pmid: 28584737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–64. doi: 10.2337/diacare.26.9.2653. pmid: 12941734. [DOI] [PubMed] [Google Scholar]
- 9.Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80(5):468–77. doi: 10.1034/j.1600-0420.2002.800503.x. pmid: 12390156. [DOI] [PubMed] [Google Scholar]
- 10.Paget C, Lecomte M, Ruggiero D, Wiernsperger N, Lagarde M. Modification of enzymatic antioxidants in retinal microvascular cells by glucose or advanced glycation end products. Free Radic Biol Med. 1998;25(1):121–9. doi: 10.1016/s0891-5849(98)00071-9. pmid: 9655530. [DOI] [PubMed] [Google Scholar]
- 11.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48(10):1899–906. doi: 10.2337/diabetes.48.10.1899. pmid: 10512352. [DOI] [PubMed] [Google Scholar]
- 12.Bazzoni G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost. 2006;95(1):36–42. pmid: 16543959. [PubMed] [Google Scholar]
- 13.Deissler HL, Deissler H, Lang GE. Inhibition of vascular endothelial growth factor (VEGF) is sufficient to completely restore barrier malfunction induced by growth factors in microvascular retinal endothelial cells. Br J Ophthalmol. 2011;95(8):1151–6. doi: 10.1136/bjo.2010.192229. doi: 10.1136/bjo.2010.192229 pmid: 21273213. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Salinas R, Garcia-Gutierrez MC, Garcia-Aguirre G, Morales-Canton V, Velez-Montoya R, Soberon-Ventura VR, et al. Evaluation of VEGF gene polymorphisms and proliferative diabetic retinopathy in Mexican population. Int J Ophthalmol. 2017;10(1):135–9. doi: 10.18240/ijo.2017.01.22. doi: 10.18240/ijo.2017.01.22 pmid: 28149790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawashima H, Mizukawa K, Kiryu J. Factors associated with visual recovery after sub-Tenon injection of triamcinolone acetonide in diabetic macular edema. Clin Ophthalmol. 2012;6:1307–14. doi: 10.2147/OPTH.S34631. doi: 10.2147/OPTH .S34631 pmid: 22927745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142(3):405–12. doi: 10.1016/j.ajo.2006.04.023. doi: 10.1016/j.ajo .2006.04.023 pmid: 16935584. [DOI] [PubMed] [Google Scholar]
- 17.Sambhav K, Grover S, Chalam KV. The application of optical coherence tomography angiography in retinal diseases. Surv Ophthalmol. 2017;62(6):838–66. doi: 10.1016/j.survophthal.2017.05.006. doi: 10.1016/j.survophthal.2017.05.006 pmid: 28579550. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH. Optical Coherence Tomography Angiography of Diabetic Retinopathy in Human Subjects. Ophthalmic Surg Lasers Imaging Retina. 2015;46(8):796–805. doi: 10.3928/23258160-20150909-03. doi: 10.3928/23258160-201509 09-03 pmid: 26431294. [DOI] [PubMed] [Google Scholar]
- 19.Diabetic Retinopathy Clinical Research N, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–7. doi: 10.1016/j.ophtha.2007.05.062. doi: 10.1016/ j.ophtha.2007.05.062 pmid: 17698196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–405. doi: 10.2337/dc10-0493. doi: 10.2337/dc10-0493 pmid: 20980427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031. doi: 10.1016/j. ophtha.2011.01.031 pmid: 21459215. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. doi: 10.1016/j.ophtha.2011.12.039 pmid: 22330964. [DOI] [PubMed] [Google Scholar]
- 23.Arevalo JF, Lasave AF, Wu L, Acon D, Farah ME, Gallego-Pinazo R, et al. Intravitreal bevacizumab for diabetic macular oedema: 5-year results of the Pan-American Collaborative Retina Study group. Br J Ophthalmol. 2016;100(12):1605–10. doi: 10.1136/bjophthalmol-2015-307950. doi: 10.1136/bjo phthalmol-2015-307950 pmid: 26912377. [DOI] [PubMed] [Google Scholar]
- 24.Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134–46 e3. doi: 10.1016/j.ophtha.2010.03.032. doi: 10.1016/j.ophtha.2010.03.032 pmid: 20417567. [DOI] [PubMed] [Google Scholar]
- 25.EMC. Ozurdex Summary of Product Characteristics. EMC. 2017 [cited 2017] [Google Scholar]
- 26.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28(5):351–66. doi: 10.1007/s12325-011-0019-z. doi: 10.1007/s12325-011-0019-z pmid: 21494891. [DOI] [PubMed] [Google Scholar]
- 27.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–6. doi: 10.1167/iovs.10-5285. doi: 10.1167/iovs.10-5285 pmid: 20702826. [DOI] [PubMed] [Google Scholar]
- 28.Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–51. doi: 10.1016/j.ophtha.2013.02.018. doi: 10.1016/ j.ophtha.2013.02.018 pmid: 23706947. [DOI] [PubMed] [Google Scholar]
- 29.Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–14. doi: 10.1016/j.ophtha.2014.04.024. doi: 10.1016/ j.ophtha.2014.04.024 pmid: 24907062. [DOI] [PubMed] [Google Scholar]
- 30.Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473–81. doi: 10.1016/j.ophtha.2014.07.002. doi: 10.1016/j.ophtha.2014.07.002 pmid: 25155371. [DOI] [PubMed] [Google Scholar]
- 31.Mastropasqua R, Toto L, Borrelli E, Di Antonio L, De Nicola C, Mastrocola A, et al. Morphology and Function over a One-Year Follow Up Period after Intravitreal Dexamethasone Implant (Ozurdex) in Patients with Diabetic Macular Edema. PLoS One. 2015;10(12):e0145663. doi: 10.1371/journal.pone.0145663. doi: 10.1371/journal.pone.01 45663 pmid: 26720268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. doi: 10.1056/NEJM199412013312203. doi: 10.1056/NEJM19941201 3312203 pmid: 7526212. [DOI] [PubMed] [Google Scholar]
- 33.Augustin AJ, Keller A, Koch F, Jurklies B, Dick B. [Effect of retinal coagulation status on oxidative metabolite and VEGF in 208 patients with proliferative diabetic retinopathy] Klin Monbl Augenheilkd. 2001;218(2):89–94. doi: 10.1055/s-2001-12251. doi: 10.1055/s-2001-12251 pmid: 11258131. [DOI] [PubMed] [Google Scholar]
- 34.Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51(4):364–80. doi: 10.1016/j.survophthal.2006.04.005. doi: 10.1016/j.survophthal.2006.0 4.005 pmid: 16818083. [DOI] [PubMed] [Google Scholar]
- 35.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–10. doi: 10.1016/j.ajo.2004.09.046. doi: 10.1016/j.ajo.2004.09.046 pmid: 15733992. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, Yokoyama T, Ebihara N, Murakami A. Histopathologic analysis of the internal limiting membrane surgically peeled from eyes with diffuse diabetic macular edema. Jpn J Ophthalmol. 2012;56(3):280–7. doi: 10.1007/s10384-012-0130-y. doi: 10.1007/s10384-012-0130-y pmid: 22438196. [DOI] [PubMed] [Google Scholar]
- 37.Yanyali A, Bozkurt KT, Macin A, Horozoglu F, Nohutcu AF. Quantitative assessment of photoreceptor layer in eyes with resolved edema after pars plana vitrectomy with internal limiting membrane removal for diabetic macular edema. Ophthalmologica. 2011;226(2):57–63. doi: 10.1159/000327597. doi: 10.1159/000327597 pmid: 21555906. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1325–30. doi: 10.1007/s00417-009-1107-5. doi: 10.1007/s00417-009-1107-5 pmid: 19430805. [DOI] [PubMed] [Google Scholar]
- 39.Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30(5):774–80. doi: 10.1097/IAE.0b013e3181c2e0d6. doi: 10.1097/IAE.0b013e3181c2e0d6 pmid: 19996821. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Suzuma K, Ryu M, Tsuiki E, Fujikawa A, Kitaoka T. Systemic factors influence the prognosis of diabetic macular edema after pars plana vitrectomy with internal limiting membrane peeling. Curr Eye Res. 2013;38(12):1261–5. doi: 10.3109/02713683.2013.820327. doi: 10.3109/02713683.2013.820327 pmid: 23972000. [DOI] [PubMed] [Google Scholar]
- 41.Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1415–20. doi: 10.1007/s00417-012-1968-x. doi: 10.1007/s00417-012-1968-x pmid: 22354371. [DOI] [PubMed] [Google Scholar]
- 42.Romano MR, Romano V, Vallejo-Garcia JL, Vinciguerra R, Romano M, Cereda M, et al. Macular hypotrophy after internal limiting membrane removal for diabetic macular edema. Retina. 2014;34(6):1182–9. doi: 10.1097/IAE.0000000000000076. doi: 10.1097/IAE.0000000000000076 pmid: 24846134. [DOI] [PubMed] [Google Scholar]
- 43.Ichiyama Y, Sawada O, Mori T, Fujikawa M, Kawamura H, Ohji M. The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1545–51. doi: 10.1007/s00417-015-3251-4. doi: 10.1007/s00417-015-3251-4 pmid: 26780461. [DOI] [PubMed] [Google Scholar]
- 44.Kumagai K, Ogino N, Furukawa M, Demizu S, Atsumi K, Kurihara H, et al. [Internal limiting membrane peeling in vitreous surgery for diabetic macular edema] Nippon Ganka Gakkai Zasshi. 2002;106(9):590–4. pmid: 12385125. [PubMed] [Google Scholar]
- 45.Kumagai K, Hangai M, Ogino N, Larson E. Effect of Internal Limiting Membrane Peeling on Long-Term Visual Outcomes for Diabetic Macular Edema. Retina. 2015;35(7):1422–8. doi: 10.1097/IAE.0000000000000497. doi: 10.1097/IAE.000000000000 0497 pmid: 26102439. [DOI] [PubMed] [Google Scholar]
- 46.Bonnin S, Sandali O, Bonnel S, Monin C, El Sanharawi M. VITRECTOMY WITH INTERNAL LIMITING MEMBRANE PEELING FOR TRACTIONAL AND NONTRACTIONAL DIABETIC MACULAR EDEMA: Long-term Results of a Comparative Study. Retina. 2015;35(5):921–8. doi: 10.1097/IAE.0000000000000433. doi: 10.1097/IAE.0000000000000 433 pmid: 25545486. [DOI] [PubMed] [Google Scholar]
- 47.Nizawa T, Arai M, Takatsuna Y, Oshitari T, Sato E, Yamamoto S. [Comparison of visual acuity and central macular thickness after vitrectomy for diffuse diabetic macular edema with or without preoperative treatments] Nippon Ganka Gakkai Zasshi. 2013;117(10):785–92. pmid: 24354262. [PubMed] [Google Scholar]
- 48.Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, et al. Diabetic Retinopathy Clinical Research Network Writing C. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117(6):1087–93 e3. doi: 10.1016/j.ophtha.2009.10.040. doi: 10.1016/j.ophtha.2009.10.040 pmid: 20299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. PARS PLANA VITRECTOMY FOR DIABETIC MACULAR EDEMA: A Systematic Review, Meta-Analysis, and Synthesis of Safety Literature. Retina. 2017;37(5):886–95. doi: 10.1097/IAE.0000000000001280. doi: 10.1097/IAE.0000000000 001280 pmid: 27632713. [DOI] [PubMed] [Google Scholar]