Abstract
Acute stress responsiveness is a quantitative trait that varies in severity from one individual to another; however, the genetic component underlying the individual variation is largely unknown. Fischer 344 (F344) and Wistar Kyoto (WKY) rat strains show large differences in behavioral responsiveness to acute stress, such as freezing behavior in response to footshock during the conditioning phase of contextual fear conditioning (CFC). Quantitative trait loci (QTL) have been identified for behavioral responsiveness to acute stress in the defensive burying (DB) and open field test (OFT) from a reciprocal F2 cross of F344 and WKY rat strains. These included a significant QTL on chromosome 6 (Stresp10). Here, we hypothesized that the Stresp10 region harbors genes with sequence variation(s) that contribute to differences in multiple behavioral response phenotypes between the F344 and WKY rat strains. To test this hypothesis, first we identified differentially expressed genes within the Stresp10 QTL in the hippocampus, amygdala, and frontal cortex of F344 and WKY male rats using genome-wide microarray analyses. Genes with both expression differences and non-synonymous sequence variations in their coding regions were considered candidate quantitative trait genes (QTGs). As a proof-of-concept, the F344.WKY-Stresp10 congenic strain was generated with the Stresp10 WKY donor region into the F344 recipient strain. This congenic strain showed behavioral phenotypes similar to those of WKYs. Expression patterns of Gpatch11 (G-patch domain containing 11), Cdkl4 (Cyclin dependent kinase like 4), and Drc1 (Dynein regulatory complex subunit 1) paralleled that of WKY in the F344.WKY-Stresp10 strain matching the behavioral profiles of WKY as opposed to F344 parental strains. We propose that these genes are candidate QTGs for behavioral responsiveness to acute stress.
Introduction
Acute stress is a common occurrence in everybody’s life with differing severity. Individual variation in the response to acute stress depends on the sensitivity to the stressor and the ability to cope with it. Genetic studies support the assumption that acute stress responsiveness is a quantitative trait [1–3]. Its narrow-sense heritability ranges between 0.4–0.6 defined by multivariate genetic analyses in family and twin studies [4–7]. The heritability of acute stress responsiveness is estimated to be similar in rodents to that in humans [8, 9]. However, only a limited number of candidate genes have been proposed for individual variation in behavioral acute stress responsiveness to date [10–14]. The purpose of this study was to identify candidate genes with sequence variations that contribute to variations in individual stress vulnerability.
We have studied the genetic components of behavioral responsiveness to acute stress in the reciprocally crossed F2 generation of Wistar Kyoto (WKY) and Fischer 344 (F344) rat strains using the defensive burying (DB) and open field tests (OFT) [15–19]. The parental WKY strain consistently exhibits hypoactive and avoidant behavior compared to that of the F344 [19]. We argue that this consistent repertoire of behavior across various tests is not composed of discrete, individual reactions to each situation, but belies a more global response to an acute challenge with substantial genetic origin. Among the quantitative trait loci (QTL) found in these studies, a highly significant locus on chromosome 6, Stresp10, has been identified for multiple phenotypes; a potential pleiotropy for behavioral stress responsiveness (Table 1) [16]. The phenotypes associated with this QTL include latency to bury, grooming, rearing in DB, and rearing in OFT. It is notable that a discrepancy was described for the OFT and DB rearing QTLs using genetic mapping from the time of the original publication [19]. However, physical mapping using the more recent rat genome version Rnor_6.0 identified that these QTLs indeed overlap. Furthermore, rearing measures highly correlate between the two tests [19]. All of these behaviors represent a general stress response to an acute stressor, whether it be the shock in the DB or the novelty of OFT. Thus, this chromosomal region is likely to harbor one or more genes with sequence variation(s) that contribute to phenotypic variations in general stress responsiveness to acute stressors between these strains.
Table 1. List of behavioral phenotypes associated with the Stresp10 QTL.
| QTL Symbol | Position | Behavior | LOD | Pointwise significance | Variance (%) | Reference |
|---|---|---|---|---|---|---|
| Stresp10 | 6:1–35,623,029 | Latency (DB) | 3.55 | 0.00002 | 3.5 | [15] |
| Rearing (DB)*^ | 3.72 | 0.00019 | 1.6 | [19] | ||
| Grooming (DB)* | 3.1 | 0.00079 | [19] | |||
| Rearing (OFT) | 8.24 | 2.82E-10 | 8.9 | [19] |
* suggestive, trait x lineage covariates
^ Rearing (DB) QTL was originally identified at 62cM
From the Rat Genome Database version Rnor_6.0
In this study, we aimed to identify candidate quantitative trait genes (QTG) that contribute to differences in acute stress responsiveness between F344 and WKY rat strains in the Stresp10 QTL. To aid in the identification of QTG(s), first we established the differentially expressed genes (DEGs) between the two parental strains within the Stresp10 QTL using genome-wide microarray analyses in the hippocampus, amygdala, and frontal cortex. Using the Rat Genome Database (www.rgd.mcw.edu) and the original sequences of the two parental strains [12, 14], we then determined nonsynonymous sequence variations in the coding regions of DEGs between the F344 and WKY strains. We hypothesized that DEGs that show expression differences parallel to behavioral differences between the two strains are candidate quantitative trait genes. To investigate the generalizability of the findings to a different acute stress phenotype, we measured the behavioral response differences in the conditioning part of the contextual fear conditioning (CFC) paradigm between the two strains since the acute stress of the electric shock in the CFC is similar to that shock in the DB test. For proof-of-concept, we measured the transcript levels of these candidate QTGs in the brain regions of a congenic strain generated by inserting the Stresp10 WKY donor region into the F344 recipient strain. DEGs with expression profiles in the F344.WKY-Stresp10 congenic strain similar to the WKY’s, but different from the F344, suggest that the sequence variations within these genes might contribute to the Stresp10 QTL.
Materials and methods
Animal care and treatment
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee of Northwestern University. Adult male Fischer 344 (F344) and Wistar Kyoto (WKY) rats (approximately 3 months old) were obtained from Envigo (Indianapolis, IN) and Charles River Laboratories (Wilmington, MA), respectively. All animals were group-housed (2–3 per cage) in a temperature-controlled environment with 12 h light/dark cycles and allowed feed and water ad–libitum.
The experimental design required three animal cohorts. Specifically, one cohort of control F344 and WKY rats were not subjected to any behavioral testing. RNA was isolated from the three brain regions and used for the microarray experiments. The second cohort consisted of F344 and WKY rats that were subjected to CFC testing and their RNA was isolated from the three brain regions. This RNA was used for expression analyses by RT-PCR of candidate genes together with RNA isolated from the F344.WKY-Stresp10 rats. The third cohort of F344, WKY, and F344.WKY-Stresp10 rats were maintained independently and used for only behavioral testing in the OFT followed by DB three weeks later.
Construction of congenic F344.WKY-Stresp10
The F344.WKY-Stresp10 congenic strain was generated by repeated marker-directed backcrossing of the F344-WKY F1 generation into the F344 parental strain. The markers used are described before [16, 19]. The N15 generation of F344.WKY-Stresp10 congenic male animals (approximately 3 months old) were used for behavioral experimentation together with the simultaneously maintained F344 and WKY rats. The 15th generation of the F344.WKY-Stresp10 strain was homozygous from 135,053 to 6,709,713 bp and from 19,464,437 to the end of the QTL at 28,931,796 bp, and heterozygous from 6,963,239 to 15,597,330 bp (S1 Table). Additionally, after 15 generations of backcross, congenic strains are known to have less then 5% heterozygosity throughout the genome [20]. This strain was characterized using the 10K Affymetrix Targeted Genotyping Array (Affymetrix, Santa Clara, CA) [21]. Using this array, 4 μg of rat DNA was genotyped with the GeneChip Scanner 2000 Targeted Genotyping System (GTGS) following the manufacturer’s protocol (Affymetrix, Santa Clara, CA) [22]. The genotyping data was analyzed using the corresponding software for GTGS (Affymetrix, Santa Clara, CA). The 10K Affymetrix Targeted Genotyping Array used the rat genome version RGSC_3.4. The QTL locations were translated to the latest version or the rat genome, Rnor_6.0. S1 Table lists the QTL locations from both versions of the rat genome.
Behavioral tests
Contextual fear conditioning
The fear conditioning phase of the contextual fear conditioning test was carried out as described previously [23]. Briefly, male rats of the F344 and WKY strains were individually placed in the CFC chamber (Technical & Scientific Equipment, Bad Homburg, Germany) and exposed to 3 minutes of habituation followed by 3 footshocks (0.8 mA, 1 sec duration, spaced 1 minute apart). The behavior observed after the footshocks was analyzed by the TSE Videomot software version 5.75 (Bad Homburg, Germany), which measures the animals’ movement, including distance travelled, number of times rearing, and freezing duration. The rats were sacrificed by quick decapitation 24 hours later. Whole brains were collected in RNAlater (Ambion, Austin, TX) and frozen at -80°C until dissection of brain regions. Total RNA was isolated from the hippocampus, amygdala, and frontal cortex, and was used for the expression analyses of candidate genes. All rats were approximately 3 months old at the time of sacrifice and tissue sample collection.
Open field test
The open field test was carried out on another cohort of animals, as previously described [23]. Briefly, male rats of the F344, WKY, and F344.WKY-Stresp10 strains were placed in a circular arena (82 cm in diameter) with a 30 cm high wall, lit to a brightness of approximately 60 lux by indirect overhead lighting. The arena contained an inner concentric circle (50 cm in diameter) designated as the inner zone. The rats were placed in the center of the arena and allowed to move freely for 10 min while the activity was recorded and tracked by the TSE Videomot software 2 version 5.75 (Bad Homburg, Germany), which measured the number of times rearing by the animal.
Defensive burying
Three weeks after the OFT, the defensive burying test was carried out on the same animals as previously described in the QTL studies [15, 16, 19]. Briefly, male rats of the three strains were habituated to a Plexiglas chamber (40 cm square, 60 cm high) with bedding (wood shaving) (7 cm deep, 1 cm below the hole for the prod) for 15 min each day, for three consecutive days, between 10:00 AM and 2:00 PM. On the fourth day, a continuously electrified prod was introduced into the chamber, which delivered a 4.5 mA shock when the rat touched it. The shock was generated from a shock generator (Lafayette Instruments, San Diego, CA) set at 4.5 mA. The rats typically explored the novel prod and received a shock, which started the 15-minute video-taped test. Once shocked, animals typically retreated to the back of the cage, and either remained there (classic WKY behavior) or began spraying bedding toward the prod in an effort to cover it. Behaviors scored by an observer blind to the genotype of the animal include the latency to begin burying, the total time spent burying (duration of burying), the duration of grooming, and the number of times rearing by the animal. The rats were sacrificed by quick decapitation two weeks after the test. Whole brains were collected in RNAlater (Ambion, Austin, TX) and frozen at -80°C until dissection. All rats were approximately 3 months old at the time of sacrifice and tissue sample collection.
Brain dissection and RNA isolation
Brain regions (hippocampus, amygdala, and frontal cortex) were dissected from adult male F344, WKY and F344.WKY-Stresp10 congenic rats as described previously [24] and stored in RNAlater (Ambion, Austin, TX) at -80°C. Briefly, a rat brain matrix was used to prepare primary sections from which the brain regions were dissected according the Paxinos coordinates [25]: hippocampus (AP−2.12 to −6.0, ML 0 to 5.0, DV 5.4 to 7.6), amygdala (AP −0.58 to −2.18, ML 1.5 to 4.5, DV 4 to 5.75), and frontal cortex (AP 5.20 to 1.70, ML 0 to 3.3, DV 9.0 to 4.4).
Total RNA was isolated from individual tissue samples using the Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA). Briefly, individual tissue samples were homogenized in TRIzol reagent (Ambion, Austin, TX) and RNA was immediately isolated using the kit following the manufacturer’s protocol. Total RNA (2 μg) was reverse transcribed to generate double-stranded cDNA using the SuperScript VILO cDNA Synthesis Kit (ThermoFisher Scientific, Waltham, MA).
Microarray analysis
Genome-wide microarray analysis was performed from RNA isolated from hippocampi, amygdalae, and frontal cortexes of F344 and WKY adult male rats that were unstressed, without undergoing any behavioral testing, as described previously [26]. Briefly, the cDNA generated from the RNA was linearly amplified and labeled with biotinylated nucleotides in an in vitro transcription reaction using the Illumina TotalPrep RNA Amplification Kit (San Diego, CA) to make cRNA. 1.5 μg of biotin-labelled and fragmented cRNA was then hybridized onto RatRef-12 Expression BeadChips (Illumina, San Diego, CA). The BeadChips have multiple probes per transcript to mitigate hybridization bias artifacts. Probe intensity data from the BeadChips were directly read into the R software environment (http://www.R-project.org) from bead summary files produced by BeadStudio using the R/beadarray package [26, 27]. Quantile normalization was applied to the data using the R/preprocessCore package [26, 28]. Data quality was assessed using histograms of signal intensities, scatter plots, and hierarchical clustering of samples, as previously described [26]. Statistical significance of microarray expression differences between F344 and WKY was determined using ANOVA methods within the R/maanova package as previously described [26, 29]. DEGs were determined between strains with an FDR-adjusted P–value less than 0.05 and a fold change greater than 1.3 (30% increase or decrease). This criterion has been well-established to give biologically meaningful datasets when interpreting differential gene expression profiles in microarray experiments [30, 31]. The microarray data used the rat genome RGSC_v3.4 for identification of transcripts, which was translated to the latest version of rat genome, Rnor_6.0.
The differential gene expression profiles between the two strains were determined with a significance criteria of an FDR-adjusted P value less than 0.05 and an absolute fold change above 1.3 (30% increase or decrease). With these criteria, we found 1,030 DEGs in the hippocampus, 769 in the amygdala, and 976 in the frontal cortex, as listed in S3–S5 Tables.
Identification of single nucleotide polymorphisms in coding regions
The genes with non-synonymous single nucleotide polymorphisms (SNPs) within coding regions between the F344 and WKY genomes were obtained from the Rat Genome Sequencing and Mapping Consortium and Baud et al. [12, 14]. In these studies, both F344 and WKY genomes were first mapped to the Brown Norway reference genome, version RGSC_3.4. Using the Integrative Genomics Viewer (Broad Institute, Cambridge, Massachusetts), we identified the coding sequence variations between the F344 and WKY genomes and translated them to the rat genome version Rnor_6.0. For quality control, we set the criteria that the coverage for each SNPs had to be greater than 10 reads, where reads are used to reconstruct the sequence. The more reads a sequence had, the more reliable the data. Furthermore, the single nucleotide variations had to be called in at least 50% of the reads.
Among inherited gene variations in humans, nonsynonymous single nucleotide polymorphisms that lead to an amino acid change in the protein product are most relevant to human inherited diseases [32]. Therefore, as the first step in the identification of candidate QTGs, we focused on this subset of genes. Genes with non-synonymous SNPs within the heterozygous Stresp10 region were excluded from our analysis. Of the 16 DEGs mapped within the Stresp10 region, 10 genes were found to contain SNPs (Table 2). SNPs are listed in S9 Table.
Table 2. Differentially expressed genes within the Stresp10 QTL region containing coding region non-synonymous SNPs between F344 and WKY.
| Gene Symbol | Gene Description | Chr | Start | End |
P-value 0 = < 1.00E-08 |
|---|---|---|---|---|---|
| Gpatch11h,a,f | G-patch domain containing 11 | 6 | 1410507 | 1423041 | h: 0, a: 0, f: 0 |
| Prkd3f | Protein kinase D3 | 6 | 1546018 | 1622232 | f: 1.18E-07 |
| Cyp1b1h,f | Cytochrome P450, family 1, subfamily b, polypeptide 1 | 6 | 2307808 | 2316722 | h: 0, f: 0 |
| Cdkl4h | Cyclin-dependent kinase-like 4 | 6 | 3234090 | 3254779 | h: 7.39E-05 |
| Mta3h,f | Metastasis associated 1 family, member 3 | 6 | 6908684 | 7031828 | h: 0, f: 0 |
| Ttc27a,f | Tetratricopeptide repeat domain 27 | 6 | 21735834 | 21880003 | a: 1.42E-07, f: 0 |
| Alkh,a,f | Anaplastic lymphoma receptor | 6 | 22696397 | 23203775 | h: 0, a: 0, f: 0 |
| Rbksh | Ribokinase | 6 | 26051396 | 26128906 | h: 0 |
| Drc1a | Dynein regulatory complex subunit 1 | 6 | 27425235 | 27460038 | a: 4.74E-08 |
| Dtnbh,a,f | Dystrobrevin, beta | 6 | 27975417 | 28177214 | h: 5.09E-07, a: 3.79E-05, f: 1.56E-05 |
h: hippocampus, a: amygdala, f: frontal cortex
From the Rat Genome Database version Rnor_6.0
Real-time RT-PCR
The real-time reverse transcription-polymerase chain reaction (qRT-PCR) was carried out using RNA from the second cohort of F344 and WKY rats that were subjected to CFC testing, as well as RNA from the F344.WKY-Stresp10 rats. Primers for each gene were designed using Primer Express Software version 3.0 (Applied Biosystems, Carlsbad, CA). The default setting was used to design primers that amplify 80 to 150 bp regions. The primer sequences for the candidate quantitative trait genes are listed in S2 Table. Five ng of cDNA were amplified in 20 μL reactions (1X SYBR Green Master Mix (ThermoFisher Scientific), 250 μM primers) in QuantStudio 7 Flex Real-Time PCR System (ThermoFisher Scientific, Waltham, MA) using the relative quantification (-ΔΔCt) method, with Gapdh as the housekeeping gene and a general calibrator. We have established that there was no change in hippocampal Gapdh expression across strains and conditions.
Statistical analysis
All data were presented as mean ± standard error of mean. Outliers from the quantitative RT-PCR data were determined as being more than two standard deviation away from the mean. Therefore, the number of samples per group differ between target genes. All statistical analyses were performed using GraphPad Prism v 7.0 (GraphPad Software, La Jolla, CA). Statistical significance of differences between strains were determined by ANOVA, followed by post-hoc analysis with the Bonferroni’s correction for multiple comparisons. Statistical significance was considered at an adjusted P-value of less than 0.05. When significant main effects were indicated by the ANOVA, but the Bonferroni’s multiple comparisons test did not show significance, hypothesis testing by Student’s t-test was carried out between groups. Our decision to apply the Student’s t-test was based on an increasing number of discussions arguing that P-values are not as reliable as it is previously thought [33] and that while a three-group comparison ANOVA may not result in significance, two groups of the three can differ from each other at the P < 0.05 level [34]. ANOVA results are given in the results and post-hoc significances are noted in the figures.
Results
Microarray analysis
Genome-wide microarrays were performed on hippocampal, amygdalar, and frontal cortex RNA from unstressed F344 and WKY male rats. To identify potential QTGs, DEGs were mapped within the Stresp10 QTL chromosomal region. This region is associated with multiple behavioral phenotypes in response to acute stress (Table 1). The chromosomal location of these QTGs was mapped from RGSC_v3.4 to the latest version of rat genome Rnor_6.0. Of the DEGs, 14 genes in the hippocampus, 12 in the amygdala, and 14 in the frontal cortex were found within the Stresp10 region (S6–S8 Tables). Since many of these genes overlap in two or more brain regions, there were a total of 18 genes to investigate. Precisely, there were six genes (Gpatch11, Slc3a1, Camkmt, Alk, Dtnb, Klhl29) that overlapped in all three brain regions: Rasgrp3 between the hippocampus and amygdala, Cyp1b1 and Mta3 between the hippocampus and frontal cortex, and Ttc27 between the amygdala and frontal cortex.
Single nucleotide polymorphisms in coding regions of DEGs mapped within Stresp10
To determine which genes contribute to the phenotypic variation in acute stress responsiveness, we identified the genes with non-synonymous single nucleotide polymorphisms (SNPs) within coding regions between the F344 and WKY genomes, which were obtained from Baud et al. [12, 14]. The SNPs were identified first between the WKY, F344 and the Brown Norway reference genome and then between the WKY and F344 genome using Rnor_6.0. The candidate SNPs had to be greater than 10 number of reads and the single nucleotide variations in greater than 50% of the reads. Of the 16 DEGs mapped within the homozygous Stresp10 region, 10 genes were found to contain non-synonymous SNPs in coding regions (Table 2). These SNPs are listed in S9 Table.
Acute stress responsiveness in F344 and WKY rat strains
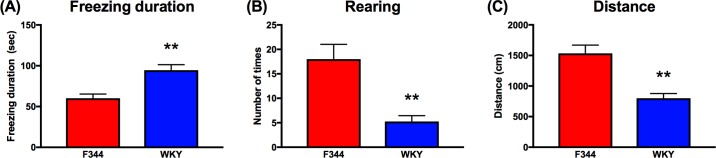
To investigate the generalizability of the findings to a different acute stress phenotype, we measured the acute stress response differences between the two strains in the conditioning part (day 1) of the CFC (Fig 1). Similar to behaviors in the DB and OFT [15, 16, 19], WKY rats exhibited significantly more freezing in response to the stress of the footshock (t(30) = 4.066; P < 0.01), which is a clear indication of a more passive stress response (Fig 1A). Furthermore, WKYs also exhibited a significantly lower frequency of rearing (t(29) = 4.00; P < 0.01), which is an avoidance response [19, 35]. WKYs also exhibited a more hypoactive response to the footshocks, measured by significantly shorter distance travelled in the chamber compared to the F344 rats (t(30) = 1.813; P < 0.01) (Fig 1B and 1C).
Fig 1. Behavioral responses to footshock of F344 and WKY adult male rats.
(A) WKY adult male rats froze longer (freezing duration in seconds) following the footshocks in the CFC conditioning chamber. (B) Number of rears are significantly lower for WKYs compared to F344s. (C) F344s move around the chamber after the footshock significantly more than WKYs measured by distance traveled in cm. Data are presented as mean ± standard error of mean. N = 16 per group. ** P < 0.01 by Student’s t-test.
Confirmation of Stresp10 phenotypes in F344 and WKY parental strains, and in the F344.WKY-Stresp10 congenic strain
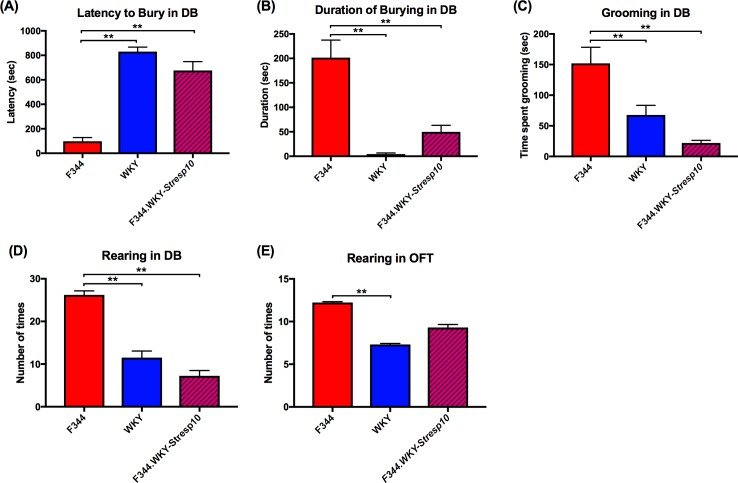
The phenotypes associated with Stesp10 include latency to bury, grooming, and rearing in the DB test, and rearing in the OFT; all of which represent an acute stress response. To confirm that the Stresp10 QTL contributes to the variation in these behavioral acute stress responses, a congenic strain, F344.WKY-Stresp10, was generated (S1 Table). Fig 2 shows the phenotypic differences between the F344, WKY, and F344.WKY-Stresp10 strains. In the DB test, both the WKY and F344.WKY-Stresp10 rats exhibited enhanced avoidance and hypoactive responses to acute stress, measured by significantly longer latency to bury (F[3,46] = 44.55; P < 0.01) and shorter duration of burying the electrified prod (F[2,43] = 23.96; P < 0.01) compared to the F344s (Fig 2A and 2B). Furthermore, WKY and F344.WKY-Stresp10 rats were less active showing less grooming (strain: F[2,43] = 11.80, P < 0.01) and rearing (strain: F[2,25] = 34.04, P < 0.01) compared to the F344 rats (Fig 2C and 2D). In the OFT, WKYs reared significantly less (F[2,87] = 14.79, P < 0.01) and the number of rearing for the F344.WKY-Stresp10 were intermediary not differing from either parental strain significantly (Fig 2E).
Fig 2. Phenotypic differences between the F344, WKY, and F344.WKY-Stresp10 strains in the defensive burying and open field tests.
(A, B) Both WKY and F344.WKY-Stresp10 adult male rats exhibited significantly longer latency to bury and shorter duration of burying the electrified prod compared to the F344s. (C, D) Both WKY and F344.WKY-Stresp10 rats groomed less (time spent grooming in seconds) and reared less compared to the F344s in the DB paradigm. (E) WKYs also rear significantly less than F344s in the OFT, while the number of rearing of F344.WKY-Stresp10 did not differ significantly from either parental strain. Data are presented as mean ± standard error of mean. N = 13–18 per group. ** P < 0.01 by two-way ANOVA followed by Bonferroni’s multiple comparisons test.
Quantitative expression of DEGs with sequence variations in the brain regions of F344, WKY, and F344-WKY.Stresp10 adult male rats
Quantitative expression analyses of the 10 genes (Table 2) were carried out using RNA from the hippocampus, amygdala, and frontal cortex of F344, WKY, and congenic strain F344.WKY-Stresp10 male rats.
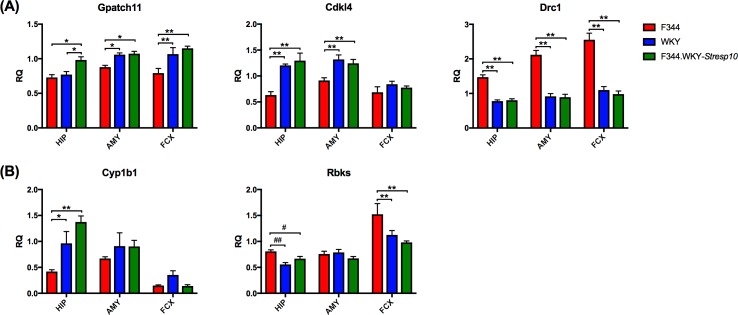
Genes with similar expression between the WKY and F344.WKY-Stresp10 strains, but different from the F344, in two or more brain regions were considered to be strong candidate QTGs that likely contribute to the variation in acute stress responsiveness between the parental strains. These candidate QTGs include Gpatch11 (G-patch domain containing 11), Cdkl4 (Cyclin dependent kinase like 1), and Drc1 (Dynein regulatory complex subunit 1) (Fig 3A). Expression of Gpatch11 differed significantly by brain region and strain (brain region: F[2,51] = 12.75, P < 0.01; strain: F[2,51] = 21.82, P < 0.01). Expression of Drc differed significantly by brain region and strain as well the interaction between the two (brain region: F[2,43] = 15.39, P < 0.01; strain: F[2,43] = 96.56, P < 0.01; interaction: F[4,43] = 3.844, P < 0.01). Both Gpatch11 and Drc1 showed significant differences in expression between the F344s and WKYs and F344s and F344.WKY-Stresp10 rats in all three brain regions. Expression of Cdlk4 differed significantly by brain region and strain as well as the interaction between the two (brain region: F[2,44] = 22.85, P < 0.01; strain: F[2,44] = 21.82, P < 0.01, interaction: F[4,44] = 3.34, P < 0.05). Cdkl4 also showed the same pattern of expression in the hippocampus and amygdala, while transcript levels in the frontal cortex were relatively low.
Fig 3. Expression analyses of candidate quantitative trait genes in the hippocampus, amygdala, and frontal cortex of F344, WKY, and F344.WKY-Stresp10 adult male rats.
(A) Candidate genes with similar expression between the WKY and F344.WKY-Stresp10 strains, but different from the F344, in two or more brain regions. (B) Genes that express a similar pattern in only one brain region. Data are presented as mean ± standard error of mean. N = 5–7 per group. * P < 0.05 and ** P < 0.01 by two-way ANOVA followed by Bonferroni’s multiple comparisons test. # P < 0.05 and ## P < 0.01 by Student’s t-test.
Additionally, genes that express the same pattern but only in one brain region were considered. Sequence variations in these genes can interfere with gene expression by interacting with brain region-specific modulators, such as miRNAs, transcription factors, and binding proteins, present or absent in the specific brain region, which may or may not induce transcription [36, 37]. Only two genes, Cyp1b1 (Cytochrome P450 family 1 subfamily B member 1) and Rbks (ribokinase), showed this pattern of expression. (Fig 3B). Brain region- and strain-specific expression of Cyp1b1 showed a complex pattern (brain region: F[2,45] = 26.01, P < 0.01; strain: F[2,45] = 7.465, P < 0.01; interaction: F[4,45] = 3.567, P < 0.05). Cyp1b1 expression was significantly greater in the WKY and the F344.WKY-Stresp10 strains compared to F344, but only in the hippocampus. Similar to the expression of Cyp1b1, Rbks transcript levels were regulated in a brain region- and strain-specific manner (brain region: F[2,50] = 48.67, P < 0.01; strain: F[2,50] = 10.43, P < 0.01; interaction: F[4,50] = 4.041, P < 0.01). Rbks was expressed at significantly lower levels in the WKY and F344.WKY-Stresp10 rats relative to the F344 rats in the frontal cortex; the hippocampal expression profile was similar but F344 and the F344.WKY-Stresp10 showed significance only as hypothesis testing (t(11) = 2.57, P < 0.01). There were no significant differences in the expression of this gene in the amygdala. Prkd3, Mta3, Ttc27, Alk, and Dtnb showed no strain-specific effects.
Discussion
The major findings of this study point to candidate QTGs that can contribute to differences in behavioral responsiveness to acute stress between the F344 and WKY strains across multiple paradigms and phenotypes. Brain region-specific gene expression differences between the F344 and WKY strains and a congenic strain, incorporating a WKY donor chromosomal regions mapped to multiple stress responsive QTLs into the F344 recipient background, identified candidate genes within this locus. Those genes that showed sequence variations between the two parental strains are proposed to be candidate QTGs. These candidate QTGs were identified using a multifaceted approach that, to our knowledge, has not been used previously. Specifically, this approach included genome-wide microarray analyses to identify DEGs within the QTL in question, followed by non-synonymous SNPs within these DEGs, the generation of the congenic strain for the behavioral consequences associated with the specific QTL, and finally the expression analysis involving all three strains with a different analytical method, the qPCR.
Human studies have identified few genetic variations associated with individual differences in behavioral responses to acute stress (for example, [38, 39]). All of these candidate genetic variations were associated with responses to fearful faces measured by multiple imaging and other methods. In contrast, animal studies have identified multiple QTL for behavioral responsiveness to acute stress, but very few QTGs have been proposed [11–14, 40, 41]. The usual methods to detect QTL, such as backcrosses, F2 crosses, and consomic strains [42–44], usually identify large genomic regions, with large number of genes mapped to them. Other techniques such as recombinant inbred strains, congenic strains, and heterogeneous stocks [10, 45] are able to identify smaller genomic regions, but still have no confirmed QTGs for acute stress responsiveness.
In our previous QTL studies, using the recombinant F2 generation of the reciprocally crossed F344 and WKY, we identified multiple QTL for behavioral responsiveness to acute stress in different paradigms. Specifically, the chromosomal region of the Stresp10 QTL was associated with latency to bury, grooming, and rearing in the DB test, and rearing in the OFT from this cross. All of these phenotypes describe a behavioral response of the animal either to a novel environment or to an aversive shock stimulus, which are characteristically either active or passive. In the DB test, these options lead to the active behaviors of avoiding the shock by increasing the duration of burying and of rearing, or the passive response to the shock manifested by freezing and thereby increased latency to bury [46, 47]. The acute stress in the OFT is the novel environment from which the animal is trying to escape by rearing; an active response to this stress. These overlapping QTL within the Stresp10 locus may represent pleiotropy, or multiple sequence variations interacting, resulting in a common genetic architecture underlying different behavioral responses to acute stress.
In the fear conditioning component of the CFC test, the animal can either freeze (parallel to latency to bury in the DB), explore (parallel to burying), or rear, after it receives the footshock. Exploration is an active behavior that is inversely related to freezing, while rearing is a risk-assessing behavior that encompasses exploratory, activity, and excitability components [15, 16, 19]. In both the CFC and the DB tests, the initial period of exploration allows the animal to form an associative memory between the context and the conditioning stimulus. This association has been proven to be necessary to trigger conditioned fear responses in the CFC and is essential to the learning component of the fear conditioning paradigm [48, 49]. Thus, the individual, genetic differences in these responses likely influence the degree of fear memory recall and, thereby, are of major significance. Regarding the DB test, re-exposure to the DB chamber without the shock, similar to CFC, suggested that this is also a learning paradigm [50]. Although the CFC phenotypes may not be mapped to Stresp10, considering the abovementioned parallels and the third rearing phenotype showing similar differences between the strains, we presumed that these acute stress phenotypes are relevant to the current study. Indeed, the patterns of candidate gene expressions reflect the behavioral measures in the CFC, OFT, and DB tests, suggesting that the candidate QTGs contribute to variations in the general behavioral response to acute stress, as we had hypothesized.
In this study, we aimed to identify candidate QTGs within the Stresp10 QTL. We mined our previously collected genome-wide transcriptomic data to find genes in this region with brain region-specific expression that parallel the behavioral responses to acute stress in the F344 and WKY strains. The brain regions explored are the hippocampus, amygdala, and frontal cortex, which are all intimately involved in the behaviors discussed. The neural circuitry connecting the amygdala and frontal cortex are involved in the emotional responses to acute stress [51–54]. The ventral hippocampus is involved in anxiety-like responses, while the dorsal hippocampus is more involved in fear learning [55]. Because the sorting of behavioral responses to these categories is not feasible, we examined the whole hippocampus in this study. Since the goal of this study was to identify QTGs for acute stress responsiveness, the assumption was that steady-state expression of DEGs between the F344 and WKY strains will differ in all three brain regions due to sequence variations in the candidate QTGs.
As a proof-of-concept, we hypothesized that the expression of candidate QTGs in the brain of the congenic strain F344.WKY-Stresp10 would parallel that of the WKY and differ from the F344 parental strain. This pattern would mirror the strain differences in behavioral phenotypes obtained either in the DB or the OFT. We identified sequence variations within 12 genes, of which a total of five candidate QTGs were identified. Expression of three of the genes (Cdkl4, Drc1, and Gpatch11) were parallel to the behavior in two or more brain regions, while the other two (Cyp1b1 and Rbks) were parallel to the behavior in at least one brain region. Cdkl4 belongs to the cyclin-dependent protein kinase family and is responsible for cell cycle progression, including transferase activity, transferring phosphorous-containing groups and protein tyrosine kinase activity [56]. Drc1 encodes a key component of the nexin-dynein complex that regulates the assembly of ciliary dynein [57]. Gpatch11 is involved in nucleic acid binding [58]. Cyp1b1 encodes a member of the cytochrome P450 superfamily of enzymes, which catalyze reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids [59–61]. In retinal endothelial cells, expression of Cyp1b1 has been shown to reduce intracellular oxidative stress; although this has not been shown in neurons [62]. Rbks encodes a member of the carbohydrate kinase PkfB family and is known to catalyze the phosphorylation of ribose [63]. Although none of these genes have been directly implicated for acute stress responsiveness, their genomic location within QTLs for acute stress response suggests that they may underlie some common mechanisms of these phenotypic traits.
Interestingly, the individual candidate QTGs are link to stress-related immunoregulatory genes, including Il5 (interleukin 5), Btnl2 (butyrophilin-like 2), Ifna2 (interferon alpha 2), and Ifnl1 (interferon lambda 1) [64–67]. Both Il5 and Btnl2 can activate candidate QTG Drc1, which is known to encode a key component of the nexin-dynein complex that regulates the assembly of ciliary dynein [64, 65, 68]. While Drc1 has never been implicated in stress responses or stress-related disorders, Il5 has been reported to be differentially expressed in the frontal cortex of rats exposed to acute stress [69]. In a human study, elevated levels of Il5 were associated with an increased likelihood of major depressive disorder [70, 71]. Additionally, candidate QTG Drc1 can be activated by Cd38 (Cd38 molecule), which encodes a multifunctional protein involved in glucose-mediated insulin secretion and immune system functioning [64, 65, 72]. In the brain, Cd38 regulates the secretion of the neuropeptide oxytocin and is associated with several stress-related phenotypes, including social impairments in humans such as autism spectrum disorder [73]. Additionally, the rs3796863 SNP is associated with heightened stress sensitivity and predicting social anxiety and depression in humans [73]. The other candidate QTG Gpatch11 can also be activated by Ifna2 and Ifnl1, which are both interferon immunosuppressor genes. Interestingly, Ifna2 is a pleiotropic cytokine that triggers immune responses, hypothalamic-pituitary-adrenal axis abnormalities and disturbances in brain metabolism resembling those in depressive states [74]. Ifna2 is also known to induce memory, concentration, and mood disturbances when administered as a therapeutic [74]. In differentiating neurons, the expression of Ifna2 affects their response to inflammatory cytokines, which is consistent with molecular mechanisms involved in schizophrenia and autism spectrum disorder [75].
One inherent limitation of this study stems from the heterozygous region of the congenic strain, which we excluded from our analyses. Other candidate QTGs could be mapped to this region. Among the other limitations of this study is that it focuses on candidate QTGs with non-synonymous sequence variations in their coding regions. It is known that the large majority of sequence variations are in non-coding regions that may act as cis-regulatory and/or trans-acting modules. This makes identification of candidate QTGs for behavioral and psychiatric phenotypes more difficult [76]. However, our presumption that the SNPs within the QTGs affect the expression of these genes prompted us to first investigate coding region non-synonymous sequence variations. Furthermore, among inherited gene variations in humans, the non-synonymous SNPs in coding regions that lead to changes in amino acid in protein expression are most relevant to human inherited diseases [32]. Additionally, these candidate QTGs with cis-regulated expression changes can affect gene expression in trans, as described in Bryois et al. [77]. Future studies will examine sequence variations between F344 and WKY within the QTL in non-coding sequences conserved across species, as described in Yoshihara et al. [78]. We will also focus on sequence variations in microRNAs with known gene targets (www.targetscan.org and www.exiqon.com/microrna-target-prediction).
Taken together, our findings indicate that strain differences in acute stress responsiveness are generalizable across multiple behavioral paradigms. The unique approach of transcriptomics combined with sequence variations within a specific QTL in the different parental and congenic strains identified unique QTGs that might contribute to variations in the behavioral responses to acute stress. The role of these candidate genes in the behavioral response to stress should be confirmed in future studies requiring brain region- and strain-specific transgenic approaches.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
This material is based on research sponsored by the Air Force Research laboratory under agreement number FA8650-15-2-5518. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Research Laboratory or the U.S. Government.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This material is based on research sponsored by the Air Force Research laboratory (http://www.wpafb.af.mil/afrl/) under agreement number FA8650-15-2-5518. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Research Laboratory or the U.S. Government.
References
- 1.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of internal medicine. 1993;153(18):2093–101. Epub 1993/09/27. . [PubMed] [Google Scholar]
- 2.Claessens SEF, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology. 2011;214(1):141–54. doi: 10.1007/s00213-010-2118-y ; PubMed Central PMCID: PMCPMC3045508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebner K, Singewald N. Individual differences in stress susceptibility and stress inhibitory mechanisms. Current Opinion in Behavioral Sciences. 2017;14:54–64. http://dx.doi.org/10.1016/j.cobeha.2016.11.016. [Google Scholar]
- 4.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual review of neuroscience. 2001;24:1161–92. Epub 2001/08/25. doi: 10.1146/annurev.neuro.24.1.1161 . [DOI] [PubMed] [Google Scholar]
- 5.Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. The Journal of clinical endocrinology and metabolism. 2004;89(12):6244–50. Epub 2004/12/08. doi: 10.1210/jc.2004-0981 . [DOI] [PubMed] [Google Scholar]
- 6.Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Human Genetics. 2009;126(1):215–32. doi: 10.1007/s00439-009-0655-4 [DOI] [PubMed] [Google Scholar]
- 7.Trampush JW, Yang MLZ, Yu J, Knowles E, Davies G, Liewald DC, et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry. 2017. doi: 10.1038/mp.2016.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shumake J, Furgeson-Moreira S, Monfils MH. Predictability and heritability of individual differences in fear learning. Animal Cognition. 2014;17(5):1207–21. doi: 10.1007/s10071-014-0752-1 ; PubMed Central PMCID: PMCPMC4138434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thifault S, Ondrej S, Sun Y, Fortin A, Skamene E, Lalonde R, et al. Genetic determinants of emotionality and stress response in AcB/BcA recombinant congenic mice and in silico evidence of convergence with cardiovascular candidate genes. Human molecular genetics. 2008;17(3):331–44. Epub 2007/10/05. doi: 10.1093/hmg/ddm277 . [DOI] [PubMed] [Google Scholar]
- 10.Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, et al. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet. 2006;38(8):879–87. Epub 2006/07/13. doi: 10.1038/ng1840 . [DOI] [PubMed] [Google Scholar]
- 11.Valdar W, Solberg LC, Gauguier D, Cookson WO, Rawlins JN, Mott R, et al. Genetic and environmental effects on complex traits in mice. Genetics. 2006;174(2):959–84. Epub 2006/08/05. doi: 10.1534/genetics.106.060004 ; PubMed Central PMCID: PMCPMC1602068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baud A, Guryev V, Hummel O, Johannesson M, Flint J. Genomes and phenomes of a population of outbred rats and its progenitors. 2014;1:140011 doi: 10.1038/sdata.2014.11 https://www.nature.com/articles/sdata201411—supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hettema JM, Webb BT, Guo AY, Zhao Z, Maher BS, Chen X, et al. Prioritization and association analysis of murine-derived candidate genes in anxiety-spectrum disorders. Biological psychiatry. 2011;70(9):888–96. Epub 2011/08/30. doi: 10.1016/j.biopsych.2011.07.012 ; PubMed Central PMCID: PMCPMC3191234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rat Genome S, Mapping C, Baud A, Hermsen R, Guryev V, Stridh P, et al. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nature genetics. 2013;45(7): doi: 10.1038/ng.2644 PMC3821058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE. X-linked and lineage-dependent inheritance of coping responses to stress. Mammalian genome official journal of the International Mammalian Genome Society. 2003;14(11):748–57. Epub 2004/01/15. doi: 10.1007/s00335-003-2292-x . [DOI] [PubMed] [Google Scholar]
- 16.Ahmadiyeh N, Churchill GA, Solberg LC, Baum AE, Shimomura K, Takahashi JS, et al. Lineage is an epigenetic modifier of QTL influencing behavioral coping with stress. Behavior genetics. 2005;35(2):189–98. Epub 2005/02/03. doi: 10.1007/s10519-004-1018-5 ; PubMed Central PMCID: PMCPMC3764451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadiyeh N, Slone-Wilcoxon JL, Takahashi JS, Redei EE. Maternal behavior modulates X-linked inheritance of behavioral coping in the defensive burying test. Biological psychiatry. 2004;55(11):1069–74. Epub 2004/05/26. doi: 10.1016/j.biopsych.2004.02.014 ; PubMed Central PMCID: PMCPMC3760164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solberg LC, Ahmadiyeh N, Baum AE, Vitaterna MH, Takahashi JS, Turek FW, et al. Depressive-like behavior and stress reactivity are independent traits in a Wistar Kyoto x Fisher 344 cross. Molecular psychiatry. 2003;8(4):423–33. Epub 2003/05/13. doi: 10.1038/sj.mp.4001255 . [DOI] [PubMed] [Google Scholar]
- 19.Baum AE, Solberg LC, Churchill GA, Ahmadiyeh N, Takahashi JS, Redei EE. Test- and behavior-specific genetic factors affect WKY hypoactivity in tests of emotionality. Behavioural brain research. 2006;169(2):220–30. Epub 2006/02/24. doi: 10.1016/j.bbr.2006.01.007 ; PubMed Central PMCID: PMCPMC3762875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nature Genetics. 1997;17:280 doi: 10.1038/ng1197-280 [DOI] [PubMed] [Google Scholar]
- 21.The SC. SNP and haplotype mapping for genetic analysis in the rat. Nature Genetics. 2008;40:560 doi: 10.1038/ng.124 https://www.nature.com/articles/ng.124—supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardenbol P, Yu F, Belmont J, MacKenzie J, Bruckner C, Brundage T, et al. Highly multiplexed molecular inversion probe genotyping: Over 10,000 targeted SNPs genotyped in a single tube assay. Genome Research. 2005;15(2):269–75. doi: 10.1101/gr.3185605 PMC546528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tunc-Ozcan E, Harper KM, Graf EN, Redei EE. Thyroxine administration prevents matrilineal intergenerational consequences of in utero ethanol exposure in rats. Hormones and Behavior. 2016;82:1–10. doi: 10.1016/j.yhbeh.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Molecular psychiatry. 2005;10:961 doi: 10.1038/sj.mp.4001694 [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. Journal of neuroscience methods. 1980;3(2):129–49. [DOI] [PubMed] [Google Scholar]
- 26.Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, et al. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Molecular psychiatry. 2012;17(1):49–61. doi: 10.1038/mp.2010.119 PMC3117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunning MJ, Smith ML, Ritchie ME, Tavare S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics (Oxford, England). 2007;23(16):2183–4. Epub 2007/06/26. doi: 10.1093/bioinformatics/btm311 . [DOI] [PubMed] [Google Scholar]
- 28.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics (Oxford, England). 2003;19(2):185–93. Epub 2003/01/23. . [DOI] [PubMed] [Google Scholar]
- 29.Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Solberg Woods LC, et al. Context and strain-dependent behavioral response to stress. Behavioral and brain functions BBF. 2008;4:23 Epub 2008/06/04. doi: 10.1186/1744-9081-4-23 ; PubMed Central PMCID: PMCPMC2424057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huggins CE, Domenighetti AA, Ritchie ME, Khalil N, Favaloro JM, Proietto J, et al. Functional and metabolic remodelling in GLUT4-deficient hearts confers hyper-responsiveness to substrate intervention. Journal of Molecular and Cellular Cardiology. 2008;44(2):270–80. doi: 10.1016/j.yjmcc.2007.11.020 [DOI] [PubMed] [Google Scholar]
- 31.McCarthy DJ, Smyth GK. Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics (Oxford, England). 2009;25(6):765–71. Epub 2009/01/30. doi: 10.1093/bioinformatics/btp053 ; PubMed Central PMCID: PMCPMC2654802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, et al. Human Gene Mutation Database (HGMD): 2003 update. Human mutation. 2003;21(6):577–81. Epub 2003/05/20. doi: 10.1002/humu.10212 . [DOI] [PubMed] [Google Scholar]
- 33.Nuzzo R. Scientific method: Statistical errors: P values, the ‘gold standard’of statistical validity, are not as reliable as many scientists assume. Nature, 506 (February), 150–152. 2014. doi: 10.1038/506150a [DOI] [PubMed] [Google Scholar]
- 34.Hsu J. Multiple comparisons: theory and methods: CRC Press; 1996. [Google Scholar]
- 35.Shishimi A, Imada H. Discriminated and nondiscriminated avoidance conditioning of the rearing response in rats. Animal Learning & Behavior. 1977;5(3):259–64. doi: 10.3758/bf03209237 [Google Scholar]
- 36.Hovatta I, Zapala MA, Broide RS, Schadt EE, Libiger O, Schork NJ, et al. DNA variation and brain region-specific expression profiles exhibit different relationships between inbred mouse strains: implications for eQTL mapping studies. Genome Biology. 2007;8(2):R25 doi: 10.1186/gb-2007-8-2-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yh Taguchi. MicroRNA-mediated regulation of target genes in several brain regions is correlated to both microRNA-targeting-specific promoter methylation and differential microRNA expression. BioData Mining. 2013;6:11–. doi: 10.1186/1756-0381-6-11 PMC3693885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almli LM, Stevens JS, Smith AK, Kilaru V, Meng Q, Flory J, et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. American journal of medical genetics Part B, Neuropsychiatric genetics the official publication of the International Society of Psychiatric Genetics. 2015;168b(5):327–36. Epub 2015/05/20. doi: 10.1002/ajmg.b.32315 ; PubMed Central PMCID: PMCPMC4844461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woudstra S, Bochdanovits Z, van Tol MJ, Veltman DJ, Zitman FG, van Buchem MA, et al. Piccolo genotype modulates neural correlates of emotion processing but not executive functioning. Translational psychiatry. 2012;2:e99 Epub 2012/07/27. doi: 10.1038/tp.2012.29 ; PubMed Central PMCID: PMCPMC3337071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, et al. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet. 2006;38(8):879–87. http://www.nature.com/ng/journal/v38/n8/suppinfo/ng1840_S1.html. doi: 10.1038/ng1840 [DOI] [PubMed] [Google Scholar]
- 41.Barth A, Bilkei-Gorzo A, Drews E, Otte DM, Diaz-Lacava A, Varadarajulu J, et al. Analysis of quantitative trait loci in mice suggests a role of Enoph1 in stress reactivity. Journal of neurochemistry. 2014;128(6):807–17. Epub 2013/11/19. doi: 10.1111/jnc.12517 . [DOI] [PubMed] [Google Scholar]
- 42.Sokoloff G, Parker CC, Lim JE, Palmer AA. Anxiety and fear in a cross of C57BL/6J and DBA/2J mice: mapping overlapping and independent QTL for related traits. Genes, Brain and Behavior. 2011;10(5):604–14. doi: 10.1111/j.1601-183X.2011.00699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brigman JL, Mathur P, Lu L, Williams RW, Holmes A. Genetic relationship between anxiety- and fear -related behaviors in BXD recombinant inbred mice. Behavioural pharmacology. 2009;20(2):204–9. doi: 10.1097/FBP.0b013e32830c368c PMC2701299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponder CA, Kliethermes CL, Drew MR, Muller J, Das K, Risbrough VB, et al. Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes, Brain and Behavior. 2007;6(8):736–49. doi: 10.1111/j.1601-183X.2007.00306.x [DOI] [PubMed] [Google Scholar]
- 45.Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. American Journal of Physiology—Renal Physiology. 2010;298(6):F1484–F91. doi: 10.1152/ajprenal.00002.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacology Biochemistry and Behavior. 1996;54(1):113–6. http://dx.doi.org/10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- 47.De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European journal of pharmacology. 2003;463(1–3):145–61. Epub 2003/02/26. . [DOI] [PubMed] [Google Scholar]
- 48.Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17(1):16–39. https://doi.org/10.1016/0023-9690(86)90018-4. [Google Scholar]
- 49.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110(1):73–81. https://doi.org/10.1016/S0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 50.Sandbak T, Murison R, Sarviharju M, Hyytia P. Defensive Burying and Stress Gastric Erosions in Alcohol-Preferring AA and Alcohol-Avoiding ANA Rats. Alcoholism: Clinical and Experimental Research. 1998;22(9):2050–4. doi: 10.1111/j.1530-0277.1998.tb05915.x [PubMed] [Google Scholar]
- 51.Andolina D, Maran D, Valzania A, Conversi D, Puglisi-Allegra S. Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection. Neuropsychopharmacology. 2013;38(10):2057–67. Epub 2013/05/03. doi: 10.1038/npp.2013.107 ; PubMed Central PMCID: PMCPMC3746690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andolina D, Maran D, Viscomi MT, Puglisi-Allegra S. Strain-dependent variations in stress coping behavior are mediated by a 5-HT/GABA interaction within the prefrontal corticolimbic system. Int J Neuropsychopharmacol. 2014;18(3). Epub 2014/12/19. doi: 10.1093/ijnp/pyu074 ; PubMed Central PMCID: PMCPMC4360254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cerebral cortex (New York, NY 1991). 2007;17(12):2796–804. Epub 2007/02/27. doi: 10.1093/cercor/bhm008 . [DOI] [PubMed] [Google Scholar]
- 54.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. Epub 2011/08/03. doi: 10.1016/j.neuropharm.2011.07.014 ; PubMed Central PMCID: PMCPMC3196296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang WN, Bast T, Xu Y, Feldon J. Temporary inhibition of dorsal or ventral hippocampus by muscimol: distinct effects on measures of innate anxiety on the elevated plus maze, but similar disruption of contextual fear conditioning. Behavioural brain research. 2014;262:47–56. Epub 2013/11/12. doi: 10.1016/j.bbr.2013.10.044 . [DOI] [PubMed] [Google Scholar]
- 56.Lin M, Zhang Y, Li A, Tang E, Peng J, Tang W, et al. High-throughput RNAi screening of human kinases identifies predictors of clinical outcome in colorectal cancer patients treated with oxaliplatin. Oncotarget. 2015;6(18):16774–85. doi: 10.18632/oncotarget.3736 ; PubMed Central PMCID: PMCPMC4599307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wirschell M, Olbrich H, Werner C, Tritschler D, Bower R, Sale WS, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45(3):262–8. Epub 2013/01/29. doi: 10.1038/ng.2533 ; PubMed Central PMCID: PMCPMC3818796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohta S, Bukowski-Wills JC, Sanchez-Pulido L, Alves Fde L, Wood L, Chen ZA, et al. The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell. 2010;142(5):810–21. Epub 2010/09/04. doi: 10.1016/j.cell.2010.07.047 ; PubMed Central PMCID: PMCPMC2982257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aklillu E, Oscarson M, Hidestrand M, Leidvik B, Otter C, Ingelman-Sundberg M. Functional analysis of six different polymorphic CYP1B1 enzyme variants found in an Ethiopian population. Molecular pharmacology. 2002;61(3):586–94. Epub 2002/02/21. . [DOI] [PubMed] [Google Scholar]
- 60.Bailey LR, Roodi N, Dupont WD, Parl FF. Association of cytochrome P450 1B1 (CYP1B1) polymorphism with steroid receptor status in breast cancer. Cancer research. 1998;58(22):5038–41. Epub 1998/11/21. . [PubMed] [Google Scholar]
- 61.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. American journal of human genetics. 2002;70(2):448–60. Epub 2002/01/05. doi: 10.1086/338709 ; PubMed Central PMCID: PMCPMC384919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma S, Saxena R, Siddiqui MH, Santha K, Sethupathy S. Evaluation of CYP1B1 Expression, Oxidative Stress and Phase 2 Detoxification Enzyme Status in Oral Squamous Cell Carcinoma Patients. Journal of Clinical and Diagnostic Research JCDR. 2017;11(3):Bc01–5. doi: 10.7860/JCDR/2017/22196.9324 ; PubMed Central PMCID: PMCPMC5427294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, van Koeverden P, Singh B, Gupta RS. Identification and characterization of human ribokinase and comparison of its properties with E. coli ribokinase and human adenosine kinase. FEBS letters. 2007;581(17):3211–6. Epub 2007/06/26. doi: 10.1016/j.febslet.2007.06.009 . [DOI] [PubMed] [Google Scholar]
- 64.Horikawa K, Takatsu K. Interleukin-5 regulates genes involved in B-cell terminal maturation. Immunology. 2006;118(4):497–508. doi: 10.1111/j.1365-2567.2006.02382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swanson RM, Gavin MA, Escobar SS, Rottman JB, Lipsky BP, Dube S, et al. Butyrophilin-like 2 Modulates B7 Costimulation To Induce Foxp3 Expression and Regulatory T Cell Development in Mature T Cells. The Journal of Immunology. 2013;190(5):2027–35. doi: 10.4049/jimmunol.1201760 [DOI] [PubMed] [Google Scholar]
- 66.Marcello T, Grakoui A, Barba–Spaeth G, Machlin ES, Kotenko SV, Macdonald MR, et al. Interferons α and λ Inhibit Hepatitis C Virus Replication With Distinct Signal Transduction and Gene Regulation Kinetics. Gastroenterology. 2006;131(6):1887–98. doi: 10.1053/j.gastro.2006.09.052 [DOI] [PubMed] [Google Scholar]
- 67.Hultcrantz M, Hühn MH, Wolf M, Olsson A, Jacobson S, Williams BR, et al. Interferons induce an antiviral state in human pancreatic islet cells. Virology. 2007;367(1):92–101. doi: 10.1016/j.virol.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Elledge SJ. DRC1, DNA replication and checkpoint protein 1, functions with DPB11 to control DNA replication and the S-phase checkpoint in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences. 1999;96(7):3824–9. doi: 10.1073/pnas.96.7.3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Pardo M, de Souza Armini R, Martinez A, Mouhsine H, Zagury J-F, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain, behavior, and immunity. 2016;53:207–22. doi: 10.1016/j.bbi.2015.12.012 PMC4783243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elomaa A-P, Niskanen L, Herzig K-H, Viinamäki H, Hintikka J, Koivumaa-Honkanen H, et al. Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC Psychiatry. 2012;12(1):2 doi: 10.1186/1471-244x-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Molecular psychiatry. 2011;16 doi: 10.1038/mp.2010.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nata K, Takamura T, Karasawa T, Kumagai T, Hashioka W, Tohgo A, et al. Human gene encoding CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase): organization, nucleotide sequence and alternative splicing. Gene. 1997;186(2):285–92. Epub 1997/02/28. . [DOI] [PubMed] [Google Scholar]
- 73.Tabak BA, Vrshek-Schallhorn S, Zinbarg RE, Prenoveau JM, Mineka S, Redei EE, et al. Interaction of CD38 Variant and Chronic Interpersonal Stress Prospectively Predicts Social Anxiety and Depression Symptoms Over Six Years. Clinical psychological science a journal of the Association for Psychological Science. 2016;4(1):17–27. doi: 10.1177/2167702615577470 ; PubMed Central PMCID: PMCPMC4779340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoyo-Becerra C, Huebener A, Trippler M, Lutterbeck M, Liu ZJ, Truebner K, et al. Concomitant Interferon Alpha Stimulation and TLR3 Activation Induces Neuronal Expression of Depression-Related Genes That Are Elevated in the Brain of Suicidal Persons. PLoS ONE. 2013;8(12):e83149 doi: 10.1371/journal.pone.0083149 PMC3877033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Lin M, Hrabovsky A, Pedrosa E, Dean J, Jain S, et al. ZNF804A Transcriptional Networks in Differentiating Neurons Derived from Induced Pluripotent Stem Cells of Human Origin. PLoS One. 2015;10(4):e0124597 Epub 2015/04/24. doi: 10.1371/journal.pone.0124597 ; PubMed Central PMCID: PMCPMC4408091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao X, Chang H, Li M. Molecular mechanisms underlying noncoding risk variations in psychiatric genetic studies. Molecular psychiatry. 2017;22:497 doi: 10.1038/mp.2016.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryois J, Buil A, Evans DM, Kemp JP, Montgomery SB, Conrad DF, et al. Cis and Trans Effects of Human Genomic Variants on Gene Expression. PLOS Genetics. 2014;10(7):e1004461 doi: 10.1371/journal.pgen.1004461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshihara M, Saito D, Sato T, Ohara O, Kuramoto T, Suyama M. Design and application of a target capture sequencing of exons and conserved non-coding sequences for the rat. BMC genomics. 2016;17:593 Epub 2016/08/11. doi: 10.1186/s12864-016-2975-9 ; PubMed Central PMCID: PMCPMC4979189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.