Summary
Background
EGFR antibodies have been shown to increase survival in patients with advanced non-small cell lung cancer (NSCLC), particularly with squamous cell (SC) histology. Our prior work suggested that EGFR copy number by fluorescence in-situ hybridization (FISH) identifies patients most likely to benefit from these agents combined with chemotherapy. This study aimed to explore the activity of cetuximab with chemotherapy in EGFR-FISH positive (EGFR-FISH+) patients.
Methods
S0819 was a multicenter, open-label, Phase III study. Eligible patients with treatment-naïve Stage IV NSCLC were randomized with equal probability to receive paclitaxel (200 mg/m2; q21d) plus carboplatin (AUC = 6; q21d) or carboplatin plus paclitaxel and bevacizumab (15 mg/kg; q21d) with or without cetuximab (250 mg/m2 weekly after loading dose). Randomization was stratified by bevacizumab treatment, smoking status, and M-substage using a dynamic balancing algorithm. Co-primary objectives were progression-free survival (PFS) in patients with EGFR-FISH+ cancers and overall survival (OS) in the overall study population. Intention-to-treat analyses were employed for primary and secondary outcomes; the safety population included patients who received at least one dose of study drug. This completed study is registered with ClinicalTrials.gov (NCT00946712).
Findings
Between August 2009 and June 2014, 1,333 patients were enrolled and 1,313 patients were randomized (control group: 277/380 bevacizumab-treated [BT]/no bevacizumab [BN]; cetuximab group: 283/373 BT/BN). EGFR FISH was assessable in 976 patients; 400 (41%) were EGFR-FISH+. PFS was not significantly different between the arms among the EGFR-FISH+ subpopulation (HR=0·92 [0·75–1·12], P=0·40). OS was not significantly different among treatment arms in the overall study population (HR=0·93 [0·83–1·04], P=0·22). In a prespecified analysis among patients with EGFR-FISH+ cancers with SC histology, OS was significantly improved in the cetuximab group (HR=0·58 [0·39–0·86], P=0·01): median OS of 11·8 months (95% CI: 8·6–13·5) and 6·1 months (95% CI: 4·2–8·7) in cetuximab and control arms, respectively. The most common grade 3–4 AEs were neutrophils (n=210 cetuximab; n=158 control), leukocytes (n=103 cetuximab; n=74 control), fatigue (n=81 cetuximab; n=74 control) and rash (n=52 cetuximab; n=1 control). Grade 5 AEs occurred in 5% (n=32) of cetuximab and 2% (n=13) of controls.
Interpretation
Although this study did not meet its primary endpoints, subgroup analyses of patients with EGFR-FISH+ cancers by histology suggests further study of cetuximab is warranted in those with SC cancers. These data, together with other recent trials, support continued evaluation of anti-EGFR antibodies in this subpopulation.
Funding
National Cancer Institute and Eli Lilly and Company
Introduction
Though the incidence and mortality rates associated with lung cancer have steadily declined, lung cancer continues to be the leading cause of cancer death in the United States (US); over 158,000 associated deaths were estimated for 2016.1 Non-small cell lung cancer (NSCLC) constitutes approximately 80% of lung cancers. The standard of care for advanced NSCLC has evolved, from best supportive care to the use of multiple regimens including platinum-based chemotherapy, molecularly targeted agents, and immunotherapy.2 Of the targeted agents, those inhibiting the epidermal growth factor receptor (EGFR) have experienced multiple generations of development.3–6 The tyrosine kinase inhibitors (TKIs) erlotinib, gefitinib, and afatinib confer dramatic clinical responses in the subset of patients who harbor EGFR tyrosine kinase domain mutations, and have shown only modest efficacy in unselected previously-treated patients3, but have been less effective as first-line agents in combination with chemotherapy in EGFR unmutated patients with advanced NSCLC.7
Cetuximab, a highly specific, chimerized, monoclonal antibody targeting EGFR, has been investigated in combination with platinum-based chemotherapies for the treatment of chemotherapy-naïve patients with advanced NSCLC based upon biological evidence demonstrating the EGFR pathway plays a role in lung cancer development and progression.8, 9 Furthermore, the lack of efficacy of oral EGFR TKIs in combination with chemotherapy suggested an alternative EGFR-directed approach was needed and preclinical models showed synergy with chemotherapy and cetuximab.5, 10, 11 Though in EGFR mutated patients using varied dosing schedules there were hints of activity for EGFR TKI and chemotherapy. SWOG conducted two Phase II clinical trials to evaluate the efficacy and safety of adding cetuximab to first-line treatment of advanced NSCLC – S0342 and S0536.12–14 The S0342 trial evaluated if there was sufficient activity of cetuximab concurrently with or sequentially after chemotherapy in treatment-naïve advanced NSCLC in order to select one of these regimens for further study.12 A secondary analysis of this trial indicated that EGFR copy number, as assessed by fluorescence in situ hybridization (EGFR FISH), may be associated with improved survival in this patient population.13 The S0536 trial was designed to assess the safety and feasibility of a chemotherapy doublet (carboplatin/paclitaxel) given concurrently with a biologic doublet consisting of the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab and cetuximab as first-line therapy in advanced NSCLC.14 This trial also assessed EGFR FISH as a biomarker of response. The primary safety endpoint evaluating Grade 4–5 hemorrhage-related toxicities was met, with only 2% (n = 2) of the study population experiencing Grade 5 pulmonary hemorrhage, with all other toxicities similar to previous cetuximab combinations.12, 14, 15 The overall response rate (RR) was 56% (52 of 95 patients) and the overall disease control rate was 77%; moreover, the median PFS was 7 months and OS was 15 months.14 The results supported a potential relationship between EGFR FISH-positivity and enhanced clinical outcome.14
Given the overall safety, efficacy, and biomarker results from the S0342 and S0536 studies, this Phase III biomarker validation study investigated the safety and effectiveness of first-line therapy with cetuximab plus carboplatin/paclitaxel chemotherapy with or without bevacizumab in patients with advanced NSCLC and was designed to validate EGFR FISH as a predictive biomarker for cetuximab in this population.16 We hypothesized that EGFR FISH-positivity would be associated with increased PFS and/or OS.
Methods
Study design and participants
This was a randomized, multicenter, Phase III study of carboplatin plus paclitaxel or carboplatin plus paclitaxel and bevacizumab with or without cetuximab in patients with advanced NSCLC (list of centers in Appendix page 1). Patients had histologically/cytologically proven Stage IV primary NSCLC that was newly diagnosed or recurrent after previous surgery and/or irradiation. Patients were excluded if they received prior chemotherapy for NSCLC, platinum-based chemotherapy for any purpose, any drug targeting the EGFR or VEGF pathways, any chimerized or mouse monoclonal antibody therapies, or had documented presence of human anti-mouse antibodies. Patients were required to have CT or MRI scans to document the extent of their disease; measurable disease was assessed within 28 days prior to registration and non-measurable disease was assessed within 42 days of registration. CT or MRI scans were required within 42 days prior to registration in order to determine the extent of central nervous system disease; patients with adequately treated, controlled brain metastases were allowed if the patient had no residual neurological dysfunction off corticosteroids for ≥ 1 day. At least 28 days must have passed since major surgery was performed on patients. Laboratory and clinical tests were performed within 14 days prior to registration and results had to meet the following requirements: absolute neutrophil count ≥ 1,500/mcl; platelet count ≥ 100,000/mcl; hemoglobin ≥ 9 g/dL; serum creatinine ≤ the institutional upper limit of normal (IULN) and a calculated or measured creatinine clearance ≥ 50 cc/min; adequate hepatic function (serum bilirubin ≤ 2x IULN and either SGOT or SGPT ≤ 2x IULN; for patients with liver metastases, bilirubin and either SGOT or SGPT must be ≤ 5x IULN); Zubrod performance status of 0 (fully active, able to carry on all pre-disease performance without restriction) to 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature); < Grade 2 symptomatic neuropathy-sensory; no evidence of active infection or acute hepatitis; no history (within the prior 6 months) of cerebrovascular accident, myocardial infarction or unstable angina and no evidence of uncontrolled hypertension, NYHA Grade 2 or higher congestive heart failure, serious cardiac arrhythmia requiring medication or clinically significant vascular disease. No other prior malignancy was allowed except adequately treated basal cell or squamous cell (SC) skin cancer, in situ cervical cancers, adequately treated Stage I or II cancer from which the patient is in complete remission, or any other cancer from which the patient has been disease-free for 5 years. Patients provided prior smoking history and were prohibited from becoming pregnant or nursing due to the increased risk of fetal harm from the chemotherapeutic agents.
Patients were bevacizumab inappropriate if they had any of the following: ≥ 50% SC tumor component; history of hemoptysis (≥ ½ tsp per event within the past year); cavitary pulmonary lesion; history of documented hemorrhagic diathesis or coagulopathy; non-healing wound, or bone fracture, abdominal fistula, gastrointestinal perforation or intra-abdominal abscess; patients receiving anticoagulation. Patients were placed in the “bevacizumab no” (BN) stratum if they did not receive bevacizumab; reasons could include being bevacizumab inappropriate or if the patient or physician decided to not treat the patient with bevacizumab. Patients with central nervous system metastases were defined as bevacizumab appropriate until protocol amendment in June 2013. For patients receiving bevacizumab, if urine protein creatinine ratio was > 0.5, 24-hour urine protein was required to be < 1,000 mg for enrollment. The S0819 trial (Trial Registry: ClinicalTrials.gov Identifier: NCT00946712) was approved by the institutional review boards of the participating institutions and all patients provided informed consent.
Randomization
Patients were randomized with equal probability. The study was open label. Randomization was stratified by bevacizumab treatment status (having received bevacizumab treatment [BT] versus BN), smoking status (current or former versus never), and stage (M1a versus M1b) using a dynamic balancing algorithm.17 Patients were enrolled via a web-based application and simultaneously randomized by a computer program. Sites were automatically notified of the patient’s randomization arm at the time of enrollment.
Procedures
Patients received chemotherapy with paclitaxel (200 mg/m2; 3 hour IV infusion; q21d) and carboplatin (AUC = 6 by modified Calvert formula; 30 minute IV infusion immediately following paclitaxel; q21d) for a maximum of 6 cycles or until one of the criteria for removal from treatment was met, which included disease progression based on investigator’s assessment or symptomatic deterioration, unacceptable toxicity, or treatment delay > 4 weeks. Carboplatin and paclitaxel were chosen as the chemotherapy regimen for this trial because this regimen was acceptable for all histologies that were eligible for the trial, and because of prior SWOG data with this regimen in combination in the Phase II S0342 trial.
For the patients receiving bevacizumab (15 mg/kg; 30–90 minute IV infusion occurred 1 hour following carboplatin; q21d). The patients randomized to cetuximab received 400 mg/m2 loading dose as a 2-hour IV infusion on Week 1 of Cycle 1, followed by 250 mg/m2 weekly dosing starting on Week 2, one hour prior to paclitaxel. Patients randomized to receive cetuximab were pre-medicated with 50 mg IV diphenhydramine hydrochloride prior to the first dose of cetuximab to prevent hypersensitivity reaction. Dose reductions were allowed in the event of toxicity and all dose reductions were permanent. Laboratory monitoring occurred every cycle of treatment starting at Cycle 2 and included complete blood counts, serum creatinine, calculated or measured creatinine clearance, urine protein creatinine ratio, total bilirubin, serum glutamic oxaloacetic transaminase or serum glutamic aspartate aminotransferase, alkaline phosphatase, international normalized ratio, albumin, lactate dehydrogenase serum sodium, calcium, and magnesium. Assessment of toxicity was done by CTCAE 4.0 and was performed at every cycle of treatment starting at Cycle 2.
Patients were followed for 3 years after registration or until death. CT or MRI scans were ascertained every 6 weeks for the first 9 months and every 3 months thereafter, until disease progression.
Paraffin-embedded, formalin fixed tumor specimens or fine needle aspirate slides were submitted prior to the start of therapy for EGFR FISH analysis. EGFR FISH and KRAS mutational status were evaluated at each interim analysis.
EGFR FISH analysis was performed using the “Colorado EGFR Scoring System,” as reported.13 Tumors were considered to be EGFR FISH-positive (EGFR-FISH+) if they harbored four or more copies of EGFR in ≥ 40% of cells or if they showed EGFR amplification (defined as gene-to-chromosome ratio ≥ 2 or presence of gene cluster or ≥ 15 gene copies in ≥ 10% of cells). Tumors that were successfully tested and failed to meet these criteria were classified as EGFR FISH-negative. EGFR mutational testing was not a required test for this trial.
Outcomes
The primary co-objectives were to compare PFS by institutional review in EGFR-FISH+ patients and OS in the entire study population. Central review of PFS was specified in the protocol, however, this data will be presented in a separate manuscript. Secondary objectives included comparison of OS in EGFR-FISH+ patients; comparison of PFS in the entire study population, comparison of response rates (confirmed plus unconfirmed, complete and partial responses) by RECIST 1.1 in the subset of patients with measurable disease in the entire study population and the EGFR-FISH+ subset; assessment of the toxicity by CTCAE 4.0 of each treatment arm and by BN or BT subgroups; prospective testing of EGFR FISH as a predictive marker for the selection of patients for cetuximab plus chemotherapy; a comparison of OS and PFS within the BT and BN subgroups; an evaluation of the role of KRAS mutations in terms of cetuximab efficacy; and comparison of results of EGFR FISH with KRAS mutations, EGFR mutations, EGFR immunohistochemistry (IHC), and other purported EGFR-related biomarkers. The analysis of KRAS, EGFR IHC, and other EGFR-related biomarkers will be presented in a separate manuscript.
Statistical analysis
The basis for the statistical design of the study has been previously described.16 OS was defined as the duration from registration to death due to any cause. Patients last known to be alive were censored at the date of last contact. PFS was defined as the duration from randomization to progression, symptomatic deterioration, or death due to any cause (whichever comes first), by RECIST 1·1 as assessed by the treating investigator. Patients last known to be alive and progression free were censored at the date of last contact. The primary analysis was by intention to treat.
While the study employs co-primary objectives, the sample size was based on the primary objective within the EGFR-FISH+ population. The study-wide type I error is 2·5%, with 2% allocated to the EGFR-FISH+ objective. The original design for the EGFR-FISH+ objective was based on the following specifications: a design with 92% power to detect a hazard ratio (HR) of 0·75 at the 1-sided 2% level requires 297 PFS events. Then, assuming exponential survival, 50% of patients are BT, a median PFS of 4 months among BN patients, a median PFS of 6 months in BT patients, uniform accrual over 4 years, and 1 year of follow-up, 618 EGFR-FISH+ patients are needed.
The total target sample size was 1,546 patients, which was based on the assumption that 80% of patients would be evaluable for EGFR FISH status, and of them, 50% would be EGFR-FISH+. The level of testing within the entire study population was set to be 0·015, accounting for the correlation between the two co-primary objectives. Under the design assumptions above, and assuming a median OS of 10 and 12 months within BN and BT patients respectively, the study had 86% power to detect a HR of 0·83 for OS using a 1-sided 0·015 level log-rank test within the entire study population. As the sample size for the study was determined by the number of EGFR-FISH+ patients accrued, the study protocol included specification that the study may be modified based on the observed prevalence of EGFR FISH-positivity.
As discussed in the results section, the observed prevalence of EGFR FISH-positivity was 41% and the percentage of patients with known EGFR FISH status was lower than specified (74% with known FISH status). On June 1st, 2014, the study design was amended to account for the lower than estimated percentage of accrued patients known to be EGFR-FISH+. The accrual rate to the study was also lower than anticipated. The amended accrual goal was 400 EGFR-FISH+ patients; based on a design with 80% power, all other design parameters remained unchanged.
The interim analysis plan is fully described in Redman et al.16 Interim analyses were to take place when 30%, 67%, and 85% of the expected PFS within the EGFR-FISH+ population were observed. Interim analyses evaluated early stopping for either efficacy or futility both in the overall population and by FISH grouping.
OS and PFS were analyzed using a two-sided log-rank test stratified according to the factors listed above. HRs and corresponding confidence intervals (CIs) were estimated using a stratified Cox proportional-hazards model, with randomized group as a single covariate. The proportional hazards assumption was not evaluated. Survival curves for each treatment group were estimated by Kaplan–Meier method. Survival rates were derived from the Kaplan–Meier estimates. ORRs were compared using a two-sided, stratified Cochran–Mantel–Haenszel test, with exact 95% CIs calculated with the use of the Clopper–Pearson method. SAS (version 9.4) was used for all statistical analyses. Secondary analyses used a significance level of 5%. Analysis of clinical outcomes employed the intention-to-treat principle including all randomized patients excluding those found ineligible centrally after randomization. Analysis of toxicity included patients who received at least one dose of protocol treatment. The study was overseen by the SWOG Data Safety Monitoring Committee (DSMC) on a twice a year basis.
Role of the funding source
This trial was sponsored by SWOG/NCI cooperative group of which the authors are members. The funder contributed to study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
From August 13, 2009 to May 30, 2014, 1,333 patients were enrolled in the trial; 20 were deemed ineligible, resulting in 1,313 patients in the eligible study population: chemotherapy plus cetuximab arm (n = 656) and the chemotherapy without cetuximab arm (control) (n = 657) (Figure 1). The median follow-up duration for patients last known to be alive is 35.2 months with a range of 0.3–60 months and interquartile range of 22.9–39.9 months. Patient demographics and characteristics by treatment group are described in Table 1. Specimens for EGFR FISH testing were evaluated in 1,190 (91%) of the 1,313 eligible patients. Specimens from 1,034 patients (87%) were adequate for testing. The FISH assay failed in specimens from 58 patients leaving 976 patients (94%) with assessable specimens. Of these 976 specimens, 400 (41%) were EGFR-FISH+. The remaining 913 eligible patients are classified as FISH non-positive (combination of EGFR negative and EGFR status unknown).
Figure 1.
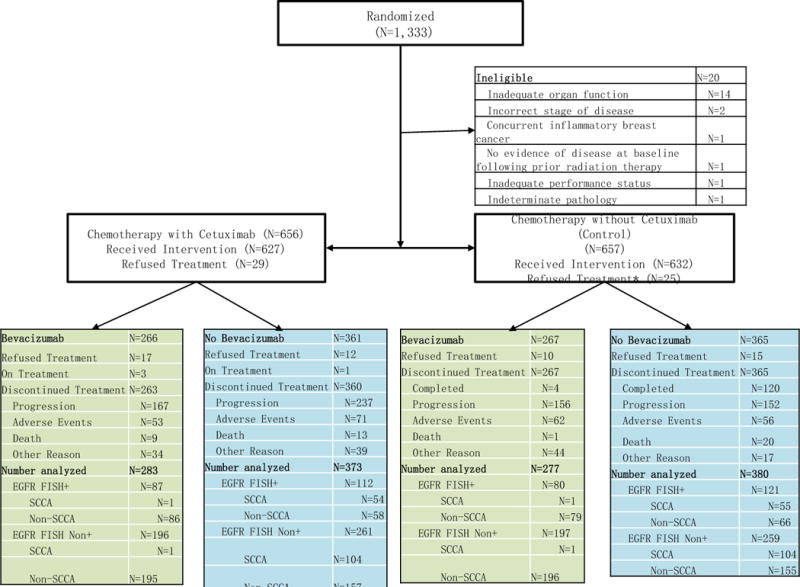
CONSORT diagram.
Table 1.
Baseline Patient Demographics and Characteristics
| EGFR FISH+ Patients | All Patients | |||
|---|---|---|---|---|
| % (n) | Control (n=201) |
+ Cetuximab (n=199) |
Control (n=657) |
+ Cetuximab (n=656) |
| Age (median, range) | 64 (34 – 84) | 62 (37 – 80) | 63 (30 – 86) | 63 (19 – 84) |
| >65 years | 94 (47) | 78 (39) | 278 (42) | 275 (42) |
| Sex | ||||
| Male | 57 (115) | 63 (125) | 55 (359) | 59 (385) |
| Female | 43 (86) | 37 (74) | 45 (298) | 41 (271) |
| M-stage | ||||
| M1a | 27 (54) | 22 (43) | 45 (297) | 45 (292) |
| M1b | 73 (147) | 78 (156) | 46 (303) | 46 (305) |
| Bevacizumab treatment | ||||
| Bevacizumab | 40 (80) | 44 (87) | 42 (277) | 43 (283) |
| No Bevacizumab | 60 (121) | 56 (112) | 58 (380) | 57 (373) |
| Smoking History | ||||
| Current | 44 (89) | 47 (94) | 45 (297) | 45 (292) |
| Former | 47 (94) | 44 (87) | 46 (303) | 46 (305) |
| Never | 9 (18) | 9 (18) | 9 (57) | 9 (59) |
| Histology | ||||
| SC | 28 (56) | 28 (55) | 25 (161) | 24 (160) |
| Adenocarcinoma | 60 (120) | 65 (130) | 62 (408) | 63 (411) |
| Other* | 12 (25) | 7 (14) | 13 (88) | 13 (85) |
| Performance Status** | ||||
| 0 | 32 (64) | 41 (81) | 35 (229) | 39 (256) |
| 1 | 68 (137) | 59 (118) | 65 (427) | 61 (400) |
| EGFR FISH Status† | ||||
| EGFR-FISH+ | 201 (100%) | 199 (100%) | 31 (201) | 30 (199) |
| EGFR FISH-negative | – | – | 45 (293) | 43 (283) |
| EGFR FISH-unknown | – | – | 25 (163) | 27 (174) |
Includes large cell, BAC, mixed, other, and not reported;
One patient on the Control arm was missing documentation.
In the control and cetuximab arms, EGFR FISH analysis failed in 4% and 5% of patients, there was inadequate specimen in 12% and 11% of cases, and there were no data in 9% and 10% of cases, respectively.
Of 1,313 patients eligible for treatment, 53 did not receive any protocol treatment and one patient withdrew consent prior to being evaluated for toxicity; the resulting 1,259 patients (627 in the cetuximab arm and 632 in the control group) were evaluated for safety events. Major protocol deviations occurred in 136 patients (some had more than one deviation): in addition to the 53 patients who did not receive treatment, 51 were deemed BN but treated with bevacizumab, 23 experienced a carboplatin dosing error, and 16 others were considered protocol deviations for miscellaneous reasons.
Results of the primary, secondary, and subgroup analyses are presented in Figures 2 and 3. The first co-primary objective was a comparison of PFS between the treatment arms in patients with EGFR-FISH+ cancers. In patients with EGFR-FISH+ cancers, the distribution of PFS was not significantly different between the treatment arms (HR = 0·92 [0·75–1·12], P = 0·40). The median PFS was 4·8 months (95% CI: 3·9–5·5) in the control arm and 5·4 months (95% CI: 4·5–5·7) in the cetuximab-containing arm (Table 2). However, OS was significantly different between the arms (HR = 0·81 [0·66–1·0], P = 0·048). The median OS was 9·8 months (95% CI: 8·7–12·1) in the control arm and 13·4 months (95% CI: 11·5–14·8) in cetuximab-containing arm. RRs in the patients with EGFR-FISH+ cancers were not significantly different between the arms (43% control arm versus 47% cetuximab-containing arm, P = 0·48).
Figure 2.
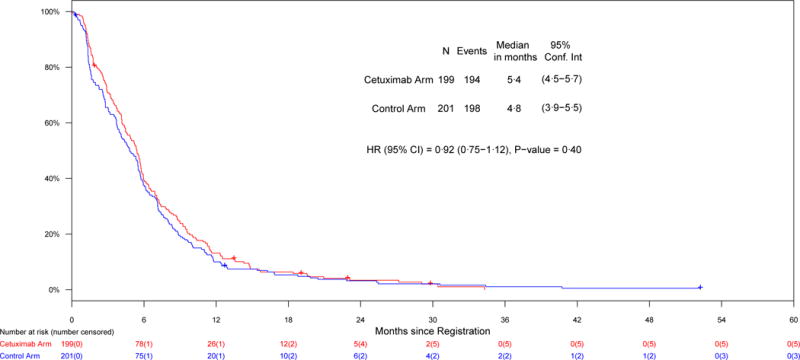
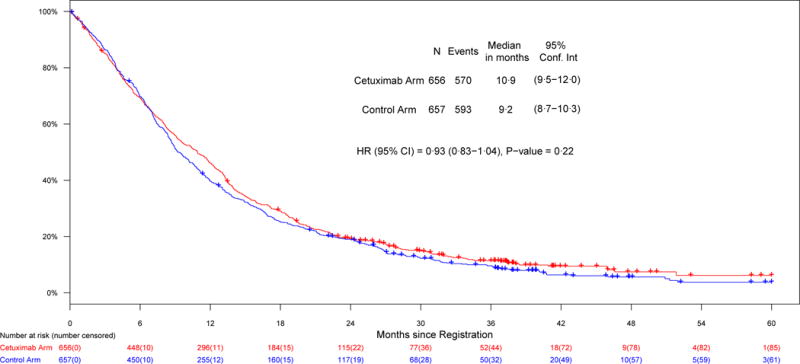
Co-primary endpoints, a) PFS in patients with EGFR-FISH+ cancers; b) OS in the entire study population
Figure 3.
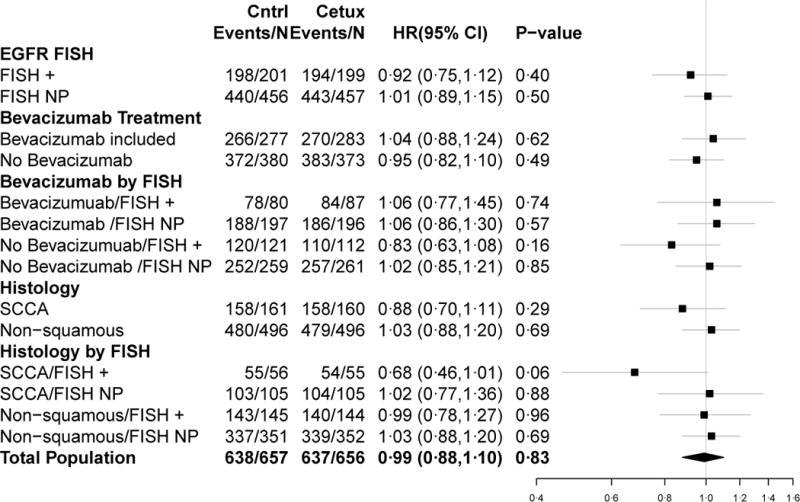
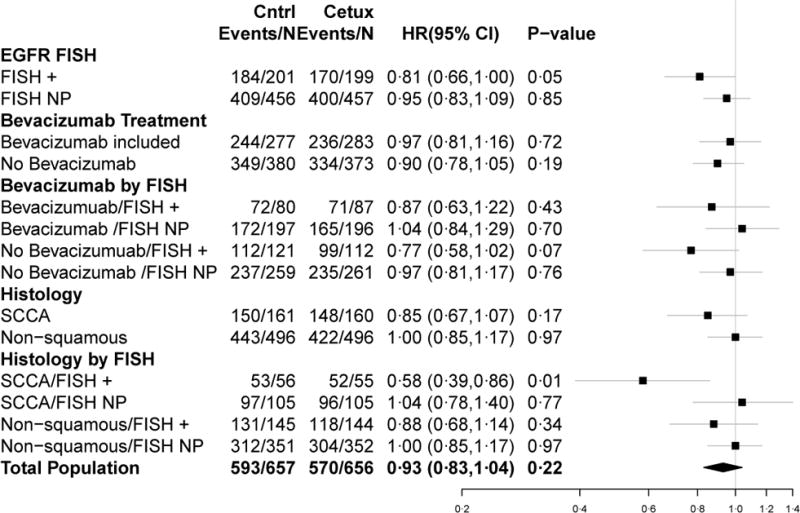
Forest plots for PFS (a) and OS (b) HRs
Table 2.
Median progression-free and overall survival estimates for the entire study population and by EGFR FISH status
| Analysis Group | Outcome (mos) | All Patients Median (95% CI) |
EGFR-FISH+ Median (95% CI) |
EGFR-FISH-NP Median (95% CI) |
|
|---|---|---|---|---|---|
|
| |||||
| All Patients
|
OS
|
Cetuximab Arm | 10·9 (9·5–12·0)
|
13·4 (11·5–14·8)
|
9·5 (8·2–10·9)
|
| Control Arm | 9·2 (8·7–10·3) | 9·8 (8·7–12·1) | 8·9 (8·4–10·2) | ||
|
| |||||
|
|
PFS | Cetuximab Arm
|
4·6 (4·2–5·2)
|
5·4 (4·5–5·7)
|
4·4 (4 0–4 8)
|
| Control Arm | 4·5 (4 2–5·1) | 4·8 (3·9–5·5) | 4·3 (4·1–4·9) | ||
|
| |||||
| Bevacizumab | OS | Cetuximab Arm | 12·7 (10·9–13·4) | 15·9 (13·4–19·1) | 10·9 (9·1–12·5) |
| Control Arm | 11·6(10·5–13·6) | 13·2 (11·2–19·9) | 11·2 (9·9–13·0) | ||
| PFS | Cetuximab Arm | 5·7 (5·3–6·1) | 6·2 (5·7–8·0) | 5·4 (4·6–5·8) | |
| Control Arm | 5·9 (5·5–6·7) | 6·7 (5·7–8·0) | 5·6 (5·3–6·4) | ||
|
| |||||
| No Bevacizumab
|
OS | Cetuximab Arm
|
9·2 (8·1–10·9)
|
11·2 (8·6–12·9)
|
8·4 (7·2–10·3)
|
| Control Arm | 8·1 (7·2–8·7) | 8·7 (5·9–10·2) | 8·1 (7·2–8·6) | ||
|
| |||||
|
|
PFS | Cetuximab Arm
|
4·1 (3·5–4·4)
|
4·4 (3·8–5·2)
|
4·0 (3·1–4·3)
|
| Control Arm | 3·8 (3·1–4·2) | 3·7 (2·8–4·6) | 4·0 (3·0–4·2) | ||
|
| |||||
| Squamous Cell Histology | OS | Cetuximab Arm | 9·6 (8·2–11·5) | 11·8 (8·6–13·5) | 8·5 (6·4–10·4) |
| Control Arm | 8·0 (7·1–8·8) | 6·1 (4·2–8·7) | 8·4 (7·2–9·9) | ||
|
|
PFS | Cetuximab Arm
|
4·2 (3·7–4·6)
|
4·5 (3·8–5·2)
|
4·0 (2·9–4·5)
|
| Control Arm | 3·7 (2·8–4·3) | 2·8 (2·6–4·1) | 4·1 (3·0–4·8) | ||
|
| |||||
| Non-Squamous Histology
|
OS | Cetuximab Arm
|
11·2 (9·6–12·5)
|
14·3 (11·4–17·7)
|
10·0 (8·4–11·7)
|
| Control Arm | 10·2 (9·0–11·2) | 12·1 (9·7–13·8) | 9·2 (8·5–10·8) | ||
|
| |||||
|
|
PFS | Cetuximab Arm
|
5·1 (4·4–5·4)
|
5·7 (5·2–6·5)
|
4·6 (4·1–5·3)
|
| Control Arm | 4·9 (4·3–5·5) | 5·5 (4·6–6·1) | 4·5 (4·1–5·3) | ||
Footnote: mos = months, NP = non-positive (includes EGFR FISH negative and EGFR FISH status unknown).
The second co-primary objective was a comparison of OS between the cetuximab-containing and control arms within the entire study population. There was no difference in the survival distribution between the arms (HR = 0·93 [0·83–1·04], P = 0·22). The median OS was 9·2 months (95% CI: 8·7–10·3) in the control arm and 10·9 months (95% CI: 9·5–12·0) in the cetuximab-containing arm (Table 2). In addition, PFS was not different between the arms (HR = 0·99 [0·88–1·10], P = 0·83). The median PFS was 4·5 months (95% CI: 4·2–5·1) in the control arm and 4.6 months (95% CI: 4·2–5·2) in cetuximab-containing arm. RRs were not significantly different between the arms (36% control arm versus 42% cetuximab-containing arm, P = 0·06)
Secondary objectives included a comparison of treatment arm outcomes within the BT and BN subsets. These analyses are summarized in Figure 2. In BT patients, there was no difference in OS (P = 0·72) or PFS (P = 0·62) between the arms. In addition, among BT patients with EGFR-FISH+ cancers, there was no difference in OS (P = 0·43) or PFS (P = 0·74) between the arms. Among BN patients, there was no statistically significant difference in OS (P = 0·19) or PFS (P = 0·49) between the arms. In addition, among the subpopulation of BN patients with EGFR-FISH+ cancers, the difference in OS between the arms was non-significant (P = 0·07) and the difference in PFS between the arms was not statistically significant (P = 0·16).
Prior to analysis of the data, the study team added a pre-specified analysis of OS or PFS and treatment among patients with SC histology, both overall and stratified by EGFR FISH status based on results from the SQUIRE trial.18 In the subpopulation of patients with SC histology, there was no difference in OS (P = 0·17), PFS (P = 0·29) or RR (35% versus 39%, P = 0·42) between the treatment arms. However, among the EGFR-FISH+ subpopulation with SC histology, OS was significantly improved in the cetuximab-containing arm compared with the control arm (P = 0·01) with a median OS of 6·1 months (95% CI: 4·2–8·7) in the control arm and 11·8 months (95% CI: 8·6–13·5) in the cetuximab-containing arm. There was not a significant difference in PFS between the arms in this subgroup (P = 0·06) and the respective medians were 2·8 (95% CI: 2·6–4·1) and 4·5 (95% CI: 3·8–5·2) for the control arm and the cetuximab-containing arm. RRs were not significantly different between treatment arms among the EGFR-FISH+ subpopulation with SC histology (P = 0·46). RRs were not significantly different between the treatment arms stratified by SC and non-SC histology (SC: P = 0·42 and non-SC: P = 0·09). In addition, RRs were not significantly different between treatment arms among the EGFR-FISH+ subpopulation with non-SC histology (P = 0·72). The HRs and associated CIs from all of the primary and sub-group analyses are graphically summarized in forest plots in Figure 3. Response rates from the overall population and sub-group analyses and associated statistics are summarized in Table 3.
Table 3.
Response rates in subset of patients with measureable disease (per RECIST) at baseline
| Analysis Group | Outcome | All Patients | EGFR FISH-Positive | EGFR FISH Non-Positive |
|---|---|---|---|---|
| All Patients | Cetuximab Arm RR, 95% CI | 257/617 = 42% (38% – 46%) | 87/187 = 47% (39% – 54%) | 170/430 = 40% (35% – 44%) |
| Control Arm RR, 95% CI | 227/623 = 36% (33% – 40%) | 82/191 = 43% (36% – 50%) | 145/432 = 34% (29% – 38%) | |
| p-value | 0.06 | 0.48 | 0.07 | |
| Bevacizumab | Cetuximab Arm RR, 95% CI | 126/266 = 47% (41% – 53%) | 44/83 = 53% (42% – 64%) | 82/183 = 45% (38% – 52%) |
| Control Arm RR, 95% CI | 118/255 = 46% (40% – 52%) | 46/75 = 61% (50% – 72%) | 72/180 = 40% (33% – 47%) | |
| p-value | 0.80 | 0.29 | 0.35 | |
| No Bevacizumab | Cetuximab Arm RR, 95% CI | 131/351 = 37% (32% – 42%) | 43/104 = 41% (32% – 51%) | 88/247 = 36% (30% – 42%) |
| Control Arm RR, 95% CI | 109/368 = 30% (25% – 34%) | 36/116 = 31% (23% – 39%) | 73/252 = 29% (23% – 35%) | |
| p-value | 0.03 | 0.11 | 0.11 | |
| Squamous Cell Histology | Cetuximab Arm RR, 95% CI | 59/150 = 39% (32% – 47%) | 23/50 = 46% (32% – 60%) | 36/100 = 36% (27% – 45%) |
| Control Arm RR, 95% CI | 54/155 = 35% (27% – 42%) | 21/54 = 39% (26% – 52%) | 33/101 = 33% (24% – 42%) | |
| p-value | 0.42 | 0.46 | 0.62 | |
| Non-Squamous Histology | Cetuximab Arm RR, 95% CI | 198/467 = 42% (38% – 47%) | 64/137 = 47% (38% – 55%) | 134/330 = 41% (35% – 46%) |
| Control Arm RR, 95% CI | 173/468 = 37% (33% – 41%) | 61/137 = 45% (36% – 53%) | 112/331 = 34% (29% – 39%) | |
| p-value | 0.09 | 0.72 | 0.07 |
Footnote: RR = Response rate; CI = Confidence Interval
Grade 5 adverse events were higher in the cetuximab-containing arm (4·4% BN and 6·0% BT) compared to the control arm (2·5% BN and 1·5% BT). Grade 3/4 adverse events occurred more frequently in the BT group (76·3% cetuximab BT and 67·4% control BT group) compared to patients who did not receive bevacizumab (66·5% cetuximab BN and 55·6% control BN group) (Table 4). As expected, 90% of patients with cetuximab experienced some sort of skin rash. Forty-three percent of patients who progressed on the control arm (254/594), and 44% (257/590) on the cetuximab arm reported receiving therapy after progression on this study. Post-progression therapy appears to be balanced between the arms.
Table 4.
Treatment-related Adverse Events, Excluding Events with Maximum Grade < 4 and Occurring in < 10% of Patients
| Cetuximab Arm, No Bevacizumab (N=361) | Cetuximab Arm, Bevacizumab (N=266) | Control Arm, No Bevacizumab (N=365) | Control, Bevacizumab (N=267) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | Grade | |||||||||||||||||||||||||||||
| 1–2 | 3 | 4 | 5 | 1–2 | 3 | 4 | 5 | 1–2 | 3 | 4 | 5 | 1–2 | 3 | 4 | 5 | |||||||||||||||||
| Hematologic | ||||||||||||||||||||||||||||||||
| Hemoglobin | 180 | 50% | 32 | 9% | 5 | 1% | 131 | 49% | 10 | 4% | 1 | <1% | 203 | 56% | 30 | 8% | 5 | 1% | 144 | 54% | 21 | 8% | 4 | 1% | ||||||||
| Neutrophils | 38 | 11% | 62 | 17% | 59 | 16% | 47 | 18% | 32 | 12% | 57 | 21% | 48 | 13% | 38 | 10% | 47 | 13% | 41 | 15% | 29 | 11% | 44 | 16% | ||||||||
| Platelets | 105 | 29% | 12 | 3% | 10 | 3% | 76 | 29% | 16 | 6% | 5 | 2% | 113 | 31% | 22 | 6% | 8 | 2% | 100 | 37% | 17 | 6% | 4 | 1% | ||||||||
| Leukocytes | 94 | 26% | 57 | 16% | 11 | 3% | 76 | 29% | 28 | 11% | 7 | 3% | 104 | 28% | 31 | 8% | 10 | 3% | 73 | 27% | 25 | 9% | 8 | 3% | ||||||||
| Lymphopenia | 52 | 14% | 30 | 8% | 5 | 1% | 39 | 15% | 20 | 8% | 5 | 2% | 50 | 14% | 21 | 6% | 3 | <1% | 39 | 15% | 12 | 4% | 3 | 1% | ||||||||
| Febrile neutropenia | 14 | 4% | 4 | 1% | 9 | 3% | 2 | <1% | 6 | 2% | 3 | <1% | 2 | <1% | 13 | 5% | 3 | 1% | ||||||||||||||
| Non-Hematologic | ||||||||||||||||||||||||||||||||
| Acne/Rash | 243 | 67% | 19 | 5% | 180 | 68% | 33 | 12% | 24 | 7% | 1 | <1% | 31 | 12% | ||||||||||||||||||
| Fatigue | 216 | 60% | 36 | 10% | 4 | 1% | 152 | 57% | 39 | 15% | 2 | <1% | 209 | 57% | 41 | 11% | 1 | <1% | 181 | 68% | 30 | 11% | 2 | <1% | ||||||||
| Neuropathy-sensory | 178 | 49% | 21 | 6% | 134 | 50% | 14 | 5% | 152 | 42% | 32 | 9% | 2 | <1% | 157 | 59% | 22 | 8% | 1 | <1% | ||||||||||||
| Dermatology-other | 199 | 55% | 5 | 1% | 163 | 61% | 9 | 3% | 173 | 47% | 153 | 57% | ||||||||||||||||||||
| Hypomagnesemia | 157 | 43% | 13 | 4% | 6 | 2% | 143 | 54% | 8 | 3% | 1 | <1% | 93 | 25% | 2 | <1% | 69 | 26% | 1 | <1% | 3 | 1% | ||||||||||
| Nausea/Vomiting | 153 | 42% | 19 | 5% | 123 | 46% | 13 | 5% | 150 | 41% | 11 | 3% | 126 | 47% | 17 | 6% | ||||||||||||||||
| Myalgias | 120 | 33% | 13 | 4% | 95 | 36% | 17 | 6% | 1 | <1% | 112 | 31% | 23 | 6% | 99 | 37% | 21 | 8% | ||||||||||||||
| Anorexia | 109 | 30% | 15 | 4% | 1 | <1% | 77 | 29% | 9 | 3% | 100 | 27% | 13 | 4% | 98 | 37% | 9 | 3% | ||||||||||||||
| Mucositis | 103 | 29% | 4 | 1% | 91 | 34% | 6 | 2% | 33 | 9% | 2 | <1% | 59 | 22% | ||||||||||||||||||
| Diarrhea | 95 | 26% | 13 | 4% | 89 | 33% | 5 | 2% | 1 | <1% | 78 | 21% | 7 | 2% | 71 | 27% | 8 | 3% | ||||||||||||||
| Constipation | 121 | 34% | 1 | <1% | 92 | 35% | 3 | 1% | 91 | 25% | 3 | <1% | 88 | 33% | 1 | <1% | ||||||||||||||||
| Lung Hemorrhage | 22 | 6% | 1 | <1% | 1 | <1% | 74 | 28% | 1 | <1% | 1 | <1% | 10 | 3% | 1 | <1% | 1 | <1% | 63 | 24% | 2 | <1% | 1 | <1% | ||||||||
| Hypertension | 10 | 3% | 1 | <1% | 1 | <1% | 35 | 13% | 15 | 6% | 10 | 3% | 4 | 1% | 51 | 19% | 23 | 9% | 1 | <1% | ||||||||||||
| Weight Loss | 79 | 22% | 3 | <1% | 70 | 26% | 1 | <1% | 61 | 17% | 3 | <1% | 68 | 25% | 5 | 2% | ||||||||||||||||
| Hypokalemia | 72 | 20% | 16 | 4% | 4 | 1% | 39 | 15% | 14 | 5% | 1 | <1% | 30 | 8% | 12 | 3% | 2 | <1% | 36 | 13% | 6 | 2% | 2 | <1% | ||||||||
| Pulmonary-other | 55 | 15% | 12 | 3% | 4 | 1% | 3 | <1% | 50 | 19% | 13 | 5% | 3 | 1% | 1 | <1% | 51 | 14% | 6 | 2% | 2 | <1% | 4 | 1% | 47 | 18% | 9 | 3% | 1 | <1% | 1 | <1% |
| Taste alteration | 76 | 21% | 67 | 25% | 58 | 16% | 67 | 25% | ||||||||||||||||||||||||
| Hypoalbuminemia | 87 | 24% | 3 | <1% | 59 | 22% | 4 | 2% | 54 | 15% | 7 | 2% | 51 | 19% | 1 | <1% | ||||||||||||||||
| Infection | 43 | 12% | 12 | 3% | 8 | 2% | 3 | <1% | 44 | 17% | 16 | 6% | 3 | 1% | 2 | <1% | 19 | 5% | 27 | 7% | 2 | <1% | 3 | <1% | 24 | 9% | 16 | 6% | 1 | <1% | ||
| Hyperglycemia | 61 | 17% | 8 | 2% | 1 | <1% | 56 | 21% | 4 | 2% | 60 | 16% | 10 | 3% | 1 | <1% | 56 | 21% | 6 | 2% | 3 | 1% | ||||||||||
| ALT | 51 | 14% | 2 | <1% | 1 | <1% | 56 | 21% | 4 | 2% | 1 | <1% | 25 | 7% | 3 | <1% | 23 | 9% | 2 | <1% | ||||||||||||
| Hypocalcemia | 73 | 20% | 7 | 2% | 45 | 17% | 1 | <1% | 34 | 9% | 1 | <1% | 31 | 12% | ||||||||||||||||||
| Hyponatremia | 53 | 15% | 12 | 3% | 1 | <1% | 43 | 16% | 12 | 5% | 2 | <1% | 41 | 11% | 12 | 3% | 1 | <1% | 32 | 12% | 14 | 5% | 2 | <1% | ||||||||
| AST | 50 | 14% | 2 | <1% | 1 | <1% | 51 | 19% | 4 | 2% | 1 | <1% | 24 | 7% | 3 | <1% | 24 | 9% | 3 | 1% | ||||||||||||
| Pruritus | 58 | 16% | 3 | <1% | 47 | 18% | 4 | 2% | 14 | 4% | 21 | 8% | ||||||||||||||||||||
| Dizziness | 52 | 14% | 4 | 1% | 47 | 18% | 3 | 1% | 42 | 12% | 4 | 1% | 1 | <1% | 31 | 12% | 5 | 2% | ||||||||||||||
| Muscle weakness | 34 | 9% | 17 | 5% | 2 | <1% | 35 | 13% | 14 | 5% | 35 | 10% | 10 | 3% | 1 | <1% | 32 | 12% | 9 | 3% | 1 | <1% | ||||||||||
| Alkaline phosphatase | 64 | 18% | 46 | 17% | 1 | <1% | 46 | 13% | 2 | <1% | 32 | 12% | ||||||||||||||||||||
| Cough | 36 | 10% | 2 | <1% | 43 | 16% | 36 | 10% | 1 | <1% | 30 | 11% | ||||||||||||||||||||
| Allergic reaction | 20 | 6% | 16 | 4% | 8 | 2% | 24 | 9% | 12 | 5% | 5 | 2% | 8 | 2% | 6 | 2% | 8 | 3% | 2 | <1% | 1 | <1% | ||||||||||
| Heartburn | 40 | 11% | 40 | 15% | 1 | <1% | 19 | 5% | 33 | 12% | ||||||||||||||||||||||
| Dehydration | 34 | 9% | 20 | 6% | 28 | 11% | 9 | 3% | 2 | <1% | 34 | 9% | 14 | 4% | 1 | <1% | 27 | 10% | 11 | 4% | 1 | <1% | ||||||||||
| Thrombosis/embolism | 3 | <1% | 4 | 1% | 3 | <1% | 4 | 2% | 16 | 6% | 14 | 5% | 2 | <1% | 1 | <1% | 4 | 1% | 1 | <1% | 2 | <1% | 16 | 6% | 5 | 2% | ||||||
| Proteinuria | 6 | 2% | 26 | 10% | 5 | 2% | 1 | <1% | 2 | <1% | 19 | 7% | 4 | 1% | ||||||||||||||||||
| Neuropathy-motor | 34 | 9% | 5 | 1% | 18 | 7% | 4 | 2% | 18 | 5% | 9 | 2% | 19 | 7% | 9 | 3% | ||||||||||||||||
| Nail changes | 36 | 10% | 2 | <1% | 25 | 9% | 2 | <1% | 10 | 3% | 26 | 10% | ||||||||||||||||||||
| GI Pain | 17 | 5% | 5 | 1% | 20 | 8% | 6 | 2% | 12 | 3% | 2 | <1% | 14 | 5% | 4 | 1% | ||||||||||||||||
| Muscle weakness: low. extrem. | 18 | 5% | 6 | 2% | 1 | <1% | 18 | 7% | 2 | <1% | 18 | 5% | 3 | <1% | 21 | 8% | 3 | 1% | ||||||||||||||
| Cardiac-other | 24 | 7% | 5 | 1% | 1 | <1% | 1 | <1% | 17 | 6% | 1 | <1% | 4 | 2% | 1 | <1% | 15 | 4% | 3 | <1% | 1 | <1% | 1 | <1% | 10 | 4% | 1 | <1% | 1 | <1% | ||
| GI Hemorrhage | 3 | <1% | 2 | <1% | 10 | 4% | 2 | <1% | 1 | <1% | 1 | <1% | 11 | 4% | 4 | 1% | 2 | <1% | ||||||||||||||
| Cytokine release syndrome | 12 | 3% | 3 | <1% | 3 | <1% | 12 | 5% | 3 | 1% | 1 | <1% | 8 | 2% | 5 | 2% | ||||||||||||||||
| Pneumonitis | 3 | <1% | 4 | 2% | 4 | 2% | 1 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||
| Cardiac Arrhythmia | 6 | 2% | 2 | <1% | 7 | 3% | 1 | <1% | 6 | 2% | 1 | <1% | 1 | <1% | 1 | <1% | 2 | <1% | 1 | <1% | 2 | <1% | ||||||||||
| Hyperkalemia | 5 | 1% | 2 | <1% | 6 | 2% | 2 | <1% | 8 | 2% | 1 | <1% | 7 | 3% | ||||||||||||||||||
| Confusion | 2 | <1% | 2 | <1% | 1 | <1% | 7 | 3% | 1 | <1% | 2 | <1% | 1 | <1% | ||||||||||||||||||
| Ocular-other | 2 | <1% | 7 | 3% | 1 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||||
| Hypoxia | 4 | 1% | 5 | 1% | 1 | <1% | 2 | <1% | 1 | <1% | 2 | <1% | ||||||||||||||||||||
| Nasal/paranasal reactions | 2 | <1% | 4 | 2% | 1 | <1% | 1 | <1% | 6 | 2% | ||||||||||||||||||||||
| Death, NOS | 6 | 2% | 5 | 2% | 1 | <1% | ||||||||||||||||||||||||||
| GI Perforation | 1 | <1% | 2 | <1% | 2 | <1% | 1 | <1% | 2 | <1% | ||||||||||||||||||||||
| GI-other | 4 | 1% | 1 | <1% | 3 | 1% | 1 | <1% | 1 | <1% | 4 | 1% | 1 | <1% | ||||||||||||||||||
| CNS ischemia | 1 | <1% | 1 | <1% | 2 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||||
| Bronchospasm | 3 | <1% | 2 | <1% | 2 | <1% | 1 | <1% | 3 | 1% | ||||||||||||||||||||||
| Hypoglycemia | 2 | <1% | 1 | <1% | 1 | <1% | 4 | 2% | 1 | <1% | 2 | <1% | 3 | 1% | ||||||||||||||||||
| Neurology-other | 3 | <1% | 1 | <1% | 1 | <1% | 2 | <1% | 3 | <1% | 2 | <1% | ||||||||||||||||||||
| Syndromes-other | 2 | <1% | 2 | <1% | 1 | <1% | ||||||||||||||||||||||||||
| Allergy-other | 2 | <1% | 1 | <1% | 2 | <1% | ||||||||||||||||||||||||||
| GI Ulcer | 1 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||||||
| Hyperuricemia | 2 | <1% | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||
| Aspiration | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Pericardial effusion | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Airway obstruction: bronchus | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Cardiopulmonary arrest | 1 | <1% | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||
| Adrenal insufficiency | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Colitis | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Cardiac ischemia/infarction | 1 | <1% | 1 | <1% | ||||||||||||||||||||||||||||
| Visceral arterial ischemia | 1 | <1% | ||||||||||||||||||||||||||||||
| MAX GRADE ANY AE | 96 | 27% | 143 | 40% | 97 | 27% | 16 | 4% | 43 | 16% | 119 | 45% | 84 | 32% | 16 | 6% | 133 | 36% | 137 | 38% | 66 | 18% | 9 | 2% | 79 | 30% | 110 | 41% | 70 | 26% | 4 | 1% |
One patient receiving Cetuximab + Bevacizumab + chemotherapy experienced syncope of unknown grade
In the control arm, 593 patients died. There were 13 treatment-related deaths: four due to infection/febrile neutropenia, two due to lung hemorrhage, one due to dyspnea, one due to decreased carbon monoxide diffusing capacity (DLCO), one due to DLCO and respiratory failure, one due to respiratory failure, one due dyspnea, asystole, and cardiac arrest, one due to CNS ischemia and one for whom the exact cause of death could not be determined. An additional 10 patients died due to adverse events unrelated to treatment. These include: pneumonitis (n = 1), pulmonary disease (n = 3), cardiac disease (n = 2), infection (n = 1), perforation of the stomach (n = 1), DLCO (n = 1), aorta injury (n = 1), and thrombosis/embolism with cardiac disease (n = 1) (one death attributed to two adverse events). The remaining 570 deaths in this arm were due to disease progression.
In the cetuximab arm, 570 patients died. There were 32 treatment-related deaths: five due to infection, three due to hemorrhage, two due to perforation of the colon, two due to multi -organ failure, two due to DLCO, one due to respiratory failure, one due to cardiac arrest, one due to seizure, one due to a pneumoperitoneum, one due to thrombosis/embolism, one due to thrombosis/embolism in combination with other unidentifiable causes, one due to hypotension, one due to dyspnea, one due to cytokine release syndrome, one due to CNS ischemia, and eight for whom the exact cause of death could not be determined. An additional 13 patients have died due to adverse events unrelated to treatment. These include: CNS ischemia with cardiac ischemia/infarction (n = 1), DLCO (n = 2), thrombosis/embolism (n = 1), thrombotic microangiopathy (n = 1), dyspnea (n = 2), cardiac disease (n = 1), pleural effusion (n = 2), lung hemorrhage (n = 1), pulmonary disease (n = 1), and aspiration (n = 1). The remaining 525 deaths were due to disease progression.
Severe adverse events (SAEs) were defined as all grade 5 and unexpected grade 4 adverse events related to treatment. The cetuximab arm had 59 (9%) patients experience SAEs with allergic reaction (n=8) and death (n=11) being the most common. The control arm had 31 (5%) patients experience SAEs with pulmonary (n=6) and febrile neutropenia (n=5) being the most common. (Appendix page 6)
In terms of safety and tolerability, on the control arm, 632 patients received protocol treatment, with 262 (41%) having at least one dose reduction of one of the agents during the entire course of their protocol treatment. This included 142 (53%) of 267 patients receiving bevacizumab, and 120 (33%) of patients not receiving bevacizumab. Of the 627 patients who received protocol treatment on the cetuximab arm, 365 (58%) had at least one dose reduction of one of the agents during the entire course of their protocol treatment. This included 188 (71%) of 266 patients receiving bevacizumab, and 177 (49%) of patients not receiving bevacizumab. Patients who discontinued treated for drug-related toxicity are summarized in Figure 1, with detailed causes available in Appendix page 8.
Discussion
In this randomized, multicenter, Phase III trial, the addition of cetuximab to standard chemotherapy did not meet its co-primary objectives of prolonging PFS in the patients with EGFR-FISH+ cancers or OS in the entire population. The scientific premise of this study was based on the hypothesis, and prior preclinical/clinical data, that EGFR antibodies (as opposed to EGFR TKIs) synergize with chemotherapy, and that this synergy is enhanced in patients with EGFR-FISH+ cancers.5, 10, 11
The EGFR pathway plays a role in non-mutated NSCLC as evidenced by the small benefit of erlotinib versus placebo in the 2nd–3rd -line treatment setting.3 However, when EGFR TKIs were evaluated concurrently with chemotherapy in untreated patients, no clinical benefit was seen.7, 19.EGFR monoclonal antibodies represent alternatives for EGFR inhibition in non-mutated NSCLC due to additional effects on receptor internalization and antibody-dependent cellular toxicity not seen with EGFR TKIs.20, 21 Indeed, cetuximab has produced favorable efficacy with platinum-based chemotherapy in early lung cancer studies and is well recognized for its activity in combination with chemotherapy and/or radiotherapy in other tumor types such as colorectal cancer and head and neck cancer.22 Cetuximab produced favorable efficacy with platinum-based chemotherapy in several early phase NSCLC studies.20 Two NSCLC Phase III trials investigating chemotherapy plus cetuximab revealed contradictory results: the FLEX trial met its primary OS endpoint, while the BMS-099 trial did not meet its primary PFS endpoint.8, 9 A meta-analysis of cetuximab trials in NSCLC concluded that the addition of cetuximab to a platinum doublet significantly improved RR, PFS, and OS with a manageable toxicity profile.23 Of additional interest, in the FLEX trial the greatest benefit in OS was achieved in those patients with SC histology cancers, especially those with high EGFR protein expression.24 While SC histology NSCLC rarely harbors EGFR mutation, it is known to have a high incidence of EGFR expression. Following this observation, a recently completed Phase III trial (SQUIRE) in advanced stage SC lung cancer of gemcitabine-cisplatin with or without the newer EGFR monoclonal antibody necitumumab also demonstrated improved OS, resulting in approval of this regimen in the US and Europe.18
The current trial, S0819, was the largest study conducted to definitively evaluate the role of cetuximab in patients with EGFR-FISH+ cancers and in unselected patients with advanced NSCLC. In the unselected patient population, OS was similar between the arms. The median OS and PFS were similar to those in the BMS-099 trial. These results may have been predictable given that no „all-comer’ frontline, randomized, Phase III trial of chemotherapy plus a targeted agent has shown a survival advantage with the exception of the modest survival benefit with bevacizumab plus paclitaxel and carboplatin demonstrated in the E4599 trial.15 Building on this triplet therapy, S0536 sought to evaluate if the addition of cetuximab to the regimen would be safe and enhance efficacy.14 S0536 did indeed demonstrate a non-overlapping and a manageable toxicity profile coupled with a numerically higher RR, PFS, and OS over the triplet therapy garnering further support for cetuximab as an active agent in lung cancer. However, our study did not show a survival benefit of the four-drug regimen. The highly selective patient population enrolled in S0536 may have accounted for the positive findings not confirmed in this larger randomized trial. In our study, a total of 554 (41·5%) patients received chemotherapy and bevacizumab with or without cetuximab.
Success with targeted therapies in lung cancer is largely attributable to identifying a predictive biomarker; therefore, incorporating a biomarker-driven co-primary endpoint was essential to this study. As such, and to the best of our knowledge, S0819 is the first cooperative group, Phase III trial to utilize this strategy. EGFR FISH-positivity was selected as a promising predictive biomarker of outcome for the treatment of advanced NSCLC with EGFR inhibitors based on several trials.16, 25, 26 In the Canadian, Phase III BR·21 study comparing erlotinib with placebo in patients whose disease progressed despite chemotherapy, the authors found statistically significant associations between objective response and polysomy or amplification of EGFR.26 A similar result was ascertained from the Phase III FLEX study, which showed a significant benefit of cetuximab in addition to cisplatin/vinorelbine chemotherapy in extending survival in patients whose cancers exhibited high levels of EGFR.25 As mentioned earlier, EGFR FISH-positivity was associated with significantly longer median OS and PFS in patients treated with cetuximab, carboplatin, and paclitaxel in S0342.13, 26–30
Tissue acquisition was critical for this study and efforts aimed at improving its quality were central to this protocol. Of all tissue specimens received, 91% were determined to be in usable condition, 87% were analyzable for EGFR FISH, and the assay was successful in 94% of specimens. Specific efforts were made to improved rates of usable and analyzable tissue over the course of study. A separate manuscript is in development to describe these efforts and their impact.
Although EGFR FISH was not a predictive marker for the overall patient population in our study, we identified a subpopulation of patients with SC histology in which EGFR FISH-positivity predicted a beneficial response to treatment with cetuximab. As previously stated, SC histology is known to have a high incidence of EGFR expression.28 In this select group of patients, the addition of cetuximab to chemotherapy improved OS (HR = 0·58 [0·39–0·86], P = 0·01) over the control group. These results are very similar to those observed in the EGFR-FISH+ cohort in the SC SQUIRE trial using necitumumab with gemcitabine-cisplatin (HR = 0·79 [0·69–0·92], P = 0·002 for OS and HR = 0·84 [0·72–0·97], P = 0·018 for PFS versus gemcitabine-cisplatin without necitumumab) in which EGFR FISH was assessed by the same methodology in the same laboratory.18 These findings indicate a different predictive mechanism for EGFR monoclonal antibodies compared to EGFR TKIs that could be beneficial in distinct biological subsets.
Cetuximab treatment was associated with an increase in Grade 3/4 adverse events for acne/rash and allergic reaction. However, the safety profile was similar to that reported in other Phase III trials of cetuximab treatment.8, 9 With respect to the quadruple drug combination, the addition of bevacizumab did increase cetuximab toxicity with regard to some events, including fatigue, acne/rash, thrombosis/embolism, hypertension, and myalgias. Future clinical trials investigating combinations with immune checkpoint inhibitors would be an important next step for these agents. Potential limitations of this study are that it was designed and conducted with cetuximab before data with the fully humanized antibody necitumumab were available that recognized that EGFR monoclonal antibodies are likely best directed against SC lung cancer.
In conclusion, there was no significant benefit in adding cetuximab to platinum-based chemotherapy with or without bevacizumab in patients with EGFR-FISH+ cancers or in the unselected intent-to-treat patient population. The trend observed in the subset of patients with EGFR-FISH+ SC carcinoma, though interesting, highlights the need to further characterize subpopulations of patients who may benefit from EGFR inhibitor therapies in the chemotherapy-naïve advanced NSCLC setting.
Research in Context.
Evidence before this study
The treatment of NSCLC has changed significantly in recent years. While many patients benefit from targeted therapy and/or immunotherapy there remain a large number who receive chemotherapy in the frontline setting. Improving upon this is of critical importance and targeting EGFR is one such approach. We searched PubMed for clinical trials published in English from January 1, 2007 to July 30, 2017 using the terms “lung cancer and EGFR”; 515 results were retrieved. Of these, several published after the beginning of our trial accrual were of particular relevance. The Phase II Lung Cancer Cetuximab Study and the Phase III FLEX study demonstrated the benefit of using EGFR antibodies plus chemotherapy over chemotherapy alone in patients with EGFR-positive advanced NSCLC. Subsequent analyses of FLEX revealed the utility of EGFR expression level as a biomarker for survival benefit with cetuximab. Another such biomarker, EGFR FISH-positivity, has shown predictive potential in several prior clinical trials, thus warranting further analysis in larger patient populations. Most recently, these findings have been reinforced by the results of the SQUIRE trial of the anti-EGFR antibody necitumumab plus chemotherapy in patients with EGFR-expressing tumors. However, analyses from other studies of cetuximab plus chemotherapy produced conflicting results, finding no correlation between EGFR expression and outcome. Our study sought to determine the role of EGFR FISH-positivity to predict the efficacy of anti-EGFR antibody therapy with cetuximab in combination with chemotherapy.
Added value of this study
The current Phase III trial is the largest biomarker validation study to date designed to assess the ability of EGFR FISH-positivity to predict outcomes to first-line cetuximab treatment in combination with platinum-based doublet chemotherapy, with or without bevacizumab. The co-primary objectives comparing PFS in EGFR-FISH+ patients and OS in the entire study population were not significantly different between cetuximab and non-cetuximab treatment arms. Among secondary objectives, there were no significant differences in OS or PFS between cetuximab and non-cetuximab treatment arms with or without bevacizumab treatment and stratified by EGFR FISH-positivity. Though EGFR FISH-positivity was not predictive in the overall NSCLC patient population, patients with SC histology who were EGFR-FISH+ experienced longer OS (P = 0·01) with cetuximab treatment than those patients who did not receive cetuximab.
Implications of all the available evidence
Cetuximab added to carboplatin and paclitaxel, with or without bevacizumab, did not significantly extend OS in the overall study population or PFS in the patients with EGFR-FISH+ cancers. The findings of this study, in addition to recent data from the SQUIRE trial, suggest that the biology of SC NSCLC may be fundamentally different from that of non-squamous NSCLC in its responsiveness to EGFR inhibition.
Acknowledgments
The authors would like to acknowledge Dr. John Crowley for his input on the statistical design of the trial and Angela M. Incassati and Yang Zhou on writing and copyediting assistance.
Acknowledgment of financial support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers (SWOG) CA180888, CA180819; (ECOG/ACRIN) CA180820; (Alliance) CA180821; Other: CA180846; CA189830, CA189971, CA189972, CA180818, C189853, CA180835, CA189957, CA189858, CA180834, CA189952, CA189954, CA189860, CA180826, CA189872, CA189953, CA 189808, CA189854, CA180858, CA189829, CA180830, CA189804, CA189997, CA189809, CA189848, CA180828, CA189856, CA189822, CA189821, CA180798, CA189817, CA189960 and in part by Eli Lilly and Company. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Eli Lily and Company. None of the authors are employed by the National Institutes of Health.
Declaration of Interests
PC reports grants from NCI BIQSFP Funding, during the conduct of the study; grants from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from BMS, personal fees from Novartis, personal fees from Guardant Health, from MolecularMD, outside the submitted work; KK reports personal fees from Clovis, grants, personal fees and other from Genentech, personal fees from Boehringer Ingelheim, grants and personal fees from Lilly, grants and personal fees from Transgene, grants and personal fees from Celgene, personal fees and other from Roche, personal fees from AstraZeneca, personal fees from Ariad, other from UpToDate, personal fees from BMS, personal fees from Merck, personal fees from G1 Therapeutics, personal fees from Regeneron, grants from Novartis, grants from EMD Serono, grants from AbbVie, grants from Gilead, grants from Five Prime, outside the submitted work; DG reports grants and other from Lilly and Bristol-Myers Squibb, outside the submitted work; LB reports personal fees from Genentech, outside the submitted work; EK reports other (consulting) from Celgene, Boehringer Ingelheim, Eli Lilly, AstraZeneca, outside the submitted work; FH (relevant COI): Scientific Advisory Board for Lilly, Genentech/Roche, Bristol-Myers Squibb; research grant (through University of Colorado) from Lilly and Bristol-Myers Squibb; co-investigator of patent through University of Colorado: “The Role of EGFR, IHC and Fish for Predicting Outcome to EGFR Inhibitors”; MV-G reports she is a co-inventor in a patent to use
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RH, MR, JM, PM, KO, DG, TS, EK, FH contributed to study design; RH, MR, JM, KK, PG, PC, DG, LB, AK, EK, CB contributed to writing; MR and JM prepared figures; RH, MW, JM, PM, SA, CR, DG, LB, EK, M V-G contributed to data collection; RH, MR, JM, PM, SA, CR, DG, AK, TS, GM, EK, FH, CB, MV-G, KK contributed to data analysis; RH, MR, JM, PG, PM, KO, SA, CR, DG, LB, AK, TS, EK, FH, CB, MV-G, KK contributed to data interpretation; FH responsible for conducting biomarker analysis; RH, PG, KO, SA, CR, DG, LB, GM, EK, KK, TS contributed to provision of study materials/patients; All authors contributed to critical review, editing and revision of manuscript draft, and approval the final version.
EGFR molecular testing for selecting lung cancer patients to targeted therapies; RH, MR, JM, PG, KO, CR, SA, AK, TS, GM, CB have nothing to disclose.
Disclaimers: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Lynch TJ, Sandler AB. Beyond doublet chemotherapy for advanced non-small-cell lung cancer: combination of targeted agents with first-line chemotherapy. Clinical lung cancer. 2009;10(1):20–7. doi: 10.3816/CLC.2009.n.003. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. The New England journal of medicine. 2005;353(2):123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. The Lancet Oncology. 2015;16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(25):5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(6):911–7. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 9.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(21):7413–7. doi: 10.1158/1078-0432.CCR-04-1045. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Kelly K, Chansky K, et al. Phase II selection design trial of concurrent chemotherapy and cetuximab versus chemotherapy followed by cetuximab in advanced-stage non-small-cell lung cancer: Southwest Oncology Group study S0342. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(31):4747–54. doi: 10.1200/JCO.2009.27.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(20):3351–7. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim ES, Moon J, Herbst RS, et al. Phase II trial of carboplatin, paclitaxel, cetuximab, and bevacizumab followed by cetuximab and bevacizumab in advanced nonsquamous non-small-cell lung cancer: SWOG S0536. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8(12):1519–28. doi: 10.1097/JTO.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 16.Redman MW, Crowley JJ, Herbst RS, Hirsch FR, Gandara DR. Design of a phase III clinical trial with prospective biomarker validation: SWOG S0819. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(15):4004–12. doi: 10.1158/1078-0432.CCR-12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 18.Paz-Ares L, Socinski MA, Shahidi J, et al. Correlation of EGFR-expression with safety and efficacy outcomes in SQUIRE: a randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin plus necitumumab versus gemcitabine-cisplatin alone in the first-line treatment of patients with stage IV squamous non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2016;27(8):1573–9. doi: 10.1093/annonc/mdw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(5):785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 20.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2008;19(2):362–9. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 21.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(5):1552–61. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 22.Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32(3):437–53. doi: 10.1016/j.clinthera.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Pujol JL, Pirker R, Lynch TJ, et al. Meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment for advanced non-small cell lung cancer. Lung cancer. 2014;83(2):211–8. doi: 10.1016/j.lungcan.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. The Lancet Oncology. 2012;13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 25.Douillard JY, Pirker R, O’Byrne KJ, et al. Relationship between EGFR expression, EGFR mutation status, and the efficacy of chemotherapy plus cetuximab in FLEX study patients with advanced non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9(5):717–24. doi: 10.1097/JTO.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 26.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer-molecular and clinical predictors of outcome. The New England journal of medicine. 2005;353(2):133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 27.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. Journal of the National Cancer Institute. 2005;97(9):643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(20):3798–807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 29.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(26):4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(28):6838–45. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]


