Abstract
Potential for on-target, but off-tissue toxicity limits therapeutic application of genetically modified T cells constitutively expressing chimeric antigen receptors (CARs) from tumor-associated antigens expressed normal tissue, such as epidermal growth factor receptor (EGFR). Curtailing expression of CAR via modification of T cells by in vitro-transcribed mRNA species is one strategy to mitigate such toxicity. We evaluated expression of an EGFR-specific CAR coded from introduced mRNA in human T cells numerically expanded ex vivo to clinically significant numbers via co-culture with activating and propagating cells (AaPC) derived from K562 pre-loaded with anti-CD3 antibody. The density of AaPC could be adjusted to impact phenotype of T cells such that reduced ratio of AaPC resulted in higher proportion of CD8+ and central memory T cells that were more conducive to electro-transfer of mRNA than T cells expanded with high ratios of AaPC. RNA-modified CAR+ T cells produced less cytokine, but demonstrated similar cytolytic capacity as DNA-modified CAR+ T cells in response to EGFR-expressing glioblastoma cells. Expression of CAR by mRNA transfer was transient and accelerated by stimulation with cytokine and antigen. Loss of CAR abrogated T-cell function in response to tumor and normal cells expressing EGFR. We describe a clinically-applicable method to propagate and modify T cells to transiently express EGFR-specific CAR to target EGFR-expressing tumor cells that may be used to limit on-target, off-tissue toxicity to normal tissue.
Keywords: EGFR, chimeric antigen receptor, mRNA, glioblastoma, artificial antigen presenting cells
Introduction
T cells exhibiting redirected specificity to a tumor-associated antigen (TAA) via genetic modification to enforce expression of a chimeric antigen receptor (CAR) can be adoptively transferred to patients for the purpose of immunotherapy.1, 2 Clinical trials administering CD19-specific CAR+ T cells for investigational treatment of B-cell malignancies have reported persistent normal B-cell aplasia concomitant with tumor responses.3, 4 CD19 is restricted to the B-cell lineage, thus this on-target, off-tissue toxicity is expected and tolerable. Because TAAs on solid tumors which are restricted or absent from normal tissue are rare, the potential of on-target, but off-tissue clinically-significant toxicity restricts broad application of CAR+ T cells.
Glioblastoma is the most common and aggressive primary brain and central nervous system malignancy, with overall survival approximately 1 year from diagnosis with conventional therapy.5 EGFR is aberrantly overexpressed on ~60% of adult primary glioblastoma and contributes to gliomagenesis by promoting cell division, invasion, angiogenesis, and inhibiting apoptosis.6, 7 The relatively homogeneous expression of EGFR in a large proportion of glioblastoma patients renders it an attractive target for therapy over other glioma-associated antigens, such as EGFRvIII.8 However, current methods to target EGFR signaling in glioblastoma, including tyrosine kinase inhibitors (TKI) or monoclonal antibody (mAb) therapy, have been clinically disappointing due to the molecular complexity and heterogeneity of glioblastoma, emergence of drug resistance, and poor penetration of systemically-administered agents across the blood brain barrier.9–11 Furthermore, targeting EGFR can cause toxicity, primarily observed in skin, gastrointestinal system, and kidney, due to its associated distribution on such normal tissues.12 We have shown that EGFR-directed CAR T cells can exert potent in vivo therapeutic effects in murine models of intracerebral gliomas13. To move toward a clinical viable strategy with enhanced safety, we are proposing to further reduce potential toxicity by expressing EGFR-specific CAR as an in vitro-transcribed mRNA.
Modification of T cells to express CAR by mRNA transfer has the potential to temporally limit the targeting capacity of genetically modified T cells due to transient CAR expression and therefore to reduce the potential of sustained killing of normal cells that also express the targeted TAA. Expression of CAR from introduced mRNA has been shown to transiently redirect T-cell specificity to a desired TAA and mediate tumor regression in preclinical murine models of mesothelioma and leukemia and in two patients with mesothelioma.14–16 Propagation of T cells prior to introduction of mRNA species is necessary to achieve clinically relevant numbers for infusion and re-infusion. Furthermore, T-cell activation is apparently a prerequisite for successful introduction of RNA17 and crossing linking CD3 and CD28 using mAbs conjugated to beads has been used to activate T cells prior to mRNA transfer. While such mAbs mediate robust expansion of CD4+ T cells, they may not as effectively numerically expand CD8+ T cells.18, 19 Development of a cell-based artificial platform derived from the erythroid leukemia cell line K562 for ex vivo activation and propagation of expansion T cells has advantages over bead-based approaches, such as endogenous expression of ICAM-1 and LFA-3, and ability to be genetically modified to enforce expression of desired co-stimulatory molecules.18, 20 Enforced expression of CD64, the high-affinity Fc receptor, allows mAbs to be “loaded” on the surface of K562 via Fc binding to CD64 to cross-link CD321, and can sustain the propagation of CD8+ T cells.22–24 Therefore, we sought to transiently express an EGFR-specific CAR by electro-transfer of mRNA to human primary T cells that had undergone numeric expansion by stimulation with OKT3-loaded activating and propagating cells (AaPC) derived from K562.
Materials and Methods
DNA plasmids
GFP under control of a T7 promoter followed by 64 A-T base pairs (pGEM/GFP/A64) was used to in vitro transcribe GFP RNA25. Cetux-CAR is composed of the scFv of cetuximab was fused to the IgG4 hinge/Fc region26, CD28 transmembrane and modified cytoplasmic domains, and CD3-ζ cytoplasmic domain to form a second generation CAR (see Table, Supplemental Digital Content 1, which shows sequence derivation for portions of CAR). Cetux-CAR was human codon optimized (GENEART) and cloned as Sleeping Beauty (SB) transposons under control of hEF1-α promoter, as previously described27. Cetux-CAR was cloned into the pGEM/A64 vector for in vitro transcription under the T7 promoter by replacing GFP from pGEM/GFP/A64 with Cetux-CAR from the SB transposon. Human codon-optimized truncated human EGFR (amino acids 1–668, NP_005219.2) containing extracellular and transmembrane domains was synthesized by GeneArt (Regensburg, Germany) and cloned under expression of hEF1-α promoter followed by F2A cleavable peptide and neomycin phosphotransferase.
Cell lines and propagation
EL4 (2009), NALM-6 (2011), U87 (2012), T98G (2012), LN18 (2012) and A431 (2012) were obtained from ATCC. K562 clone 9 and clone 420 were a kind gift from Dr. Carl June (University of Pennsylvania), obtained in 2007. Human renal cortical epithelial (HRCE) cells were obtained from Lonza in 2012. All cell lines were maintained in Dulbecco’s Modified Eagle Medium (Gibco, Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (HyClone) and 2 mM glutamax (Gibco), except where indicated. K562 clone 4, modified to express tCD19, CD86, CD137L, and a membrane IL-15-GFP fusion protein was received as a kind gift from Carl June, M.D. at the University of Pennsylvania and has been previously described18, 20. To “load” an anti-CD3 antibody, clone OKT3 (ebioscience), to CD64 high affinity Fc receptor, K562 cells are cultured overnight in X-VIVO serum free media (Lonza) with 2% N-acetylcysteine at a density of 1×106 cells/mL. The following day, cells are washed and resuspended at 1×106 cells/mL in X-VIVO media with 2% N-acetylcysteine and irradiated at achieve 100 Gy, then resuspended at 1×106 cells/mL in PBS and OKT3 (eBioscience) is added at a concentration of 1 mg/mL and incubated on roller at 4°C for 30 minutes. Cells are washed again, stained to verify expression of costimulatory molecules and OKT3 by flow cytometry, and cryopreserved. K562 clone 9, modified to express tCD19, CD86, CD137L, and CD64 (produced in collaboration with Dr. Carl June, University of Pennsylvania)18, 20, to co-express truncated CD19, CD86, CD137L, and CD64 (in collaboration with Dr. Carl June, University of Pennsylvania). K562 clone 27 was derived from clone 9 by limiting dilution after genetic modified by SB system to stably express a membrane-bound IL15-IL15Rα fusion protein28. Clone 27 was modified to express truncated EGFR by SB transfection of tErbB1-F2A-Neo/pSBSO. NALM-6, U87, T98G, LN18, and A431 were all obtained from ATCC and cultured as described for cell lines. EL4 were obtained from ATCC and modified to express tCD19-F2A-Neo or tEGFR-F2A-Neo by SB non-viral gene modification using electroporation via Amaxa Nucelofector (Lonza) and primary mouse T cell kit (Lonza) according to manufacturer’s instructions. Transfectants were selected by addition of 1 mg/mL G418 24 hours post electroporation. HRCE were obtained from Lonza, and cultured in complete Renal Growth Media (Lonza, cat# CC-3190) supplemented with recombinant human epidermal growth factor (rhEGFR), epinephrine, insulin, triiodothyronine, hydrocortisone, transferrin, 10% heat-inactivated FBS (HyClone), and 2mM Glutamax-100 (Gibco). All cell line identities were validated by STR DNA fingerprinting in 2012, at the time of the study, using the AmpF_STR Identifier kit according to manufacturer's instructions (Applied Biosystems, cat #4322288). The STR profiles were compared to known ATCC fingerprints (ATCC.org), and to the Cell Line Integrated Molecular Authentication database (CLIMA) version 0.1.200808.29 The STR profiles matched known DNA fingerprints.
Expansion of T cells on OKT3-loaded K562
Peripheral blood mononuclear cells (PBMC) from healthy donors were purchased from Gulf Coast Regional Blood Bank and isolated by Ficoll-Paque (GE Healthcare) and cryopreserved. T cells were cultured in RPMI-1640 (HyClone) supplemented with 10% FBS (HyClone) and 2mM Glutamax (Gibco). Numeric expansion of T cells was achieved by culture with 100 Gy-irradiated K562 clone 4, which expresses 41BBL, CD86, CD64 and a membrane bound form of IL15, loaded with OKT3. aAPC were added at a density of 10:1 or 1:2 (T cells: aAPC) every 7–9 days, and 50 U/mL IL-2 (Aldeleukin) was added every 2–3 days. Media changes were performed throughout culture to maintain density between 0.5–2×106 cells/mL. Inferred cell count was calculated by multiplying fold expansion following a stimulation cycle to the total number of T cells prior to stimulation cycle.
RNA-modification of T cells
CetuxCD28mZ/pGEM-A64 or GFP/pGEM-A64 was digested with SpeI linearize template for in vitro RNA transcription, performed using T7 mMACHINE mMESSAGE Ultra (Ambion, Life Technologies, cat#AM1345) according to manufacturer’s protocol and incubated at 37°C for 2 hours. Following transcription, excess DNA template was degraded by addition of Turbo DNAse at 1 unit/µg DNA template and incubated 30 minutes at 37°C. Transcribed RNA was purified using RNeasy Mini kit (Qiagen). Concentration and purity were determined by spectrophotometry and frozen in single-thaw aliquots at −80°C. T cells expanded via OKT3-loaded AaPC were electro-transferred with mRNA 3–5 days after the second stimulation by suspension in P3 Primary Cell 4D-Nucleofector buffer (Lonza, cat#V4XP-3032) to a concentration of 108/mL. 2×107 T cells were mixed with 3 µg of in vitro transcribed RNA electroporated in Amaxa 4D Nucleofector (Lonza) using program DQ-115, then rested in the cuvette up to 15 minutes. T cells recovered from electroporation in phenol-free RPMI 1640 (HyClone) supplemented with 2mM Glutamax-100 and 20% heat-inactivated FBS for 4 hours, after which 50 U/mL IL-2 and 30 ng/mL IL-21 were added to the T cells. Twenty-four hours after RNA transfer, T cells were analyzed for CAR expression by flow cytometry and functional evaluation.
DNA Modification of T cells
PBMC (Gulf Coast Regional Blood Bank) isolated by Ficoll-Paque were thawed and rested in complete RPMI-1640 for 2 hours, suspended at 2×108/mL in human T cell electroporation buffer (Lonza, cat# VPA-1002), then 100 µL of cell suspension was mixed with 15 µg transposon and 5 µg SB11 transposase in an electroporation cuvette and electroporated via Amaxa Nucleofector (Lonza) using program U-014 for unstimulated human T cells. Following electroporation, cells were immediately transferred to phenol-free RPMI supplemented with 20% heat-inactivated FBS (HyClone), and 2 mM Glutamax-100 (Gibco) to recover overnight. T cells were stimulated with 100 Gy-irradiated tEGFR+ K562 clone 27 artificial antigen presenting cells (aAPC)30 at a ratio of 2 CAR+ T cells:1 aAPC 24 hours after electroporation, continuing every 7–9 days. IL-21 (30 ng/mL) (Peprotech) was added to culture every 2–3 days. IL-2 (50 U/mL) was added to culture after second stimulation every 2–3 days. At day 14, cultures were evaluated for the presence of NK cells, defined as CD3negCD56+ and NK cell depletion was performed if NK cells represented >10% of cell population, by labeling NK cells with CD56-specific magnetic beads (Miltenyi Biotec) and sorting on LS column (Miltenyi Biotec). Flow cytometry of negative flow through containing CAR+ T cells verified successful depletion of NK cell subset from culture. Cultures were evaluated for function when CAR was expressed on >85% of CD3+ T cells, usually following 5 stimulation cycles.
Immunostaining and flow cytometry
Data were collected on FACS Calibur (BD Biosciences) using CellQuest software (version 3.3, BD Biosciences) and analyzed using FlowJo software (version x.0.6, TreeStar). Up to 106 cells were stained with mAbs (see table, Supplemental Digital Content 2, which lists all antibodies used in this study). Surface molecules were stained in FACS buffer (PBS, 2% FBS, 0.5% sodium azide) for 30 minutes in the dark at 4°C. To stain for intracellular Ki-67, T cells were fixed in cold 70% ethanol solution, per manufacturer protocol, and incubated at −20°C for at least 2 hours prior to staining and acquisition.
Multiplex digital profiling of mRNA transcripts
Cells prior to or following expansion were positively sorted for CD4 and CD8 expression by incubating with CD4 and CD8 magnetics beads (Miltenyi Biotec), respectively, and sorting on LS column. 1×106 T cells were lysed in 165 µL of RLT Buffer (Qiagen) and frozen at −80°C in single-thaw aliquots. RNA lysates were thawed and hybridized with multiplexed target-specific, color-coded reporter and biotinylated capture probes at 65°C for 12 hours. Reporter and capture probe pairs were generated from RefSeq accessions to detect mRNA for Vα and Vβ for DTEA and lymphocyte specific transcripts21, 31. Following hybridization, samples were processed in nCounter Prep (NanoString Technologies), and analyzed in nCounter Digital Analyzer (NanoString Technologies). Reference genes were identified that span wide range of RNA expression levels: ACTB, G6PD, OA21, POLR1B, RPL27, RPS13, and TBP and data were normalized to positive-, negative-, and house-keeping genes using nCounter RCC Collector (version 1.6.0, NanoString Technologies). For DTEA, percentage of TCR Vα and Vβ were derived from count data and similarity to input sequences was determined by Spearman correlation, as previously described31. For Lymphocyte-specific CodeSet, a statistical test was developed for digital gene expression profiling in which significant differential gene expression was identified by a combination of p<0.01 and a fold change greater than 1.5 in at least 2/3 pairs, as previously described32. Heat-mapping of normalized values for differentially RNA transcripts was performed by hierarchical clustering and TreeView software, version 1.133.
High-throughput CDR3 deep-sequencing
TCRβ CDR3 regions were amplified and sequenced from DNA extracted from 1×106 T cells (Qiagen DNeasy Blood and Tissue Kit, Qiagen) and carried out on ImmunoSEQ platform (Adaptive Biotechnologies, Seattle, WA), as previously described.34 CDR3 sequences in pre-expanded T cell populations were plotted versus post-expanded populations.
Intracellular cytokine production
T cells were co-cultured with target cells (1:1) for 4–6 hours in the presence of GolgiStop diluted 1:4,000 (BD Biosciences). Positive control T cells were treated with Leukocyte Activation Cocktail (BD Biosciences) diluted 1:1,000. Surface and intracellular cytokine staining were performed using Cytofix/Cytoperm Fixation and Permeabilization kit (BD Biosciences) according to manufacturer’s instructions.
Chromium release assay (CRA)
Specific cytotoxicity was assessed via standard 4-hour chromium release assay, as previously described.27
Statistical analyses
Statistical analyses were performed in GraphPad Prism, version 6.03. Statistical analyses of in vitro assays were undertaken by two-way ANOVA with donor-matching and Tukey’s post-test for multiple comparisons or two-tailed student’s t-test, as indicated in figure legends. To analyze the Nanostring bar-coded data, a statistical test was developed for digital profiling in which significant differential gene expression was identified by a combination of p<0.01 and a fold change greater than 1.5 in at least 2/3 pairs, as previously described.32 Heat-mapping of normalized values for differentially RNA transcripts was performed by hierarchical clustering and TreeView software, version 1.1.33 Analysis of TCRVα and Vβ and CDR3 repertoire changes was performed by Spearman correlation of percentage of Vα and Vβ from normalized count data. Analysis of CDR3 repertoire changes was performed by fitting a non-linear regression to determine best fit slope and 95% confidence intervals for the slope. The slope was considered significantly different from 1 if the 95% confidence interval did not contain a slope from 0.8–1.2. The slopes of the lines were compared by analysis of covariance (ANCOVA). Significance of findings defined as follows: ns = not significant, p>0.05, * p<0.05, ** p<0.01, *** p<0.001, **** p <0.0001.
Results
Numeric expansion of T cells by activating and propagating cells (AaPC)
T cell propagation was undertaken by co-culture with γ-irradiated K562-derived AaPC in the presence of IL-2 (see data, Supplemental Digital Content 3, which shows phenotype of AaPC, and schematic, Supplemental Digital Content 4, which diagrams ex vivo T cell culture conditions). T cells numerically expanded after two stimulation cycles (18 days) with a high density of AaPC (1 T cell to 2 AaPCs) achieved a statistically-superior number of T cells compared with low density of AaPC (10 T cells to 1 AaPC), p<0.0001, Fig. 1A). However, T cells stimulated with low density of AaPC contained an increased proportion of CD8+ T cells (p<0.001) (Fig. 1B). This was accounted by reduced numeric expansion of CD4+ T cells in T cell cultures stimulated with high density AaPC and not an increased numeric expansion of CD8+ T cells in cultures stimulated with low density AaPC (p<0.0001) (Fig. 1C). To determine if the reduced numeric expansion of CD4+ T cells in cultures with low density of AaPC was due to reduced proliferation, we analyzed intracellular expression of Ki-67, a marker of proliferation. Significantly fewer CD4+ T cells expressed Ki-67 when cultured with low density AaPC compared to high density AaPC (p<0.05).(Fig. 1D) Expression of annexin V and propidium iodide (PI) was not different between CD4+ T cells stimulated with low or high density AaPC, indicating no difference in apoptosis (Fig. 1E). These data indicate that recursive additions of T cells with the low density of AaPC results in an inferior total number of T cells, but preferential outgrowth of CD8+ T cells due to reduced proliferation, but not increased apoptosis, of CD4+ T cells..
Fig. 1. Numeric expansion of T cells by AaPC.
(A) Numeric expansion of CD3+ T cells following stimulation with low (10:1) or high (1:2) density of OKT3-loaded K562. Data represented as mean±SD, n=6. (B) Expression of CD4 and CD8 in T cells expanded with AaPC measured by flow cytometry following two stimulation cycles. Data represented as mean±SD, n=6. (C) Numeric expansion of CD4+ and CD8+ T cells following stimulation with AaPC. Data represented as mean±SD, n=6. (D) Ki-67 measured by intracellular flow cytometry 9 days after second stimulation cycle. Data shown as representative histogram and mean±SD, n=3. (D) Viability of cells by flow cytometry for annexin V and PI staining 9 days after second stimulation cycle. Data shown as representative dot plots and mean±SD, n=3* p<0.05, ***p<0.001, ****p<0.0001, two-way ANOVA (Sidak’ss post-test).
T cells propagated with low density AaPC tend to exhibit a memory-like immunophenotype
We assessed how the numeric expansion with low or high density AaPC impacted T-cell genotype by multiplex digital profiling (see table, Supplemental Digital Content 5, which contains the lymphocyte-specific mRNA panel and probe sequences) in T cells sorted for CD4 and CD8 expression. The purity of CD4+ and CD8+ expression after sorting is show in Fig. 2A. T cells harvested from high-density AaPC co-cultures demonstrated increased expression of genes associated with activation, such as CD38 and granzyme A in CD4+ T cells and CD38 and CD56 in CD8+ T cells35, 36 (Fig. 2B). In contrast, CD4+ and CD8+ T cells propagated with low density AaPC showed increased expression of genes associated with central memory-like or naïve-like T cells such as CCR7, CD28, and IL7Rα, and transcription factors Lef1 and Tcf7 associated with Wnt signaling pathway.37, 38 These findings were validated by flow cytometry for co-expression of CCR7 and CD45RA where CCR7+CD45RA+ indicates naïve-like, CCR7+CD45RAneg indicates central memory-like, CCR7negCD45RAneg indicates effector memory-like and CCR7negCD45RA+ indicates effector-like immunophenotype.35 CD4+ and CD8+ T cells numerically expanded with low density AaPC contained less effector memory-like cells), but more T cells with a central memory-like phenotype (see data, Supplemental Digital Content 6). In concordance with an associated increased central memory phenotype, CD4+ and CD8+ T cells expanded with low density AaPC expressed less granzyme B (p<0.01 and p<0.05, respectively) and perforin (p<0.001) when expanded with low density AaPC (Fig. 3A). Regardless of the AaPC density, CD4+ T cells produced equivalent amounts of IFN-γ, TNF-α, and IL-2; however, significantly less CD8+ T cells stimulated with low density AaPC produced IFN-γ (p<0.001) and TNF-α (p<0.05), and significantly more produced IL-2 (p<0.05) (Fig. 3B). Collectively, these data indicate that propagation with the low density of AaPC results in reduced expression of effector molecule granzyme B, perforin, IFN-γ and TNF-α in CD8+ T cells.
Fig. 2. Differential gene expression in T cells stimulated with low or high density AaPC.
(A) Purity of CD4+ and CD8+ T cell populations measured by flow cytometry after positive selection and prior to nCounter analysis. (B) Differential gene expression between CD4+ and CD8+ T cells stimulated with low or high density AaPC measured by multiplexed digital profiling of mRNA species following two cycles of stimulation. Data represented as heat-map of fold difference, n=3.
Fig. 3. T cells propagated with high density AaPC produce more effector cytokines than T cell propagated with low density AaPC.
A) Intracellular staining and flow cytometry for granzyme B and perforin in CD4+ and CD8+ expanded T cells. Data represented as mean percent±SD, n=3. (B) Cytokine production following stimulation with PMA/Ionomycin measured by intracellular cytokine staining and flow cytometry in expanded CD4+ and CD8+ T cells. Data represented as mean percent±SD, n=3. *p<0.05, ***p<0.001, two-way ANOVA (Sidak’s post-test).
Numeric expansion of T cells on AaPC results in some alteration of TCRαβ diversity
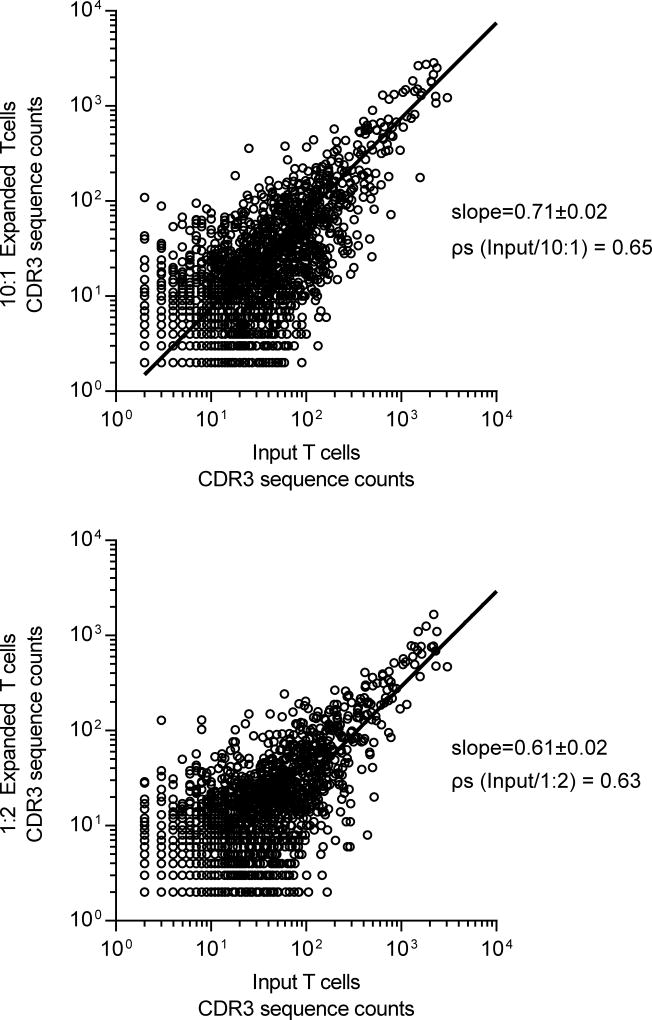
We profiled the diversity of TCRα and TCRβ expression prior to and after propagation with low and high density AaPC. This was accomplished using the direct TCR expression assay (DTEA) which employs a customized panel of bar-coded probes to quantify Vα and Vβ mRNA transcripts in a non-enzymatic digital multiplexed assay.31 TCRVα and TCRVβ diversity was similar in CD4+ and CD8+ T cells pre- and post-expansion and not influenced by density of AaPC as determined by Spearman’s correlation coefficient (ρs) (see data, Supplemental Digital Content 7 and 8). To verify these data, we assayed clonal diversity within the TCR Vβ families by high-throughput sequencing of CDR3 regions in T-cell populations before and after co-culture with AaPC. The slopes of the regressions (low density = 0.71±0.02, 95% confidence interval (CI): 0.67–0.74, ρs=0.65; high density = 0.61±0.02, 95% CI: 0.58–0.64, ρs=0.63) fitting CDR3 diversity for T cells expanded on both low and high density AaPC reveal a moderate change in CDR3 repertoire over culture time (Fig. 4). Furthermore, the slopes of the two lines were significantly different (p<0.0001), indicating greater loss of TCR repertoire in T cells expanded with high compared with low densities of AaPC. In sum, ex vivo propagation of T cells results in some contraction of endogenous TCR repertoire, which was mitigated by expanding T cells with low density of AaPC.
Fig. 4. Diversity of CDR3 sequences in T cells after numeric expansion on AaPC.
CDR3 sequences of TCR Vβ chain were determined by high-throughput sequences on ImmunoSEQ platform. Numbers of each unique sequence before numeric expansion were plotted against the numbers of the same sequence after numeric expansion with low density (10 T cells to 1 AaPC) or high density (1 T cell to 2 AaPC) AaPC. Analysis of CDR3 repertoire changes was performed by fitting a non-linear regression to determine best fit slope and 95% confidence intervals for the slope and Spearman correlation. The slopes of the lines were compared by analysis of covariance (ANCOVA). Data representative of two individual donors.
Electro-transfer of mRNA coding for EGFR-specific CAR into T cells
The electro-transfer conditions were refined by transfer of in vitro-transcribed mRNA encoding GFP. T cells expanded with high density AaPC exhibited decreased transfection efficiency and viability after RNA transfer in a variety of electroporation conditions (see data, Supplemental Digital Content 9). There was improved expression of GFP and viability after two cycles of T-cell stimulation with low density AaPC. Using this protocol, an EGFR-specific CAR that derives its specificity from the scFv of cetuximab (Cetux-CAR) was coded as in vitro transcribed mRNA, electro-transferred into T cells and compared to Cetux-CAR+ T cells genetically modified with DNA plasmids from the SB system. At time of these analyses, the SB-modified T cells were propagated for an average of 28 days on AaPC compared with 18 days for T cells modified with mRNA. Cell surface expression of Cetux-CAR in CD4+ and CD8+ T cells as measured by median fluorescence intensity (MFI) (24 hours after electro-transfer) was not statistically different (p>0.05); however, RNA-modification resulted in wider range in density of CAR expression (Fig. 5A). The average percentage of Cetux-CAR was not statistically different between CD4+ and CD8+ T cells modified with mRNA or DNA (Fig. 5B). CAR+ CD4+ and CD8+ T cells modified with mRNA contained more central memory-like cells (CCR7+CD45RAneg) than DNA-modified CAR+ T cells, but fewer T cells with effector memory-like (CCR7negCD45RAneg) immunophenotype (see data, Supplemental Digital Content 10). While CD4+ Cetux-CAR+ T cells modified by mRNA demonstrated significantly higher expression of the inhibitory receptor programmed death receptor 1 (PD-1) compared with DNA-modified Cetux-CAR+ T cells (p<0.01), both populations of CD8+ Cetux-CAR+ T cells expressed a similar low level of PD-1 (Fig. 5D). Expression of CD57, a marker of senescence39, was similarly low on CD4+ and CD8+ T cells when modified with mRNA or DNA. Expression of the cytotoxic molecules perforin and granzyme B was similar in CD4+ and CD8+ T cells modified by mRNA or DNA (Fig. 5E). In summary, modification of T cells by mRNA resulted in expression of CAR commensurate with DNA modification. The use of low density of AaPC resulted in CD8+CAR+ T cells that are clinically appealing as measured by central memory-like phenotype and low levels of PD-1 and CD57.
Fig. 5. Phenotype of Cetux-CAR+ T cells modified by DNA and mRNA.
(A) Median fluorescence intensity (MFI) of CAR expression in mRNA- or DNA-modified CD4+ and CD8+ T cells determined by flow cytometry for CAR. Data represented as mean±SD, n=8. (B) Proportion of CD4+ and CD8+ in CAR+ T-cells determined by flow cytometry. Data represented as mean±SD, n=8. (C) Expression of PD-1 and CD57 in CD4+ and CD8+ CAR+ T-cells by flow cytometry. Data represented as mean±SD, n=3. (D) Expression of granzyme B and perforin measured by intracellular cytokine staining and flow cytometry in CD4+ and CD8+ CAR+ T-cells. Data represented as mean±SD, n=3. **p<0.01, ****p<0.0001, two-way ANOVA (Tukey’s post-test).
Redirected specificity of T cells for EGFR upon expression of CAR from introduced mRNA
T-cell effector functions were assessed using the EGFR+ glioma cell lines (U87 and T98G, and LN18), epidermoid carcinoma (A431) well-characterized to express high levels of EGFR, and parental EL4 mouse T cells modified to express a truncated wild type EGFR containing only extracellular domains, (designated tEGFR+ EL4).13 Specificity for EGFR was based on using EGFRneg control cell lines, including tCD19+ EL440 and the malignant B-cell tumor NALM-6. To reduce variability, only donors in which DNA- and mRNA-modified CAR+ T cells contained a similar proportion of CD8+ T cells and expressed similar levels of CAR were analyzed (see data, Supplemental Digital Content 10). We observed that CD8+CAR+ T cells modified by mRNA produced IFN-γ or TNF-α in response to all EGFR-expressing cell lines; however, fewer mRNA-modified CAR+ T cells produced cytokine compared with DNA-modified CAR+ T cells. Stimulation with PMA/ionomycin also resulted in reduced production of cytokine from mRNA-modified compared with DNA-modified T cells, thus the reduced IFN-γ production appears due to an inherent reduced capacity of the T cells to produce these cytokines (Fig. 6A). mRNA- and DNA-modified T cells similarly and efficiently lysed tEGFR+ EL4 and A431, however, in response to the three glioma cell lines, DNA-modified CAR+ T cells demonstrated about 10% more cytotoxicity over mRNA-modified CAR+ T cells at low effector to target (E:T) ratios (Fig. 6B). In aggregate, these findings suggest that mRNA-modified CAR+ T cells exhibit redirected specificity for EGFR, but have reduced cytokine production and slightly less cytotoxicity at low E:T ratios relative to DNA-modified CAR+ T cells.
Fig. 6. Redirected specificity of T cells for EGFR upon expression of CAR from introduced mRNA.
(A) Cytokine production of CAR+ T cells was measured by intracellular staining and flow cytometry in CD8+ T cells. Data represented as mean±SD, n=3. (B) Specific cytotoxicity of CAR+ T cells was determined by CRA. Data represented as mean±SD, n=3. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-way ANOVA (Tukey’s post-test).
The physiologic decay of introduced mRNA coding for Cetux-CAR reduces T-cell effector function to EGFR
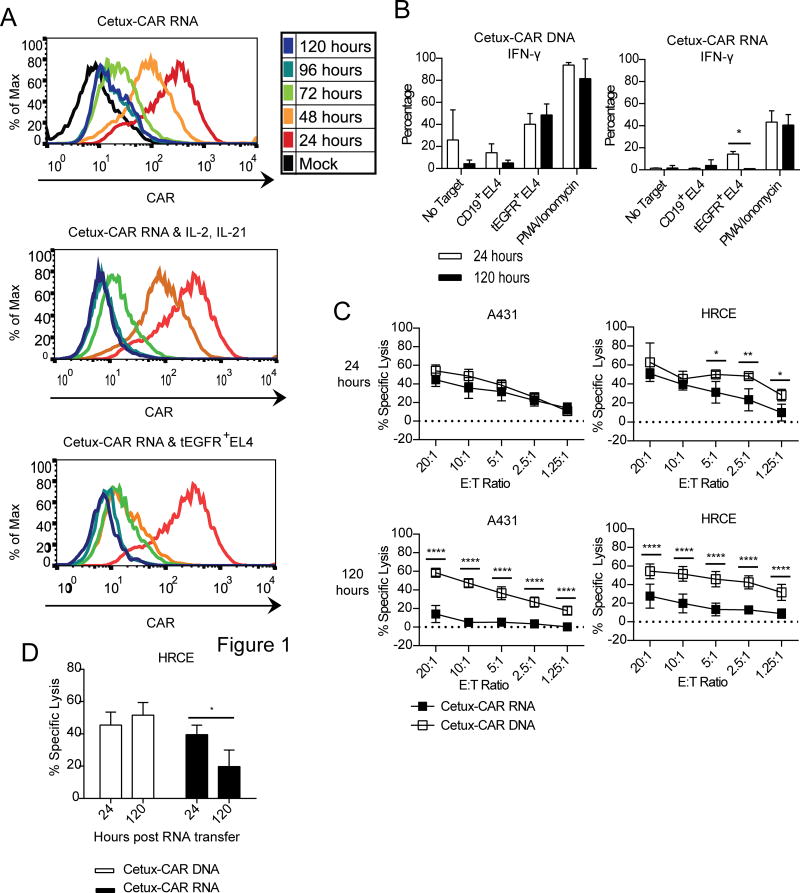
Stability of CAR expression following electro-transfer of mRNA was measured over time by flow cytometry. Expression of Cetux-CAR on mRNA-modified T cells decreased to background levels after 96 hours following electro-transfer (Fig. 7A). Addition of exogenous IL-2 and IL-21 accelerated the loss of CAR expression to background levels after 48 hours (Fig. 7B). Likewise, stimulation of RNA-modified CAR+ T cells with tEGFR+ EL4 cells accelerated the loss of CAR expression to background CAR expression after 24 hours (Fig. 7C). The effect of loss of Cetux-CAR expression on T-cell effector activity was evaluated by measuring cytokine production and specific lysis 24 and 120 hours after electro-transfer of mRNA. While mRNA-modified T cells demonstrated equivalent production of IFN-γ by PMA/ionomycin (positive control) when assessed at both time points, production of IFN-γ in response to tEGFR+ EL4 by genetically modified T cells was abrogated 120 hours after electro-transfer of mRNA (p<0.05) in contrast to DNA-modified CAR+ T cells (Fig. 8A). mRNA-modified, at 24 hours after RNA transfer, and DNA-modified CAR+ T cells exhibited equivalent specific lysis of A431 (the cell line which expressed the highest level of EGFR) and similar low-level cytotoxicity against normal human renal cortical epithelial cells (HRCE), which express low levels of EGFR (see relative levels of EGFR expression, Supplemental Digital Content 10) (Fig. 8B). However, by 120 hours (after mRNA transfer), the DNA-modified T cells mediated significantly higher specific lysis in response to A431 and HRCE (p<0.0001) (Fig. 8C). These data indicate that activity of mRNA-modified T cells in response to EGFR-expressing targets is reduced by loss of CAR expression which is expected to reduce on-target, but off-tissue toxicity.
Fig. 7. The physiologic decay of Cetux-CAR introduced by mRNA.
(A) Expression of CAR measured daily by flow cytometry for CAR with no cytokines or stimulus added to T cells (top), addition of IL-2 (50 U/mL) and IL-21 (30 ng/mL) (middle), addition of tEGFR+ EL4 cells (bottom). Stimuli were added 24 hours after mRNA transfer. Representative histogram, n=3. (B) Production of IFN-γ measured by intracellular staining and flow cytometry in CAR+ CD8+ T cells 24 hours and 120 hours after mRNA transfer. Data represented as mean±SD, n=3. (C) Specific cytotoxicity of CAR+ T cells measured by CRA 24 hours and 120 hours after RNA transfer. Data represented as mean±SD, n=3. (D) Change in specific cytotoxicity of DNA-modified and RNA-modified T cells from 24 hours to 120 hours post-mRNA transfer measured by CRA at E:T ratio of 10:1. Data represented as mean±SD, n=3. *p<0.05, **p<0.01, ****p<0.0001 two-way ANOVA (Tukey’s post-test).
Fig. 8. Transient expression of Cetux-CAR reduces T cell effector function to EGFR.
(A) Production of IFN-γ measured by intracellular staining and flow cytometry in DNA-modified and RNA-modified CD8+ T cells 24 hours and 120 hours after RNA transfer after 4 hour incubation with target cells or PMA/Ionomycin. Data represented as mean ± SD, n=3, * p<0.05, two-way ANOVA (Tukey’s post-test). (B) Specific cytotoxicity of DNA-modified and RNA-modified T cells measured by standard chromium release assay 24 hours and 120 hours after RNA transfer. Data represented as mean ± SD, n=3, * p<0.05, ** p<0.01, **** p<0.0001, two-way ANOVA (Tukey’s post-test). (C) Change in specific cytotoxicity of DNA-modified and RNA-modified T cells from 24 hours post RNA transfer to 120 hours post RNA transfer measured by standard chromium release assay at an effector to target ratio of 10:1. Data represented as mean ± SD, n=3, * p<0.05, two-way ANOVA (Tukey’s post-test).
Discussion
This report supports the contention that transient expression of CAR by electro-transfer of mRNA may reduce the potential for long-term, on-target, off-tissue toxicity of CAR+ T cells directed against TAAs, such as EGFR, with normal tissue expression. The loss of CAR due to the physiologic decay of introduced mRNA may be overcome in design of clinical trials infusing and re-infusing propagated genetically manipulated T cells. This is feasible based on co-culture with AaPC pre-loaded with OKT3. Altering the ratio of AaPC to T cells in co-culture revealed that the low density of AaPC (10 T cells to 1 AaPC) appears to be a preferred method to produce CD8+ T cells for electro-transfer of in vitro-transcribed mRNA coding for CAR. While CD8+ T cells have therapeutic benefit for anti-tumor immunotherapy, this sub-population may require CD4+ T-cell help in vivo to achieve anti-tumor response and form memory.41–43 The ideal ratio of CD4+ to CD8+ T cells is unknown. We revealed that altering the density of AaPC can skew the CD4/CD8 ratio which may be useful for evaluating impact of these T-cell subtypes in adoptive immunotherapy. In addition, the density of AaPC also appears to impact the emergence of T cells with a central memory-like immunophenotype (CCR7+CD45RAneg), which have been shown to improve the anti-tumor efficacy of T-cell therapy.26, 44–46 Future studies will examine the ability of ex vivo expansion with low density AaPC to reprogram stably genetically modified T cells or tumor-infiltrating lymphocytes to sustain a central memory phenotype over in vitro culture time for enhanced persistence in vivo. Furthermore, the adoptive transfer of transiently-modified T cells also provides an opportunity to reconstitute the immune system in recipients, such as those who are lympho-depleted. Our approach to propagate T cells revealed that the diversity and abundance of the TCRVα and TCRVβ repertoires was largely unchanged after addition of AaPC. In aggregate, these data support the use of AaPC to generate T cells for not only the electro-transfer of mRNA, but also as an approach to restore immunity based on outgrowth of T cells with favorable immunophenotype and diversity of TCR repertoire.
One concern regarding the human application of mRNA-modified T cells is that loss of CAR expression will reduce their capacity to target tumor over time. Indeed, sequential injections of T cells modified to transiently express CARs after mRNA transfer could be used to achieve an anti-tumor effect in a disseminated models of mesothelin and neuroblastoma, however the response was not durable in mice, and stably-expressed CAR achieved superior anti-tumor responses.14,47 T cells transiently expressing GD2-specific CAR demonstrated impaired tumor infiltration after systemic administration than T cells stably-expressing GD2-specific CAR in a disseminated model of neuroblastoma and may be partially responsible for reduced efficacy of transiently modified T cells. Optimization of dosing regimen by lymphodepletion prior to infusion and rational dosing intervals based on duration of CAR expression resulted in anti-tumor efficacy approaching that achieved by stably-expressed CAR.15 Other studies have described tunable expression of CAR by increasing the amount of mRNA encoding for CAR during electro-transfer to increase CAR expression, which in turn increases longevity of CAR expression15, 17. Optimal dose of mRNA and longevity of CAR expression to maximize anti-tumor activity while maintaining low toxicity has yet to be determined. We have demonstrated that loss of CAR expression from RNA-modification is accelerated following stimulation with cytokine or antigen. Loss of CAR expression may presumably be enhanced in systemic treatment models of disseminated disease, particularly in the condition of lymphodepletion, which results in increased homeostatic proliferation of infused T cells. Therefore intratumoral delivery to a compartmentalized tumor, such as glioblastoma in the CNS, may demonstrate greater benefit from RNA-modified T cells. Thus, it appears that dosing regimen and route of administration can impact the therapeutic potential of mRNA-modified T cells.
We have previously demonstrated T cells expressing this EGFR-directed CAR, derived from cetuximab, can exert potent in vivo therapeutic effects in murine models of intracerebral gliomas.13 Other TAAs targeted for glioblastoma include IL13Rα2, EGFRvIII, and HER2. A phase I clinical trial of IL13Rα2-specific CAR has demonstrated safety and feasibility of intracranial delivery of CAR-modified T cells, as patients experienced temporary, manageable inflammation of the CNS and partial anti-glioma response was detected.48 Preclinical studies with EGFRvIII-specific CAR and HER2-specific CAR have demonstrated anti-glioma efficacy in murine models of glioblastoma.49–51 Because glioblastoma is heterogeneous, it is likely that targeting multiple TAAs will be necessary to prevent emergence of TAA negative escape variants. Indeed, studies combining HER2 and IL13Rα2-specific CARs reduced the potential for antigen escape.52 Because 60% of GBM expresses wild type EGFR, addition of EGFR-specific CAR to this repertoire increases the proportion of patients that may be effectively treated by one or more of these CARs.
Although EGFR is an attractive target for immunotherapy of GBM as there is no expression of EGFR in healthy brain53, there is a risk of targeting EGFR on extra-cranial normal tissues. We demonstrated that mRNA-modification of T cells to express EGFR-specific CAR transiently redirects cytotoxicity to EGFR, and following decay of CAR cell surface expression, T cells lose specific effector function to EGFR-expressing targets, including normal EGFR-expressing cells. Nonetheless, there could still be normal tissue recognition prior to loss of CAR. These concerns are mitigated by early results from a clinical trial of mRNA-modified T cells for the treatment of mesothelin-expressing tumors that demonstrated the safety of multiple injections of mesothelin-specific CAR+ T cells in most patients, despite normal tissue expression of mesothelin at low levels of mesothelial surfaces.16 Since CAR loss is accelerated following interaction with EGFR intratumoral delivery of Cetux-CAR+ T cells to ensure encounter with tumor prior to possible extravasation and interacting with normal tissues expressing EGFR may reduce the potential for systemic toxicity.
In summary, numeric expansion of T cells prior to mRNA transfer can be achieved through co-culture with defined ratio of OKT3-loaded K562-derived AaPC. The introduced mRNA coding for Cetux-CAR redirects T cells for anti-tumor activity and the physiologic decay results is an appealing method to prevent on-target, but off-tissue deleterious effects. The electro-transfer of mRNA is another approach to the non-viral genetic modification of T cells that along with SB system, is being tested in clinical trials.
Supplementary Material
Acknowledgments
We thank flow cytometry and cellular imaging core facilities at MDACC (supported by CA016672). We thank P. Hackett (University of Minnesota) for his assistance with SB system, E. Gilboa (University of Miami) for use of pGEM/GFP/A64 vector, R. Davis and W. Ma for assistance in analyzing Nanostring data, and J. Moyes and G. McNamara for editing.
Funding: Cancer Center Core Grant (CA16672); RO1 (CA124782, CA120956, CA141303); P01 (CA148600); Center for Clinical and Translational Sciences, which is funded by National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000369 (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health); SPORE (P50 CA136411); Albert J Ward Foundation; Alex Lemonade Stand Foundation; American Legion Auxiliary, Burroughs Wellcome Fund; Cancer Answers; Cancer Prevention and Research Institute of Texas; Charles B. Goddard Foundation of Texas; CLL Global Research Foundation; DARPA (Defense Sciences Office); Department of Defense; Estate of Noelan L. Bibler; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy; Khalifa Bin Zayed Al Nahyan Foundation; Leukemia and Lymphoma Society; Lymphoma Research Foundation; Miller Foundation; Moon Shot program at MDACC, Mr. Herb Simons; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; National Foundation for Cancer Research; Pediatric Cancer Research Foundation; Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy; R.W. Butcher Foundation, University of Texas MD Anderson Cancer Center Sister Institution Network Fund and Moon Shot Fund; William Lawrence and Blanche Hughes Children's Foundation.
Footnotes
Conflict of Interest: Some of the technology described in this presentation was advanced through research conducted at the MD Anderson Cancer Center under the direction of Laurence J.N. Cooper, M.D., Ph.D. In January 2015, the technology was licensed for commercial application to ZIOPHARM Oncology., and Intrexon Corporation in exchange for equity interests in each of these companies. MD Anderson Cancer Center, Dr. Cooper, HG Caruso, H Torikai, S Maiti, L Zhang, H Singh, H Huls, DA Lee, and RE Champlin have equity interest in ZIOPHARM Oncology, Inc., and Intrexon Corporation. On May 7, 2015, Dr. Cooper was appointed as the Chief Executive Officer at ZIOPHARM. Dr. Cooper is now a Visiting Scientist at MD Anderson where he will continue to supervise the development of this technology.
References
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh H, Huls H, Kebriaei P, et al. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunological reviews. 2014;257(1):181–90. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. Journal of neuro-oncology. 2012;107(2):359–64. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 6.Nicholas MK, Lukas RV, Jafri NF, et al. Epidermal growth factor receptor - mediated signal transduction in the development and therapy of gliomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(24):7261–70. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 7.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(4):1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa R, Sugiyama T, Narita Y, et al. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain tumor pathology. 2004;21(2):53–6. doi: 10.1007/BF02484510. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(16):5814–9. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olar A, Aldape KD. Using the molecular classification of glioblastoma to inform personalized treatment. The Journal of pathology. 2014;232(2):165–77. doi: 10.1002/path.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Current molecular pharmacology. 2010;3(1):37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yano S, Kondo K, Yamaguchi M, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer research. 2003;23(5A):3639–50. [PubMed] [Google Scholar]
- 13.Caruso HGHL, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, Lee DA, Heimberger AB, Champlin RE, Cooper LJN. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent anti-tumor activity. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-15-0139. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer research. 2010;70(22):9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett DM, Liu X, Jiang S, et al. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Human gene therapy. 2013;24(8):717–27. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific Chimeric Antigen Receptor mRNA-Engineered T cells Induce Anti-Tumor Activity in Solid Malignancies. Cancer immunology research. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinovich PM, Komarovskaya ME, Wrzesinski SH, et al. Chimeric receptor mRNA transfection as a tool to generate antineoplastic lymphocytes. Human gene therapy. 2009;20(1):51–61. doi: 10.1089/hum.2008.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulos CM, Suhoski MM, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunologic research. 2008;42(1–3):182–96. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Kurlander RJ. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. Journal of translational medicine. 2010;8:104. doi: 10.1186/1479-5876-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(5):981–8. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumaresan PR, Manuri PR, Albert ND, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10660–5. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong W, Ji M, Cao Z, et al. Establishment and characterization of a cell based artificial antigen-presenting cell for expansion and activation of CD8+ T cells ex vivo. Cellular & molecular immunology. 2008;5(1):47–53. doi: 10.1038/cmi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nature biotechnology. 2002;20(2):143–8. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 24.Forget MA, Malu S, Liu H, et al. Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma. J Immunother. 2014;37(9):448–60. doi: 10.1097/CJI.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boczkowski D, Nair SK, Nam JH, et al. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer research. 2000;60(4):1028–34. [PubMed] [Google Scholar]
- 26.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer research. 2006;66(22):10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 27.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer research. 2008;68(8):2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurton LV. Doctoral Dissertation. The University of Texas Health Science Center at Houston; 2014. Tethered IL-15 to augment the therapeutic potential of T cells expressing chimeric antigen receptor: Maintaining memory potential, persistence, and antitumor activity. [Google Scholar]
- 29.Romano P, Manniello A, Aresu O, et al. Cell Line Data Base: structure and recent improvements towards molecular authentication of human cell lines. Nucleic acids research. 2009;37(Database issue):D925–32. doi: 10.1093/nar/gkn730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caruso HG, Hurton LV, Najjar A, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer research. 2015;75(17):3505–18. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Maiti S, Bernatchez C, et al. A new approach to simultaneously quantify both TCR alpha- and beta-chain diversity after adoptive immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(17):4733–42. doi: 10.1158/1078-0432.CCR-11-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor CM, Sheppard S, Hartline CA, et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Scientific reports. 2012;2:249. doi: 10.1038/srep00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins HS, Campregher PV, Srivastava SK, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nature reviews Immunology. 2012;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly-Rogers J, Madrigal-Estebas L, O'Connor T, et al. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Human immunology. 2006;67(11):863–73. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- 37.Willinger T, Freeman T, Herbert M, et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176(3):1439–46. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 38.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature medicine. 2009;15(7):808–13. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 40.Deniger DC, Switzer K, Mi T, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(3):638–47. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamphorst AO, Ahmed R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy. 2013;5(9):975–87. doi: 10.2217/imt.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourgeois C, Veiga-Fernandes H, Joret AM, et al. CD8 lethargy in the absence of CD4 help. European journal of immunology. 2002;32(8):2199–207. doi: 10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 43.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300(5617):339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature medicine. 2007;13(12):1440–9. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 46.Wu F, Zhang W, Shao H, et al. Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy. Cancer letters. 2013;339(2):195–207. doi: 10.1016/j.canlet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Singh N, Liu X, Hulitt J, et al. Nature of tumor control by permanently and transiently modified GD2 chimeric antigen receptor T cells in xenograft models of neuroblastoma. Cancer immunology research. 2014;2(11):1059–70. doi: 10.1158/2326-6066.CIR-14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown CE, Badie B, Barish ME, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T cells in Patients with Recurrent Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(4):972–84. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Science translational medicine. 2015;7(275):275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(2):474–85. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(11):2087–101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. Journal of translational medicine. 2005;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.