Abstract
Purpose
Although recurrences and toxicity occur after vaginal cuff (VC) brachytherapy, little is known about dosimetry due to the inability to clearly visualize the VC on computed tomography (CT). T2-weighted (T2W) magnetic resonance imaging (MRI) is superior to CT in this setting, and we hypothesized that it could provide previously unascertainable dosimetric information.
Methods and Materials
In a cohort of 32 patients who underwent cylinder-based brachytherapy for endometrial cancer with available MR simulation images, the VC was retrospectively contoured on T2W images, and cases were replanned to treat the upper VC to a dose of 7 Gy/fraction prescribed to 5 mm. Relevant dose-volume parameters for the VC were calculated.
Results
T2W MRI identified significant underdosing not observed on CT or T1-weighted imaging. Over two-thirds (69%) of patients had at least 1 cm3 of VC that received less than 75% of the prescription dose and half (50%) of patients had a least 1 cm3 of VC that received less than 50% of the prescription dose. The mean minimum point dose to the VC was 2.4 Gy, or 34% of the intended prescription dose (range: 0.53–6.4 Gy).
Conclusions
We identified previously unreported VC underdosing in over two-thirds of our patients, with most of these patients having volumes of undistended VC that received less than half of the prescription dose. The maximum dimension was along the craniocaudal axis in some patients or left-right/anterior-posterior axis in others, suggesting that suture material may be restricting access to the vaginal apex and that alternative applicators may be needed when the diameter of the apex is larger than the introitus. Additional follow-up will be needed to determine whether underdosing is associated with isolated VC failure or whether low failure rates across the cohort suggest that some patients are being exposed to excessive dose and unnecessary risk of toxicity.
Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States, with 54,870 new cases estimated in 2015 (1). After surgical management, 70% of failures occur in the vaginal cuff (VC) (2). Although external beam radiation therapy (EBRT) decreases risk of these failures (2, 3), brachytherapy alone achieves similar VC control in appropriately selected patients with less gastrointestinal and genitourinary toxicity (4–6).
Unique dosimetric challenges exist in brachytherapy for endometrial cancer given that the VC is both the clinical target volume (CTV) and an organ at risk. Although trials of vaginal brachytherapy required a uniform dose and prescription depth, the dose distribution to the VC CTV has historically been impossible to calculate because computed tomography (CT) or fluoroscopic imaging was used, neither of which allows for adequate delineation of the VC from surrounding tissue (7). Because VC failures are uncommon after brachytherapy (4, 8, 9), it is assumed that the at-risk tissue is being sufficiently covered. However, insufficient coverage might be the cause of failures that do occur, and this can only be investigated using imaging modalities that allow for direct visualization of the VC. Furthermore, inability to directly visualize the vaginal cuff suggests a missed opportunity to minimize toxicity by delivering only the minimum dose necessary to achieve an acceptable rate of VC control.
VC can be visualized using T2-weighted (T2W) magnetic resonance imaging (MRI) (10, 11), which provides better soft tissue contrast than CT (12) and is already used in other gynecologic cancers for defining gross disease (13, 14). This supports a novel application in endometrial cancer treatment. We hypothesize that there is significant post-operative anatomic variation in VC anatomy, and that T2W MRI can be used to provide a full and nuanced quantification of VC dosimetry.
Methods and Materials
The records of 42 patients treated with postoperative high-dose-rate (HDR) brachytherapy using vaginal cylinders at the University of Michigan from August 2013 to January 2015 were reviewed with institutional review board approval. Surgery consisted of total hysterectomy and bilateral salpingoophorectomy with or without lymphadenectomy. Eligible patients underwent vaginal cylinder–based brachytherapy with or without EBRT and had at least 1 simulation MRI. Ten patients who underwent CT-based simulation alone were excluded, leaving a total of 32 patients for analysis. A single patient had poorly differentiated mesonephric carcinoma, whereas all others had endometrial histologies. Eighty-seven MRI volumes were analyzed.
All patients were discussed at our institution’s multi-disciplinary gynecology oncology tumor board. Adjuvant radiation therapy recommendations were based on American Society for Radiation Oncology (ASTRO) and National Comprehensive Cancer Network (NCCN) guidelines, with exceptions made as appropriate based on other clinical considerations.
Vaginal brachytherapy
After surgery, patients underwent a pelvic examination to assess the diameter and length of the VC and ensure adequate VC healing before brachytherapy initiation. When EBRT was recommended, it was delivered before brachytherapy to a dose of 45 to 50 Gy in 1.8- to 2-Gy fractions using either a 4-field 3-dimensional (3D) conformal plan or intensity modulated RT (IMRT). MRI simulations were performed for each brachytherapy fraction to evaluate cylinder positioning. A specialized brief with a ring-locking mechanism was used to immobilize the cylinder. The cylinder angle was dictated by patient anatomy. A Foley catheter was placed, and the bladder was emptied before treatment. Rectal preparation was not used. Adjustments were made as needed to minimize air gaps between the cylinder and soft tissue. Brachytherapy was delivered in five 6-Gy fractions prescribed to the cylinder surface for brachytherapy alone and two 6-Gy fractions for brachytherapy after EBRT. In order to minimize the effects of anisotropy, a specialized stump cylinder was used that allowed the source to lie in closer proximity to vaginal apex (Varian Medical Systems).
For the first 25 patients, the soft tissue of the VC was not prospectively examined, as this was not part of standard practice. A preliminary analysis was performed on the first 25 patients, which revealed areas of VC that were not in contact with the vaginal cylinder, herein referred to as “undistended VC.” Dosimetric calculations demonstrated that the presence of undistended VC led to significant underdosing. Based on this finding, practice was modified for subsequent patients based on identification of the VC at the time of simulation. Applicator adjustments were made as needed, including repositioning the original applicator or replacing a standard cylinder with a stackable cylinder that extended applicator length or increased applicator diameter at the vaginal apex.
The first 3 patients had CT scans performed immediately before or after MRI, and all others underwent MRI alone. MR examinations were performed with the patient in supine position, cylinder in place, on a Skyra model wide-bore 3-T MR machine (Siemens), using an 18-channel anterior surface coil and 4, 8, or 12 elements of a posterior spine coil. Coronal and sagittal T1W and coronal, axial, and sagittal T2W images without fat saturation were obtained for each MRI, with variable slice thickness and matrix size adapted throughout the study period to optimize image quality. Intravenous contrast or antiperistaltic agents were not administered.
Data analysis
Although patients were treated with 6-Gy fractions prescribed to the cylinder surface, we re-normalized the cases for this retrospective analysis using the Post Operative Radiation Therapy in Endometrial Cancer (PORTEC)-2 regimen of 7 Gy prescribed to 5 mm from the cylinder surface (4). We selected this regimen because it represents the most commonly used regimen per the American Brachytherapy Society (ABS) Practice Survey (15), and we felt that a common regimen would be most informative for this primarily descriptive dosimetric study.
The following protocol was developed to contour the VC: (1) a point was placed 4 cm from the top of the cylinder along its central axis, which was used to indicate the most inferior level that the VC would be contoured; (2) a 3-mm rim of tissue was contoured along the cylinder surface beginning at the level of the 4-cm point, extending cranially toward the top of the cylinder and then down the opposite side, stopping at the 4-cm level; (3) when the VC did not directly contact the cylinder, the contour was adjusted to include any soft tissue felt to represent the VC and to exclude any fluid or air pockets between the VC and the cylinder. In patients with large areas of undistended VC, it was understood that this method would overestimate the total volume of at-risk VC because it involved contouring areas of VC that would have been located more caudally down the cylinder if there were no barriers to cylinder insertion. However, this method was the most reproducible, and we determined that informative calculations of underdosing could still be performed as long as absolute (as opposed to relative) volumes of vaginal cuff were calculated.
Means, medians, and standard deviations of the following parameters were reported: (1) volume of VC; (2) minimum point dose to the VC; (3) absolute volume of VC receiving less than 50%; (4) 75% of the prescription dose; and (5) maximum dose to the most underdosed 1 cm3 of VC. Preliminary analysis revealed that the minimum point dose classified all patients as having underdosing, even when MRI did not show evidence of undistended VC. The point dose was therefore felt to be overly stringent, so the maximum dose to the most underdosed 1 cm3 of VC, which is essentially equivalent to the minimum dose excluding the most underdosed 1 cm3, was intentionally chosen as a less sensitive surrogate for the minimum dose. A one-way ANOVA test was performed to assess for a correlation between cylinder size and the underdosing parameters 3 and 4 listed above.
Results
Qualitative assessment of VC
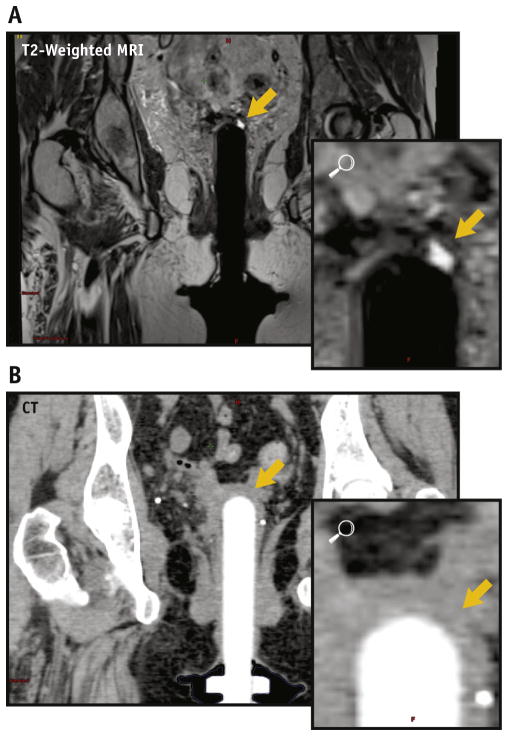
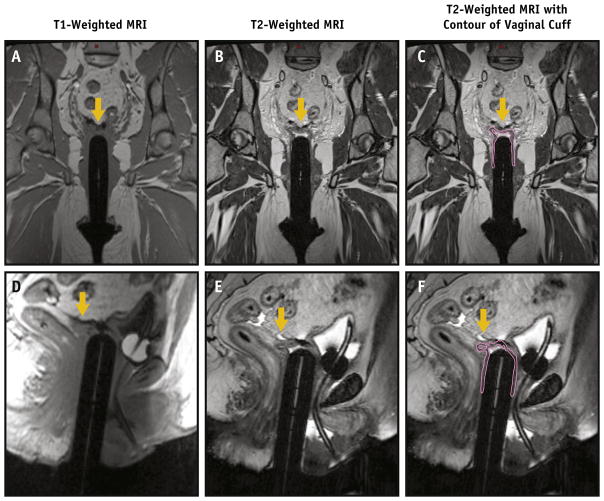
On T2W images, structures had the following appearances: vaginal cylinder-black/hypointense, lubricant, and fluid in vaginal canal-white/hyperintense, and VC-intermediate signal, hyperintense to cylinder but hypointense compared with surrounding pelvic fat. Like other muscular structures in the pelvis, the cuff had a typical striated or multilayered morphology. Normal structures were easily identified, including the bladder, urethra/Foley catheter, and rectosigmoid. Areas of undistended vaginal cuff were well seen in T2W images, but not in CT (Fig. 1) or T1-weighted MR images (Fig. 2).
Fig. 1.
(A) A coronal T2W-MRI image shows fluid (white) between undistended vaginal cuff (yellow arrow) and cylinder (black) that is not delineated on CT; (B) cylinder in white, soft tissue in gray. Abbreviations: CT = computed tomography; MRI = magnetic resonance imaging; T2W = T2-weighted. A color version of this figure is available at www.redjournal.org.
Fig. 2.
Undistended vaginal cuff (yellow arrow) cannot be easily seen on T1-weighted images ([A] coronal and [D] sagittal) but is readily identified on T2-weighted coronal (B, C [coutour added]) and sagittal (E, F [contour added]) images. A color version of this figure is available at www.redjournal.org.
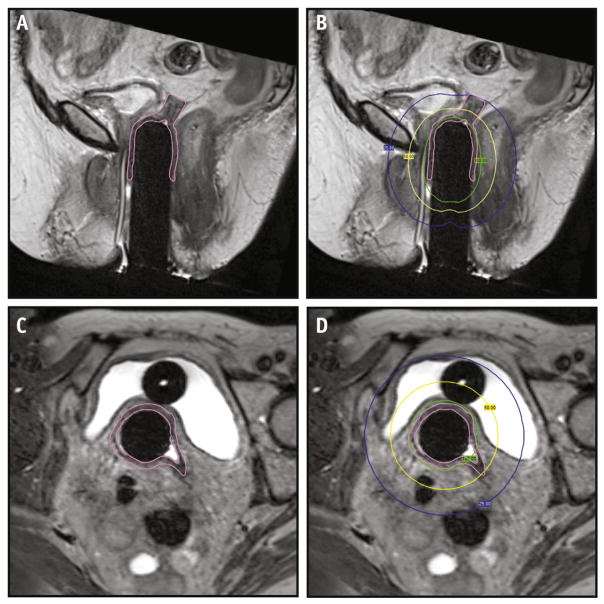
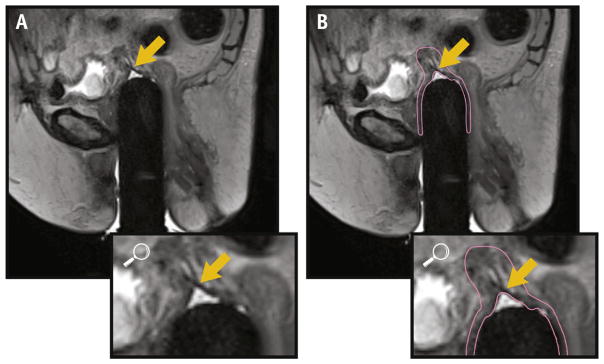
Areas of VC that extended parallel to the long axis of the cylinder were best seen on coronal or sagittal images (Fig. 3A, B) and those that extended in the direction of the radius of the cylinder (dog-ear configurations) were best seen on axial images (Fig. 3C, D). In some cases, suture material constrained the apex of the cuff in a steepled configuration, prohibiting the vaginal cylinder from being inserted further (Fig. 4).
Fig. 3.
A large area of undistended VC is contoured on sagittal T2W MRI (A), extending to the 25% isodose line ([B] blue line). Axial images best identify undistended VC (C) in another patient extending beyond the 50% isodose line ([D] yellow line). The 100% isodose line is shown in green. Abbreviations: MRI = magnetic resonance imaging; T2W = T2-weighted; VC = vaginal cuff. A color version of this figure is available at www.redjournal.org.
Fig. 4.
Sagittal images show an acute angle ([A] arrow) without contour and with contour (B), formed at the convergence of the vaginal walls, with additional VC extending superiorly, suggesting that suture is present, preventing full cylinder insertion. Abbreviation: VC = vaginal cuff.
Quantitative assessment of the VC
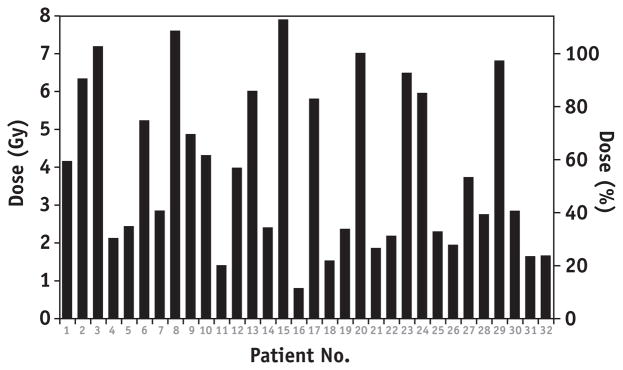
The mean volume of the VC was 18.3 cm3 (range: 9.1–33.7 cm3). The mean minimum point dose to the VC was 2.4 Gy (range: 0.53–6.4 Gy). The mean of the maximum dose to the most underdosed 1 cm3 of VC was 3.96 Gy (range: 0.81–7.9 Gy) (Fig. 5).
Fig. 5.
Maximum dose to the most underdosed 1 cm3 of VC for each patient (surrogate for minimum dose. Abbreviation: VC = vaginal cuff).
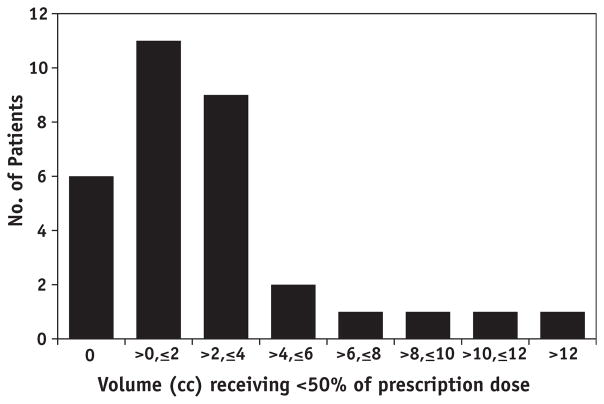
The mean volume of VC that received less than 75% of the 7-Gy prescription dose was 4.2 cm3 (range: 0–18.4 cm3). The mean volume of VC that received less than 50% of the 7-Gy prescription dose was 2.5 cm3 (range: 0–14.0 cm3). Sixteen patients (50%) and 22 patients (69%) had at least 1 cm3 of VC that received less than 75% and 50% of the prescription dose, respectively (Fig. 6). Underdosing as defined by the 50% metric occurred in the dog ears in one patient and at the apex in all others. There was no correlation between cylinder size and the volume receiving less than 50% or 75% of the prescription dose (P=.194 and .094, respectively.) Underdosing (defined by having at least 1 cm3 of VC receiving less than 50% of the prescription dose) persisted in the last 7 patients (5 of 7 [71%]), despite knowledge of the presence of underdosing obtained from analyzing the first 25 patients.
Fig. 6.
Number of patients with a given volume receiving less than 50% of the prescription dose.
Discussion
This study used emerging MRI simulation techniques to evaluate VC dosimetry in adjuvant brachytherapy for endometrial cancer. Our study has several novel findings. First, many patients had large areas of VC that were underdosed. Two-thirds of patients had at least 1 cm3 of VC that received less than 75% of the prescription dose, and half of patients had at least 1 cm3 of VC that received less than 50% of the prescription dose.
Second, there was significant heterogeneity with respect to VC configurations and dosimetry, suggesting that no single technique modification would improve the dose distribution uniformly, while maintaining appropriate doses to organs at risk. It is unknown whether these VC configurations are representative of patients on other studies and whether they will be associated with increased failure rates, but these possibilities should be considered given important implications for failure and toxicity.
We identified a subset of patients who received doses that were lower than anticipated, and must consider the scenario in which this underdosing will not be associated with increased rates of failure. The important implication in this setting is that the minimum therapeutic dose may be significantly less than the intended prescription dose, providing room for dose and toxicity reduction.
Careful analysis of quality of life data after brachytherapy reveals the rationale for investigating dose reduction. Although the side effect profile of vaginal brachytherapy is almost uniformly described as superior to that of EBRT, this is largely secondary to improved bowel and urinary incontinence scores. Long term quality of life data from the PORTEC-2 study show that brachytherapy patients were less likely than EBRT patients to report sex to be enjoyable (50% vs 87%, P=.001) (16). Furthermore, both groups had lower sexual function scores across a variety of domains than age-matched controls, suggesting an opportunity to explore strategies to reduce sexual toxicity in patients who are undergoing brachytherapy (5).
Although identification of toxicity associated with standard doses is an important first step, studies have moved beyond this stage by reporting outcomes in patients treated with lower doses. A Swedish randomized study of low-risk patients compared two brachytherapy doses and found that significant vaginal shortening was avoided in the low-dose arm without compromising VC control (9). In the United States, some centers have adopted lower dose prescriptions (eg, 6 Gy in 5 fractions or 4 Gy in 6 fractions, prescribed to the cylinder surface). These practice changes were quantified in the 2014 ABS Practice Survey (8, 15, 17), and retrospective analyses of these lower dose regimens revealed acceptable rates of VC control (8).
Given the potential for lower doses to reduce toxicity without compromising VC control, the PORTEC investigators have initiated their fourth trial, which compares the current standard of 3 fractions of 7 Gy to a lower dose regimen of three 5-Gy fractions (both prescribed to 5-mm depth) (18). Given radiation therapy’s lack of survival benefit in intermediate-risk patients, they have also included an observation arm. This study will contribute to published information by answering the question of whether dose reduction can decrease toxicity and maintain adequate control. Given that the PORTEC-4 study allows MRI-based simulation, retrospective dosimetric analysis of the VC coverage and correlation to failure rates could be highly informative.
Although the previously described studies help answer the question of whether a lower dose can be safely prescribed to a standard depth across a population, none answer the question of whether individualized dosimetry can further reduce toxicity without compromising control. Our finding of significant interpatient heterogeneity in vaginal cuff anatomy implies that maximum dose reduction requires individual planning. In order to deliver a prespecified VC minimum dose, patients with large areas of undistended VC identified on MRI will require higher standard doses at the cylinder surface to account for dose falloff across the entire VC, whereas patients with a uniformly thin VC that completely contacts the cylinder can receive far lower doses at the cylinder surface. Prospective quality of life analysis will be required to determine whether maximally safe dose reduction results in clinically significant toxicity reduction.
As dose reduction in endometrial cancer brachytherapy is explored, one must consider the possibility of higher failure rates in the absence of 3D imaging. It is possible that, in our cohort, VC control will ultimately prove to be adequate in the setting of dose reduction because imaging closely with MRI ensures that the dose distribution is consistent across fractions, even if some areas receive less than the prescription dose. Similarly, the use of 3D planning and CT simulation with each fraction could have contributed to the low rates of failure reported at other institutions that use lower doses (8). Although some institutions obtain imaging for each fraction, the ABS Practice Patterns Survey shows that 20% of respondents do not use 3D planning and that 17% do not adjust positioning of the cylinder based on pretreatment localization radiographs (15). Even with the use of CT-based planning, there is evidence that air pockets between the vaginal cylinder and mucosa are present in most fractions and that their presence varies from patient to patient and fraction to fraction (19). These air pockets result in reduced dose to the vaginal mucosa. This is relevant given that among respondents who use 3D planning, most (71%) do so only for the first fraction (15). We must therefore consider whether the current trends in post-cylinder placement imaging are sufficient given all of the sources of potential interfraction and interpatient variability.
By obtaining MR simulations for each vaginal brachytherapy fraction, we have gained initial insights into the potential causes of variation in VC anatomy. MR appearance of suture material constraining the cuff in some cases supports further evaluation of surgical technique as a contributor. Surgical technique has changed significantly since PORTEC-2 and other studies were initiated. Each of these studies report results for patients who underwent total abdominal hysterectomy, whereas many patients today undergo laparoscopic surgeries, with or without robotic assistance. Multiple series have indicated higher rates of vaginal dehiscence with laparoscopic surgeries (20). The ABS guidelines acknowledge this in stating that the amount of time necessary for healing of the VC may have increased (17). This has led to increased attention to more durable closure techniques. Some techniques focus on using different types of suture material (21), but other recommendations include “placing the suture at least 1 cm from the VC edge” (22). It is unclear how widely these recommendations have been adopted, but the technique described may inadvertently lead to poor coverage due to the creation of inaccessible areas of VC, a concept that is supported by images and dose calculations in several of our cases.
Further insight into the causes of VC underdosing will help direct efforts to identify solutions for improving brachytherapy technique. Innovations could include the use different cylinder types, including stackable cylinders, inflatable applicators, or custom applicators constructed with 3D printers (23, 24) that might better conform to different anatomic configurations. If underdosing is found to be associated with VC failure, dose escalation could be considered to improve coverage, though this could increase toxicity. For surgical technique, if dehiscence remains a concern, one could consider whether the area of the VC above the suture line can be folded back and sutured to the more proximal VC such that this area, which is presumably at the highest risk given its proximity to the uterus, would ultimately sit closer to the cylinder and within a higher isodose line.
Conclusions
In conclusion, we identified frequent and substantial VC underdosing using MRI simulation and retrospective dosimetry in women undergoing cylinder-based HDR brachytherapy after hysterectomy for endometrial cancer. The clinical implications of these findings necessitate further investigation, including associations with failure rates and toxicity. We suggest that ongoing and future prospective studies of vaginal cylinder–based brachytherapy should consider MR-based VC dosimetry to firmly establish the relationship between dosimetric data, oncologic efficacy, and toxicity-related quality of life outcomes.
Summary.
Using T2-weighted magnetic resonance imaging, we retrospectively identified the novel finding of vaginal cuff underdosing in patients undergoing cylinder-based brachytherapy. It is unclear whether this is a new phenomenon, possibly related to modern surgical technique that has the potential to increase failure rates, or whether this is an old phenomenon, in which case, existing recommendations may lead clinicians to over-treat some patients to compensate for anatomic variation in others.
Footnotes
This work was presented previously in part at the American Brachytherapy Society Annual Meeting, April 10, 2015, Orlando, FL.
Conflict of interest: none.
References
- 1.American Cancer Society. [Accessed October 23 2015];Cancer facts and figures 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- 2.Creutzberg CL, Nout RA, Lybeert ML, et al. Fifteen-year radiation therapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–e638. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiation therapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 5.Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer. 2012;48:1638–1648. doi: 10.1016/j.ejca.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Jolly S, Vargas C, Kumar T, et al. Vaginal brachytherapy alone: An alternative to adjuvant whole pelvis radiation for early stage endometrial cancer. Gynecol Oncol. 2005;97:887–892. doi: 10.1016/j.ygyno.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Prisciandaro J, Lee C, et al. Single or multi-channel vaginal cuff high-dose-rate brachytherapy: Is replanning necessary prior to each fraction? Pract Radiat Oncol. 2014;4:20–26. doi: 10.1016/j.prro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Townamchai K, Lee L, Viswanathan AN. A novel low dose fractionation regimen for adjuvant vaginal brachytherapy in early stage endometrioid endometrial cancer. Gynecol Oncol. 2012;127:351–355. doi: 10.1016/j.ygyno.2012.07.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: A randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. 2005;62:1385–1389. doi: 10.1016/j.ijrobp.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 10.Brown JJ, Gutierrez ED, Lee JK. MR appearance of the normal and abnormal vagina after hysterectomy. AJR Am J Roentgenol. 1992;158:95–99. doi: 10.2214/ajr.158.1.1727367. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Mattrey RF, Stamato S, et al. MRI of the female pelvis using vaginal gel. AJR Am J Roentgenol. 2005;185:1221–1227. doi: 10.2214/AJR.04.1660. [DOI] [PubMed] [Google Scholar]
- 12.Brocker KA, Alt CD, Eichbaum M, et al. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT: Part 1. Strahlenther Onkol. 2011;187:611–618. doi: 10.1007/s00066-011-4001-0. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MB, Dugar N, Davidson SE, et al. Magnetic resonance imaging of primary vaginal carcinoma. Clin Radiol. 2007;62:549–555. doi: 10.1016/j.crad.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image-based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Harkenrider MM, Erickson BA, Viswanathan AN, et al. Preliminary results of the American Brachytherapy Society Survey of Practice Patterns for vaginal brachytherapy for postoperative endometrial cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):S110. [Google Scholar]
- 16.de Boer SM, Nout RA, Jürgenliemk-Schulz IM, et al. Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: Results from the randomized PORTEC-2 trial. Int J Radiat Oncol Biol Phys. 2015;93:797–809. doi: 10.1016/j.ijrobp.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Small W, Jr, Beriwal S, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11:58–67. doi: 10.1016/j.brachy.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Dutch Gynecological Oncology Group. [Accessed October 6, 2015];PORTEC-4: Postoperative radiation therapy for endometrial carcinoma multicenter randomised phase III trial comparing vaginal brachytherapy (two dose schedules) with observation after surgery. Available at: https://www.msbi.nl/promise/LinkClick.aspx?fileticket=hhxkNW1k6xI%3d&tabid=125&portalid=0&mid=581.
- 19.Richardson S, Palaniswaamy G, Grigsby PW. Dosimetric effects of air pockets around high-dose rate brachytherapy vaginal cylinders. Int J Radiat Oncol Biol Phys. 2010;78:276–279. doi: 10.1016/j.ijrobp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Hur HC, Guido RS, Mansuria SM, et al. Incidence and patient characteristics of vaginal cuff dehiscence after different modes of hysterectomies. J Minim Invasive Gynecol. 2007;14:311–317. doi: 10.1016/j.jmig.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Siedhoff MT, Yunker AC, Steege JF. Decreased incidence of vaginal cuff dehiscence after laparoscopic closure with bidirectional barbed suture. J Minim Invasive Gynecol. 2011;18:218–223. doi: 10.1016/j.jmig.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Hur HC. [Accessed October 6, 2015];Vaginal cuff dehiscence after hysterectomy UptoDate. 2015 http://www.uptodate.com/contents/vaginal-cuff-dehiscence-after-hysterectomy?source=search_result&search=vaginal+cuff+dehiscence+after+hysterectomy&selectedTitle=1%7E150.
- 23.Cunha JA, Mellis K, Sethi R, et al. Evaluation of PC-ISO for customized, 3D printed, gynecologic 192-Ir HDR brachytherapy applicators. J Appl Clin Med Phys. 2015;16:5168. doi: 10.1120/jacmp.v16i1.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiebe E, Easton H, Thomas G, et al. Customized vaginal vault brachytherapy with computed tomography imaging-derived applicator prototyping. Brachytherapy. 2015;14:380–384. doi: 10.1016/j.brachy.2014.12.006. [DOI] [PubMed] [Google Scholar]