Abstract
Chronic methamphetamine use poses potentially devastating consequences for directly affected individuals and for society. Lower dopamine D2-type receptor availability has been observed in striata of methamphetamine users as compared with controls, but an analogous comparison of D1-type receptors has been conducted only on postmortem material, with no differences in methamphetamine users from controls in the caudate nucleus and putamen and higher D1 receptor density in the nucleus accumbens. Released from neurons when methamphetamine is self-administered, dopamine binds to both D1- and D2-type receptors in the striatum, with downstream effects on cortical activity. Thus, both receptor subtypes may contribute to methamphetamine-induced alterations in cortical morphology and behavior. In this study, 21 methamphetamine-dependent subjects and 23 healthy controls participated in positron emission tomography and structural magnetic resonance imaging for assessment of striatal D1- and D2-type receptor availability and cortical gray-matter thickness, respectively. Although D2-type receptor availability (BPND) was lower in the methamphetamine group, as shown previously, the groups did not differ in D1-type BPND. In the methamphetamine group, mean cortical gray-matter thickness was negatively associated with cumulative methamphetamine use and craving for the drug. Striatal D1-type but not D2-type BPND was negatively associated with global mean cortical gray-matter thickness in the methamphetamine group, but no association was found between gray-matter thickness and BPND for either dopamine-receptor subtype in the control group. These results suggest a role of striatal D1-type receptors in cortical adaptation to chronic methamphetamine use.
Introduction
It is estimated that 1.7 million people in the United States, over the age of 12, engage in the non-therapeutic use of methamphetamine (MA)1. Despite the prevalence and untoward consequences of MA use2, there is no FDA-approved medication for MA-use disorder. A better understanding of the associated neurobiological features may help guide the development of treatments.
Positron emission tomography (PET) has revealed dopaminergic abnormalities, including lower dopamine transporter availability (i.e., binding potential, BPND)3, 4, higher vesicular monoamine transporter BPND5, and lower striatal dopamine D2-type receptor BPND6, 7, in striata of MA users. Dopamine D1 receptors have been evaluated in MA users only postmortem, showing no differences vs. controls in the caudate nucleus and putamen but higher density in the nucleus accumbens8. In rats, however, administration of a D1 antagonist attenuates cocaine-seeking behavior and behavioral sensitization9, and injection of D1 antagonist into striatum attenuates the MA-induced decrease in monoamine transporter density and cerebral cortical neuronal activity10, 11. Thus, D1-type receptors may influence the untoward effects of MA.
Activities in the direct and indirect pathways from the striatum adjust the output of the basal ganglia12. D1-type receptors are expressed on direct-pathway striatal neurons projecting to the substantia nigra pars reticulata and the globus pallidus pars interna (SNr/GPi), whereas D2-type receptors are expressed on dendrites of indirect-pathway striatal neurons, projecting to SNr/GPi via the globus pallidus pars externa (GPe) and subthalamic nucleus. The two pathways have inhibitory and excitatory effects, respectively, on SNr/GPi, which provides inhibition to the thalamus, regulating glutamatergic excitatory signals to the cortex. Dopamine enhances activity in D1-type receptor-expressing neurons and inhibits neurons expressing D2-type receptors13.
MA-induced striatal dopamine release can influence cortical function. In rats, intra-striatal injection of either a D1- or D2-type receptor antagonist prevented MA-induced c-Fos protein expression in the cerebral cortex11. In contrast, intrastriatal injection of SKF 38393, a D1-type receptor agonist, increased cortical c-Fos expression, a marker of neuronal activity14, but administration of quinpirole, a D2-type agonist, did not; the increased expression was blocked by systemic administration of SCH23390, a D1-receptor antagonist15.
We compared D1-type BPND in MA-dependent and healthy-control subjects, and tested for associations of striatal BPND of D1- and D2-type receptors with cortical structure. Given prior findings6–8, we expected MA users not to differ from controls in D1-type BPND despite lower D2-type BPND. Moreover, we considered the fact that MA promotes dopamine efflux, increasing striatal concentrations of synaptic dopamine to levels adequate to stimulate D1 receptors, which have relatively lower affinity for dopamine than D2 receptors 16. Because activation of D1 receptors on medium spiny neurons of the direct pathway inhibits firing of GABAergic basal ganglia output nuclei, thereby disinhibiting thalamocortical circuitry 17, we expected cortical gray-matter thickness to be associated with cumulative MA use and with striatal D1-type BPND in MA users, reflecting adaptation to MA-induced activation.
Methods
Participants
All procedures were approved by the Institution Review Boards of the University of California Los Angeles and the Greater Los Angeles Veterans Affairs Health Care System. Participants (23 control; 21 MA) were recruited through Internet and newspaper advertisements. PET data presented here have not been published before.
After receiving a complete explanation of the study, participants gave written, informed consent. Exclusion criteria were: use of psychotropic medications; CNS, cardiovascular, pulmonary, hepatic or systemic disease; HIV seropositivity; pregnancy; lack of English fluency; MRI contraindications; and left-handedness. The Structured Clinical Interview or Mini International Neuropsychiatric Interview for DSM-IV was used to determine Axis-I diagnosis. Any Axis-I diagnosis except nicotine dependence was exclusionary for controls. MA-group participants met criteria for MA dependence and had positive urine toxicology for MA at screening; any current Axis-I diagnosis other than MA dependence or nicotine dependence was exclusionary. All participants were deemed physically healthy, according to medical history and physical examination. Participants were instructed to abstain from MA for ≥ 4 days, from marijuana for ≥ 2 days, and from cigarette smoking for 2 h before each PET scan. Abstinence from recent MA use and negative pregnancy status were determined immediately before each PET and MRI scan by urine tests, and the participants reported the duration of their abstinence. Two control-group subjects and seven MA-group subjects had urine tests positive for tetrahydrocannabinol (THC). Due to the long elimination half-life of THC18, these participants were not excluded. PET and MRI scans were performed on separate days within 3 months of one another.
Demographics and drug-use characteristics
Drug use and demographic variables were collected using a survey that queried amount, frequency and duration of MA use, and age of first use. An index of MA exposure was calculated as follows: average use (grams) × frequency (days/month)/30 × duration (months). Information was obtained regarding use of other substances of abuse in the month prior to study, and sleepiness19. Smokers completed the Fagerström Test for Nicotine Dependence (FTND)20.
MA craving was assessed using the Brief Methamphetamine Craving Scale, adapted from the Cocaine Craving Questionnaire-Brief21; with possible scores ranging from 10–70.
Brain scanning
PET data were acquired using a Philips Gemini TF PET-CT, with transverse and axial resolution of 4.8 mm FWHM in the three-dimensional scanning mode. Images were obtained with a 2-mm voxel size (field of view = 128 × 128 × 90). A low-dose CT scan was performed for attenuation correction. Participants were placed in the scanner, in the supine position with the head positioned to avoid movement during scanning. For D1-type receptor scans, emission data were collected for 90 min after a bolus injection of 14.4 ± 1.85 mCi [11C]NNC11222. D2-type receptor data were collected in two 80-min blocks, with a 20-min intermission, after a bolus injection of 5.0 ± 0.37 mCi [18F]fallypride23. Data were reconstructed using the row action maximum likelihood (RAMLA) algorithm24 for each 1-min frame.
Structural scans were acquired on a Siemens Sonata 1.5-T scanner for 39 participants (21 control; 18 MA) and a Siemens Trio 3-T scanner for 5 participants (2 control; 3 MA) due to logistical reasons. T1-weighted data, acquired with a magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence (TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, field of view = 256 × 256 × 160, 1-mm voxels) were used for co-registration with PET images and definition of volumes-of-interest (VOIs) (see below). Analyses of cortical gray-matter thickness and subcortical volumes (hippocampus, amygdala, globus pallidus, thalamus, and striatum) were performed on data from the Sonata 1.5-T scanner (n = 39).
PET data processing
Reconstructed [11C]NNC112-scan data were combined into 23 images, consisting of four 1-min frames, three 2-min frames, and sixteen 5-min frames. The reconstructed data from [18F]fallypride scans were combined into 16 images, each containing data averaged over 10 min. FSL MCFLIRT (FMRIB Centre, Dept. Clinical Neurology, University of Oxford) was used for motion correction25. The images were then co-registered to the MPRAGE image using a 6-parameter, rigid-body spatial transformation (FSL FLIRT).
VOIs were derived from individual MPRAGE images using FSL FIRST26. Caudate and putamen VOIs were combined to constitute a single striatum VOI. The cerebellum was used as the reference region27. A cerebellum VOI, including the hemispheres but not the vermis, was manually created in standard space (MNI152 template) and transformed into native space with FSL FNIRT.
Time-activity data within VOIs were extracted from PET images and imported into PMOD Kinetic Modeling (PKIN) (PMOD Technologies Ltd., Zurich). The simplified reference tissue model (SRTM)28 was used to calculate BPND with time–activity curves from VOIs as follows: CT(t) = R1CR(t) + (k2 − R1k2/(1 + BPND))CR(t) *exp(-k2t/(1 + BPND)) where CT(t) is the total radioactivity concentration in the striatum VOI measured by PET, R1 is the ratio of K1 to K1′ (K1, influx rate constant for the striatum; K1′, influx rate constant for the cerebellum), CR(t) is the radioactivity concentration in the reference region (cerebellum), and * denotes the convolution integral. The parameters R1, k2, and BPND in this model were estimated by a nonlinear curve-fitting procedure.
Morphological analysis
FreeSurfer (version 5.3) was used to assess cortical thickness and subcortical volume from MPRAGE images, as described29–31. The intensity of the images was normalized to remove bias fields, and a hybrid watershed/surface deformation procedure was applied to remove non-brain tissue32. After Talairach transformation, subcortical structures were segmented33, 34. To generate cortical surfaces, white matter was segmented, and errors in white-matter topology were corrected35. A tessellation was formed along the boundary between gray and white matter. The tessellation on the white-matter surface was grown outward towards the intensity gradient separating gray matter from cerebrospinal fluid. One control and two MA participants were excluded because of inaccurate segmentation. Following demonstration of a significant association between D1-type BPND with the global mean of cortical gray-matter thickness in the MA group, associations of D1-type BPND with thickness in individual cortical regions were tested. Frontal, temporal, occipital, and insular regions were selected for analysis because age-related gray-matter deficits in these regions were reportedly accelerated by MA use36.
Statistical analyses
Group differences in demographic data were evaluated by Student’s t or chi-squared tests. ANCOVA was used to evaluate group differences in BPND, cortical gray-matter thickness and subcortical gray-matter volumes. Age, sex and smoking status were included as covariates of no interest because of evidence that they affect dopamine-receptor density37–40 and brain structure41.
Associations of D1- and D2-type BPND in the caudate/putamen VOI with cortical gray-matter thickness and subcortical volumes were evaluated using partial correlation analysis, controlling for age, sex and smoking status. The nucleus accumbens was not included in this analysis because of evidence that the coding of direct and indirect pathways by D1 and D2 receptors, respectively, is not valid for projections from the nucleus accumbens42. Group differences in correlation were tested using Fisher’s r to z transformation. Associations of gray-matter thickness with cumulative MA use and MA craving were evaluated using partial-correlation analysis controlling for age, sex and smoking status. These analyses were conducted using SPSS IBM 19 (IBM, Armonk, NY) with p < 0.05, two-tailed, as the criterion for significance. Results are shown as mean ± SD.
Results
Participant characteristics (Table 1)
Table 1.
Characteristics of Research Participants.
| Control (n = 23) | MA (n = 21) | Group difference | |
|---|---|---|---|
| Age (years) | 33.2 ± 6.37 | 36.3 ± 10.74 | t42 = −1.139, p = 0.26 |
| Male/Female | 11/12 | 15/6 | χ2 = 2.53, p = 0.11 |
| Smoker/Nonsmoker | 14/9 | 119/2 | χ2 = 5.132, p = 0.02 |
| For smokers: | |||
| FTND | 3.4 ± 2.41 | 2.8 ± 2.42 | t31 = 0.752, p = 0.46 |
| Pack-years | 7.6 ± 7.71 | 6.7 ± 7.81 | t31 = 0.325, p = 0.75 |
| Cigarettes/day | 11.2 ± 9.82 | 8.1 ± 6.36 | t31 = 1.123, p = 0.27 |
| Stanford Sleepiness Scale | 1.8 ± 1.20 | 3.1 ± 1.53 | t42 = −3.297, p = 0.002 |
| Days of alcohol use in past month | 7.8 ± 6.87 | 6.9 ± 6.22 | t19 = 0.300, p = 0.77 |
| Days of marijuana use in past month | 3.9 ± 3.24 | 5.2 ± 5.84 | t17 = 0.542, p = 0.60 |
| Days of MA use in past month | - | 24.8 ± 8.08 | - |
| Age of first MA use (years) | - | 22.7 ± 8.40 | - |
| Average amount of current MA use per day (grams) | - | 0.7 ± 0.34 | - |
| Duration of MA use at the current amount (months) | - | 67.6 ± 88.1 | - |
Values are shown as mean ± SD. Group differences were evaluated by unpaired Student’s t-test or chi-squared test.
FTND: Fagerström Test for Nicotine Dependence
Twenty-three control and 20 MA participants had [18F]fallypride scans, and 18 control and 19 MA participants had [11C]NNC112 scans. Brain structure from 20 control and 16 MA participants, who all had [18F]fallypride scans, and from 15 control and 14 MA participants who had [11C]NNC112 scans, was analyzed. The groups did not differ in age or sex distribution, but the MA group gave higher sleepiness scores. The MA group included a higher proportion of smokers. Smokers in the groups did not differ on nicotine dependence, smoking history or cigarettes smoked per day. Nine control and 12 MA subjects reported alcohol use in the month before study. Seven control and 12 MA subjects reported marijuana use in the month before study. MA-group participants reported using MA on 24.8 ± 8.08 days in the month before study, first using MA at 22.7 ± 8.40 years of age. Abstinence time from last MA use to scanning was 10.2 ± 6.22 days, 7.2 ± 4.61 days and 8.50 ± 5.03 days for the [11C]NNC112, [18F]fallypride and MRI scans, respectively.
D1- and D2-type receptor BPND (Figure 1, Table 2)
Figure 1.
Averaged D1- and D2-type receptor BPND maps of participants in control (D1-type: n = 18; D2-type: n = 23) and MA groups (D1-type: n = 19; D2-type: n = 20) (top). No group difference was observed in D1-type BPND in striatum (caudate and putamen collectively) (F1, 32 = 0.050, p = 0.82) whereas striatal D2-type BPND differed between groups reflecting a lower mean value for the MA group (F1, 38 = 13.674, p = 0.001). In a jittered plot at bottom, blue dots represent subjects in the control group and green dots are those in the MA group.
Table 2.
D1- and D2-type receptor availability in striatal subregions.
| Control | MA | Group difference | |
|---|---|---|---|
| D1-type receptor BPND | n = 18 | n = 19 | |
| Caudate | 1.7 ± 0.32 | 1.7 ± 0.46 | 4.7%, F1, 32 = 0.732, p = 0.40 |
| Putamen | 2.0 ± 0.32 | 1.8 ± 0.49 | 7.1%, F1, 32 = 0.021, p = 0.88 |
| Nucleus accumbens | 1.6 ± 0.35 | 1.4 ± 0.38 | 15.3%, F1, 32 = 1.282, p = 0.27 |
| PET tracer: [11C]NNC112 | |||
| Injected dose | 14.4 ± 1.80 | 15.7 ± 2.81 | −8.8%, t35 = −1.614, p = 0.12 |
| Specific activity | 5.3 ± 5.85 | 3.7 ± 4.83 | 29.3%, t35 = 0.884, p = 0.38 |
|
| |||
| D2-type receptor BPND | n = 23 | n = 20 | |
| Caudate | 27.2 ± 3.35 | 21.1 ± 5.72 | 22.3%, F1, 38 = 15.956, p < 0.001 |
| Putamen | 32.8 ± 3.94 | 25.8 ± 7.29 | 21.3%, F1, 38 = 11.686, p = 0.002 |
| Nucleus accumbens | 25.6 ± 3.61 | 19.6 ± 6.31 | 23.5%, F1, 38 = 11.389, p = 0.002 |
| PET tracer: [18F]fallypride | |||
| Injected dose | 5.0 ± 0.39 | 5.0 ± 0.37 | −0.7%, t41 = −0.304, p = 0.76 |
| Specific activity | 10.3 ± 11.18 | 9.7 ± 6.50 | 5.6%, t41 = 0.201, p = 0.84 |
Values are shown as mean ± SD. Group differences were evaluated by ANCOVA controlling for age, sex and smoking status.
The groups did not differ in D1-type BPND in the caudate/putamen [1.9 ± 0.31 (control), 1.8 ± 0.47 (MA); F1, 32 = 0.050, partial η2 (ηp2) = 0.002, p = 0.82] or in striatal subregions: caudate (p = 0.40), putamen (p = 0.88), and nucleus accumbens (p = 0.27). However, D2-type BPND was lower in the MA group than the control group in the caudate/putamen [24.0 ± 6.57 VS. 30.6 ± 3.65; F1, 38 = 13.674, ηp2 = 0.265, p = 0.001] and striatal subregions: caudate (p < 0.001), putamen (p = 0.002), and nucleus accumbens (p = 0.002).
Brain structure
There were no group differences in global mean cortical gray-matter thickness [2.39 ± 0.11 (control), 2.38 ± 0.14 (MA); F1, 31 = 0.001, ηp2 < 0.001, p = 0.98], or in subcortical volumes (hippocampus: F1, 31 = 0.103, ηp2 = 0.003, p = 0.75; amygdala: F1, 31 = 0.653, ηp2 = 0.002, p = 0.43; globus pallidus: F1, 31 < 0.000, ηp2 < 0.000, p = 0.99; thalamus: F1, 31 = 3.590, ηp2 = 0.10, p = 0.07; striatum: F1, 31 = 0.889, ηp2 = 0.028, p = 0.35).
Association of striatal dopamine receptor BPND with brain structure
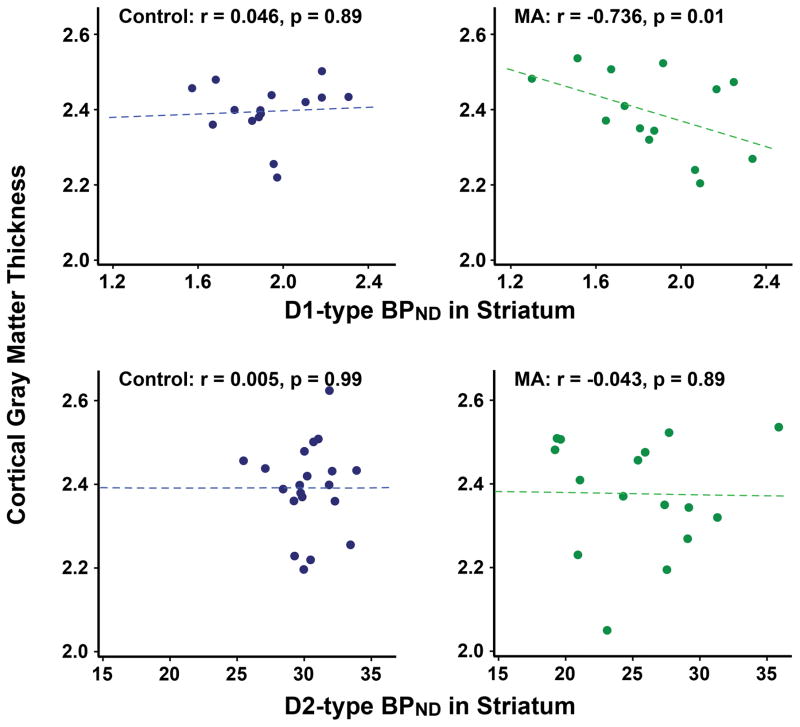
D1-type BPND was negatively correlated with global mean cortical gray-matter thickness in MA subjects (r = −0.736, p = 0.01), but not in controls (r = 0.046, p = 0.89) (Figure 2). The correlation coefficients differed significantly between groups (z = −2.50, p = 0.01). D1-type BPND was negatively correlated with gray-matter thickness in temporal (r = −0.845, p = 0.001) and occipital lobe (r = −0.748, p = 0.008) [significant after Bonferroni correction (i.e., p < 0.0125)], but not in prefrontal (r = −0.494, p = 0.12) or insular (r = −0.034, p = 0.92) regions in MA users. D2-type BPND was not correlated with cortical gray-matter thickness in either group (control: r = 0.005, p = 0.99; MA: r = −0.043, p = 0.89) (Figure 2).
Figure 2.
Striatal D1-type receptor BPND is negatively correlated with global mean cortical gray-matter thickness in the MA group. Scatter plots displaying the correlations of the cortical gray-matter thickness with D1- (top) and D2-type (bottom) receptor BPND by group. Correlation coefficients (r) and significance (p) were determined, controlling for age, sex and smoking status.
Among subcortical regions, hippocampal gray-matter volume was negatively associated with D1-type BPND in MA subjects (r = −0.790, p = 0.004) [significant after Bonferroni correction (p < 0.01)]. In addition, trends of negative association between D1-type BPND and gray-matter volume in the thalamus (r = −0.715, p = 0.013) and striatum (r = −0.659, p = 0.03) were observed. In controls, D1-type BPND was not significantly associated with hippocampal or other subcortical volumes (p’s > 0.23). The correlation coefficient between D1-type BPND and hippocampal volume showed no group difference (z = −1.21, p = 0.22). D2-type BPND was not correlated with hippocampal or any subcortical volumes in either group.
Associations of cortical gray-matter thickness with MA use and craving
Global mean cortical gray-matter thickness was negatively associated with cumulative MA use (entire sample: r = −0.843, p < 0.001; excluding one outlier: r = −0.575, p = 0.04). Post-hoc correlation analyses showed that gray-matter thickness was negatively associated with duration (r = −0.638, p = 0.02), but not amount (r = −0.107, p = 0.73) or frequency of use (r = −0.086, p = 0.75). Global cortical gray-matter thickness was also negatively correlated with MA craving (r = −0.569, p = 0.04) (Figure 3).
Figure 3.
Global mean of cortical gray-matter thickness is negatively associated with cumulative MA use and craving. Correlation coefficients (r) and significance (p) were determined controlling for age, sex and smoking status.
In post-hoc analyses, negative associations between cumulative MA use and gray-matter thickness in temporal (r = −0.839, p < 0.001) and frontal (r = −0.899, p < 0.001) lobes were significant after Bonferroni correction (i.e., p < 0.0125), with a trend in the occipital lobe (r = −0.676, p = 0.013) but not the insula (r = −0.525, p = 0.07). MA craving was negatively associated with gray-matter thickness in the temporal lobe (r = −0.759, p = 0.003), but not in other regions (p’s > 0.06).
Discussion
That striatal dopamine D1-type receptor availability, measured in vivo, did not differ between MA users and controls, is consistent with postmortem findings in the dorsal striatum but not with the finding of elevated D1-receptor density in the nucleus accumbens8. One postmortem study reported partial desensitization of D1-receptor function in MA users despite unchanged receptor density43. Studies in rodents have produced mixed results, depending on the treatment regimen. Daily administration of 4 mg/kg MA daily for 14 days produced no change in striatal D1-receptor density44, 45, but five 15-mg/kg doses at 6-h intervals lowered D1-receptor density in the caudate and putamen46. In the human postmortem study showing elevated nucleus accumbens D1-receptor density, recent MA use was confirmed in biological samples, but participants studied here were abstinent for ≥ 4 days. MA may upregulate D1 receptors in the nucleus accumbens acutely, with subsequent reduction over several days of abstinence from MA. As observed here with MA-dependent subjects, cocaine-dependent subjects, abstinent >14 days, did not differ from controls in D1-type BPND47.
Cigarette smoking, which is common among stimulant users48–50, can be a confounding factor, and there were more smokers in the MA group than the control group. However, smoking status was controlled statistically, and D1-type BPND did not differ between nonsmokers and smokers in the control group (controlled for age and sex, p = 0.926). We also controlled for the contribution of smoking to the group difference in D2-type BPND, which was seen as well in a separate comparison of smokers alone in our sample (14 controls vs. 18 MA users; controlled for age and sex, p = 0.002). Lower D2-type BPND in MA users vs. controls demonstrates similarity of our sample with those studied before6, 7, indicating that negative findings regarding D1-type BPND were not an artifact of sample selection. Recent findings suggest that lower D2-type BPND in cocaine-dependent individuals is associated with sleep disturbance51. Adenosine A2 receptors, predominantly expressed in striatum, co-localize with D2-type receptors on striatal medium spiny neurons52, which form the indirect pathway, but not with D1-type receptors. Enhanced adenosine levels due to sleep deprivation53 may potentiate internalization of D2-type receptors54, leading to reduced D2-type BPND in MA users. Greater self-reported sleepiness in MA users supports this hypothesis. Thus, sleeplessness can contribute to a deficit in D2-type BPND in MA users.
The negative associations of cortical gray-matter thickness with striatal D1-type receptor availability in MA users may reflect adaptation to striatal D1 receptor activation with chronic MA use. Most striatal neurons (≥ 77%) are GABAergic projection neurons55 that transmit signals via the direct and indirect pathways to SNr/GPi12. Striatal dopamine release enhances the activity of D1-expressing direct-pathway neurons but suppresses activity of D2-receptor-expressing indirect-pathway neurons13. Thus, chronic MA-induced striatal dopamine release may affect cortical responses and produce adaptation. That local injection of D1- but not D2-receptor agonists into the striatum increases cortical c-Fos expression in rats15, suggests a greater contribution of striatal D1 than D2 receptors to cortical activity, consistent with an association between striatal D1- but not D2-type receptors and cortical structure.
Although this study replicated a previous report of negative association of global mean cortical gray-matter thickness with cumulative MA use56, there were no group differences in the cortical gray-matter thickness. We previously found no difference in global gray-matter volume between MA users and controls recruited using inclusion/exclusion criteria identical to those reported here57. However, focal abnormalities in cerebral cortical structure have been observed in MA users58. Such group differences may be masked by averaging gray-matter thickness to generate a global mean. In addition, the greater proportion of females in the control group than the MA group included in structural analysis may have influenced a group difference, although age and sex were statistically controlled.
Post-hoc analyses indicated a negative association of gray-matter thickness in the occipital and temporal lobes with striatal D1-type BPND. The temporal lobe is the only region where gray-matter thickness was associated with cumulative MA use and MA craving. In a previous study, temporal cortical gray-matter volume in MA users was smaller than in controls matched for smoking status59. In another, the effect of MA use on age-related loss in gray-matter was substantially greater in the temporal lobe than in other cortical areas36.
This study also found no group difference in hippocampal gray-matter volume despite its negative association with D1-type BPND in the MA group, and no group difference in striatal volume despite previously reported greater volume in the striata of MA users60, 61. Some previous studies showed smaller hippocampal volumes in MA users than controls57, 62, but others did not59, 63. The largest of these studies (44 controls, 61 MA users) had a negative finding. Some previous studies also found no group difference in striatal gray-matter volume59, 63. These inconsistencies may reflect differences in methods, sample sizes, or duration of abstinence. Although previous studies suggested an effect of abstinence on recovery from cortical gray-matter deficits in stimulant users59, 64, duration of abstinence was not associated with measures of gray-matter thickness or volume here (Pearson correlation: p’s > 0.3). Finally, the negative association between global mean cortical gray-matter thickness and MA craving was consistent with the finding of negative association between MA craving and gray-matter volume in a distributed set of brain regions including temporal and occipital cortex in an independent sample of MA users65.
This study has limitations, including a modest sample size. For logistical reasons, there were some gaps in time between the self-report, PET, and MRI measures, which ideally would have been collected on the same day, given evidence for effects of abstinence on cortical gray-matter volume59. However, duration of abstinence prior to each scan was not significantly different, and including days abstinent at MRI scanning as a covariate did not change the correlation between D1-type BPND and cortical gray-matter thickness (r = −0.706, p = 0.02). That some of the participants studied had positive urine tests for marijuana even though they endorsed abstinence from marijuana use for at least 2 days before testing is a potential limitation. Indeed, dopaminergic neurons are modulated by the endocannabinoid system 66, and recent reviews indicate effects of both THC and cannabidiol 67, 68. Most relevant to our manuscript are findings related to striatal dopamine receptors. The findings are inconsistent, with one study finding that acute THC administration decreased dopamine type D1 and D2 receptor Bmax values in rat striatum 69, and another finding no effect 70. In a human PET study, cannabis users did not differ from controls in striatal D2-type receptor BPND 71. Concern regarding effects of possible recent marijuana use by participants in our study is tempered, however, by the observation that THC status, determined by urine toxicology, was not a significant covariate of no interest in the results. Other limitations are associated with the radiotracers. Some affinity of [11C]NNC112 to 5-HT2A receptors72 precludes definitive statements regarding D1-type receptors, but this nonspecificity should not be problematic for measurements in the striatum, which has a negligible density of 5-HT2A receptors73. [18F]Fallypride has high affinity for D2-type receptors, but it does not distinguish between D2 and D3 receptor subtypes74.
Finally, causal relationships among biochemical, clinical and brain structural measures cannot be claimed as this is a cross-sectional study. Nonetheless, the results suggest a possible role of striatal dopamine D1-type receptors in MA-induced neuroadaptation in cortical gray-matter structure and, in turn, MA craving. More work is needed to define the link between D1 receptors and effects on cortical gray matter and various clinical aspects of MA-use disorder.
Supplementary Material
Acknowledgments
This research was supported, in part, by grants from the National Institute on Drug Abuse (K23 DA027734, ACD) and endowments from the Thomas P and Katherine K Pike Chair in Addiction Studies (EDL) and the Marjorie M Greene Trust. KO was partly supported by Department of Psychiatry, Graduate School of Medicine, Chiba University, DOMONKAI fund. The authors acknowledge the excellent technical support provided by Karen Lazare, Josephine Ribe and Garrett Crook in PET acquisition, and Bryan Garrison and Dmitriy Gekker in radioligand preparation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA) Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015. [PubMed] [Google Scholar]
- 2.Chomchai C, Chomchai S. Global patterns of methamphetamine use. Curr opin psychiatry. 2015;28(4):269–274. doi: 10.1097/YCO.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 3.Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am j psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am j psychiatry. 2001;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 5.Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J neurosci. 2008;28(39):9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain res. 2015;1628(Pt A):174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol psychiatry. 2000;5(6):664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- 9.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271(5255):1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 10.Gross NB, Duncker PC, Marshall JF. Striatal dopamine D1 and D2 receptors: Widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse. 2011;65(11):1144–1155. doi: 10.1002/syn.20952. [DOI] [PubMed] [Google Scholar]
- 11.Gross NB, Marshall JF. Striatal dopamine and glutamate receptors modulate methamphetamine-induced cortical Fos expression. Neuroscience. 2009;161(4):1114–1125. doi: 10.1016/j.neuroscience.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci biobehav rev. 2008;32(3):333–342. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J neurosci methods. 1989;29(3):261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 15.Blandini F, Fancellu R, Orzi F, Conti G, Greco R, Tassorelli C, et al. Selective stimulation of striatal dopamine receptors of the D1- or D2-class causes opposite changes of fos expression in the rat cerebral cortex. European j neurosci. 2003;17(4):763–770. doi: 10.1046/j.1460-9568.2003.02520.x. [DOI] [PubMed] [Google Scholar]
- 16.Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30(3):767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- 17.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J neurosci. 2013;33(47):18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis GM, Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin pharmacol ther. 1985;38(5):572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- 19.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of Sleepiness: A New Approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 20.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br j addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 21.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug alcohol depend. 2006;83(3):233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, et al. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: Validation and reproducibility. J Cereb Blood Flow Metab. 2000;20(2):225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46(3):170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 24.Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J nucl med. 2007;48(3):471–480. [PubMed] [Google Scholar]
- 25.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 26.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1-and D2-dopamine receptors, and dopamine and its metabolites in the human brain. 1994 doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 28.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE trans med imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 36.Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106(8):1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42(1):48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- 38.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am j psychiatry. 2008;165(4):507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 39.Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol aging. 2000;21(5):683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 40.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am j psychiatry. 1998;155(6):768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 41.Jancke L, Merillat S, Liem F, Hanggi J. Brain size, sex, and the aging brain. Hum brain mapp. 2015;36(1):150–169. doi: 10.1002/hbm.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18(9):1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong J, Ross BM, Schmunk GA, Peretti FJ, Kalasinsky KS, Furukawa Y, et al. Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase activity in human methamphetamine users. Am j psychiatry. 2003;160(5):896–903. doi: 10.1176/appi.ajp.160.5.896. [DOI] [PubMed] [Google Scholar]
- 44.Nonaka R, Moroji T. Effects of chronic methamphetamine treatment on the binding parameters of [3H]SCH 23390, a selective D1-dopamine receptor ligand, in the rat brain. Neurosci lett. 1990;120(1):109–112. doi: 10.1016/0304-3940(90)90180-h. [DOI] [PubMed] [Google Scholar]
- 45.Ujike H, Akiyama K, Nishikawa H, Onoue T, Otsuki S. Lasting increase in D1 dopamine receptors in the lateral part of the substantia nigra pars reticulata after subchronic methamphetamine administration. Brain res. 1991;540(1–2):159–163. doi: 10.1016/0006-8993(91)90503-n. [DOI] [PubMed] [Google Scholar]
- 46.McCabe RT, Hanson GR, Dawson TM, Wamsley JK, Gibb JW. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987;23(1):253–261. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]
- 47.Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, et al. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34(7):1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addict behav. 2007;32(1):24–38. doi: 10.1016/j.addbeh.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patkar AA, Lundy A, Leone FT, Weinstein SP, Gottheil E, Steinberg M. Tobacco and alcohol use and medical symptoms among cocaine dependent patients. Subst Abus. 2002;23(2):105–114. doi: 10.1080/08897070209511480. [DOI] [PubMed] [Google Scholar]
- 51.Wiers CE, Shumay E, Cabrera E, Shokri-Kojori E, Gladwin TE, Skarda E, et al. Reduced sleep duration mediates decreases in striatal D2/D3 receptor availability in cocaine abusers. Transl psychiatry. 2016;6:e752. doi: 10.1038/tp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansson B, Georgiev V, Fredholm BB. Distribution and postnatal ontogeny of adenosine A2A receptors in rat brain: comparison with dopamine receptors. Neuroscience. 1997;80(4):1187–1207. doi: 10.1016/s0306-4522(97)00143-7. [DOI] [PubMed] [Google Scholar]
- 53.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277(20):18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 55.Graveland GA, Williams RS, DiFiglia M. A Golgi study of the human neostriatum: neurons and afferent fibers. J comp neurol. 1985;234(3):317–333. doi: 10.1002/cne.902340304. [DOI] [PubMed] [Google Scholar]
- 56.Lyoo IK, Yoon S, Kim TS, Lim SM, Choi Y, Kim JE, et al. Predisposition to and effects of methamphetamine use on the adolescent brain. Mol psychiatry. 2015;20(12):1516–1524. doi: 10.1038/mp.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann NY Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug alcohol depend. 2012;125(3):230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol psychiatry. 2005;57(9):967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am j psychiatry. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 62.Orikabe L, Yamasue H, Inoue H, Takayanagi Y, Mozue Y, Sudo Y, et al. Reduced amygdala and hippocampal volumes in patients with methamphetamine psychosis. Schizophr res. 2011;132(2–3):183–189. doi: 10.1016/j.schres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50(4):1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated Grey Matter Changes with Prolonged Addiction and Extended Abstinence in Cocaine Users. PloS one. 2013;8(3):e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol psychiatry. 2015;20(6):764–771. doi: 10.1038/mp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez-Ruiz J, Hernandez M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS neurosci ther. 2010;16(3):e72–91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Delta9-tetrahydrocannabinol on the dopamine system. Nature. 2016;539(7629):369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renard J, Norris C, Rushlow W, Laviolette SR. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci biobehav rev. 2017;75:157–165. doi: 10.1016/j.neubiorev.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Navarro M, Fernandez-Ruiz JJ, De Miguel R, Hernandez ML, Cebeira M, Ramos JA. Motor disturbances induced by an acute dose of delta 9-tetrahydrocannabinol: possible involvement of nigrostriatal dopaminergic alterations. Pharmacol biochem behav. 1993;45(2):291–298. doi: 10.1016/0091-3057(93)90241-k. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez De Fonseca F, Fernandez-Ruiz JJ, Murphy LL, Cebeira M, Steger RW, Bartke A, et al. Acute effects of delta-9-tetrahydrocannabinol on dopaminergic activity in several rat brain areas. Pharmacol biochem behav. 1992;42(2):269–275. doi: 10.1016/0091-3057(92)90526-l. [DOI] [PubMed] [Google Scholar]
- 71.Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2012;26(1):144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- 72.Andersen PH, Gronvald FC, Hohlweg R, Hansen LB, Guddal E, Braestrup C, et al. NNC-112, NNC-687 and NNC-756, new selective and highly potent dopamine D1 receptor antagonists. Eur j pharmacol. 1992;219(1):45–52. doi: 10.1016/0014-2999(92)90578-r. [DOI] [PubMed] [Google Scholar]
- 73.Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M, et al. [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2A) receptors: a PET study in healthy human subjects. J cereb blood flow and metab. 2007;27(10):1733–1741. doi: 10.1038/sj.jcbfm.9600468. [DOI] [PubMed] [Google Scholar]
- 74.Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl med biol. 1999;26(5):519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.