Abstract
Background and Purpose
Muscle weakness predisposes seniors to a fourfold increase in functional limitations and has previously been associated with reduced motor cortex excitability in the elderly. The purpose of this study was to determine if a single session of anodal transcranial direct current stimulation (tDCS) of the motor cortex would increase elbow flexion muscle strength and EMG amplitude in very old individuals.
Methods
Eleven very old individuals (85.8(4.3) years) performed 3 maximal isometric elbow flexion contractions before and after 20 min of sham or anodal tDCS on different days. Order of stimulation was randomized, and the study participants and investigators were blinded to condition. Additionally, voluntary activation capacity of the elbow flexors was determined by comparing voluntary and electrically evoked forces.
Results
Anodal tDCS did not alter muscle strength or EMG activity in comparison to sham stimulation. Elbow flexion voluntary activation capacity was very high among the study participants (99.3(1.8)%).
Conclusion
Contrary to our hypothesis, we observed no effect of anodal tDCS and no impairment in elbow flexor voluntary activation capacity in the very old. Whether anodal tDCS would exert a positive effect and support our initial hypothesis in another muscle group that does exhibit impairments in voluntary activation in older adults is a question that is still to be addressed.
Keywords: Aging, Muscle Strength, Sarcopenia
INTRODUCTION
Approximately 30% of older women and 15% of older men in the United States self-report that they are unable to lift or carry 10 pounds, and approximately 50% of women and 40% of men report difficulty in stooping, crouching, or kneeling.1 Weakness predisposes seniors to a fourfold increase in functional limitations as well as a twofold increase in mortality.2 Identifying the factors contributing to such extensive physical impairments is necessary to develop targeted interventions. Although muscle wasting (i.e., atrophy) partially explains the decline in muscle strength with age,3 recent studies have indicated that age-associated changes in nervous system form and function are a major contributor to muscle weakness in the elderly.4
Several studies have indicated that aging is associated with reductions in motor cortex excitability.5–9 We recently reported that weaker older adults exhibit motor cortex hypoexcitability and impairments in voluntary activation of the wrist flexor musculature.9 Accordingly, we hypothesized that increasing motor cortical excitability would acutely enhance muscle strength in older adults. Thus, in this experiment, we sought to determine if a single session of anodal transcranial direct current stimulation (tDCS) would increase elbow flexion muscle strength and electromyographic (EMG) amplitude in older adults.
tDCS, a form of non-invasive focal neurostimulation using weak direct electrical currents delivered via sponge electrodes on the scalp, has the capacity to elicit sustained but transient changes in cortical excitability.10 The electrical current does not directly stimulate axons causing them to discharge, but rather acutely modulates the resting membrane potential (i.e., subthreshold depolarization or hyperpolarization) of the tissue under the electrodes, thereby, adjusting the ongoing neuronal firing activity.10 Numerous studies, including our own, have demonstrated that anodal tDCS, where current flows from anode over the motor cortex to cathode over the contralateral forehead, acutely increases motor cortex excitability as measured by transcranial magnetic brain stimulation after the stimulation period.10–12 The degree and duration of the after-stimulation effects of tDCS on cortical excitability are known to depend upon the dosage of current delivered. For instance, anodal tDCS delivered for a minimum of 13 min (2.0 mA current delivered via 35 cm2 electrodes) while an individual is at rest has been shown to increase measures of intracortical facilitation and simultaneously decrease intracortical inhibition for up to 90 min after the stimulation.11,13
METHODS
Study participants
Eleven community-dwelling individuals aged 80 years and older (85.8(4.3) years; 7 women and 4 men; 66.4(17.6) kg; 161.1(15.1) cm; BMI: 25.6(6.1) kg/m2) participated in this experiment. Study participants were excluded if they reported an ADL disability (e.g., difficulty feeding), had a known neuromuscular or neurological disorder (e.g., hemiplegia, Parkinson’s disease), severe cardiac disease (e.g., class III or IV congestive heart failure), cognitive impairment (≤ 70 on the Repeatable Battery for the Assessment of Neuropsychological Status), and/or medications known to alter cortical excitability. 14 58 percent of the study participants were classified as having “minimal limitations” in lower extremity function based on the short physical performance battery (i.e., SPPB scores 10 to 12, whereas 42% had mild limitations (i.e., SPPB scores 7 to 9).15 In terms of upper extremity function, the women had a score of 9.3(2.3) on the Purdue Pegboard Test (normative score for women 80 y 10.7(2.1),16 and the men had a score of 10.2(0.6) (normative score for men 80 y 9.8(1.7).16 18 percent of the study participants were classified as “frail” based on the Fried criteria.17 The Institutional Review Board at Ohio University approved the study protocol, and all study participants provided written informed consent.
General Overview of the Study
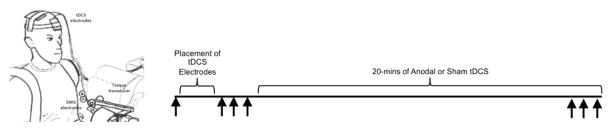
Study participants completed 3 laboratory-based testing sessions. Each of these testing sessions was separated by a minimum of 4 days and a maximum of 14 (average days between sessions: 10.0 (2.4 days)). During the first and third sessions, study participants performed 3 maximal isometric elbow flexion contractions with 30 s of rest using their non-dominant elbow flexors. Next, the study participants were randomized to receive either 20 mins of sham or anodal tDCS delivered to the motor cortex. For the first 17 min and 30 s, study participants simply rested. After 17 min and 30 s into the respective stimulation conditions, study participants performed an additional 3 maximal isometric elbow flexion contractions with 30 s rest. At the completion of these contractions, the tDCS stimulator was turned off. Study participants and investigators were blinded to condition (i.e., double blind). Study participants were provided with visual feedback of their torque output, and EMG was recorded from the long head of the biceps brachii during the contractions. Outcomes included the average of maximum voluntary strength and EMG amplitude (500 ms root mean squared value surrounding peak force) before and after the respective stimulation conditions, as well as the peak values for strength and EMG amplitude. During the other visit, voluntary activation capacity of the elbow flexor muscle group was assessed using a combination of voluntary and electrically evoked contractions (described below). We chose to assess voluntary activation capacity in order to quantify the study participant’s degree of neural impairment in muscle activation. Figure 1 illustrates the mechanical set-up for testing and the timeline of the strength-tDCS experimental sessions.
Figure 1.
Illustration of the setup and participant positioning (left) and the experimental timeline (right).
Experimental Setup and Mechanical Recordings
Study participants were seated in a BioDex dynamometer with the non-dominant arm positioned next to the body in 10–15° of abduction with the arm supported at the elbow (Biodex System 4, Biodex Medical Systems Inc., Shirley, NY). The elbow joint was positioned at 110° and aligned with the axis of rotation of the torque motor. The wrist was in a neutral position. During the testing sessions, study participants wore a prefabricated wrist-hand-thumb orthosis (Model 1000, Orthomerica, Newport Beach, CA). The orthosis was securely strapped to the lever arm. Individuals were also securely strapped to the chair using waist and shoulder straps. The resolution of the torque output was scaled on a case-by-case basis to maximize signal resolution using the Biodex Researchers Tool Kit Software. The torque signal was sampled at 625 Hz (MP150, BioPac Systems, Inc.), and it was visually displayed on a computer monitor located 1 meter in front of the participant.
Assessment of Muscle Strength
Study participants performed two practice maximal isometric contraction trials at the start of the testing sessions followed by at least 10 min of rest before the testing protocol began. Strength was assessed before and at the end of the tDCS conditions. During these assessments, study participants were asked to perform isometric maximal contractions lasting 5 s with 30 s of rest between trials. Standard verbal encouragement was provided throughout the contraction, and study participants were given visual feedback of their torque output on the computer monitor. Peak torque for each trial was calculated.
Electrical Recordings
EMG activity was recorded from the lateral head of the biceps brachii muscle using bipolar surface electrodes (Ag-AgCl, 8-mm diameter, interelectrode distance 25-mm, Trace 1, Nikomed, Huntingdon Valley, PA) located longitudinally over the lower portion of the muscle belly on shaved, abraded and cleaned skin with the reference electrode placed on the back of the dominant hand. EMG signals were amplified (1,000x), band-pass filtered (10–500 Hz), and sampled at 10,000 Hz using a 16-bit data acquisition system (MP150, BioPac Systems Inc., Goleta, CA). The root mean squared (RMS) EMG amplitude over a 500 ms window corresponding to peak torque was calculated.
Transcranial Direct Current Stimulation
Anodal or sham tDCS was delivered to the right motor cortex, opposite of the left non-dominant arm, using a constant current stimulator (NeuroConn Eldith I Channel DC Stimulator Plus, Rogue Resolutions, Cardiff, United Kingdom). For both conditions, 2 conductive-rubber electrodes (35 cm2) enclosed in saline-soaked sponges (0.9%) (McKesson USP Normal Saline Sterile 0.9%, McKesson Medical-Surgical, Richmond, VA) were placed on the participant’s pre-moistened scalp. The stimulating electrode was placed over the right motor cortex centered over the hotspot for the biceps brachii (identified via transcranial magnetic stimulation) and the active reference electrode was placed on the left forehead just above the left eyebrow/orbit as we have previously described.12,18 For the anodal stimulation condition, a continuous 1.5 mA current was delivered using 35 cm2 electrodes for 20 min (current density=0.043 mA/cm2). The stimulation began and ended with an 8-second ramp. For the sham stimulation condition, current was delivered for the first 30 seconds and then it was cessated. The post-tDCS maximal strength assessments began 18 min and 30 s after the start of the stimulation. 3 maximal contractions lasting 5 s in duration were performed with 30 s of rest between each contraction.
Voluntary Activation
Voluntary activation was quantified using a doublet interpolation technique that involved delivering electrical stimulation (0.2 ms pulses) to the elbow flexor muscles. This testing was done on a separate day (as part of a larger study) from the 2 tDCS (anodal vs. sham conditions) testing sessions and was a similar technique to that used in our prior voluntary activation studies.19 After positioning the study participants as described above, 5 cm diameter self-adhesive electrodes were placed over the motor points of the biceps brachii. Single pulses of incrementally increasing current were delivered via a Digitimer DS7AH stimulator until twitch force plateaued. Study participants then performed 2 maximal, isometric contractions separated by approximately 3 min, and during the contraction, a 100 Hz doublet was delivered at supramaximal intensity (110% of the current that produced maximum twitch force), followed by a second doublet delivered to the resting muscle. Voluntary activation was determined with the following formula: % voluntary activation = (1 − [Evoked Force During MVC/Evoked Force Following MVC]) × 100. If force did not increase, voluntary activation was recorded as 100%. The average of the 2 trials was calculated.
Statistical Analyses
SPSS was used for all statistical analyses (version 19 for Mac, SPSS Inc, Chicago, IL). A pre-set α level of significance of 0.05 was required for statistical significance. Data are reported as mean (SD) in the text and mean (SEM) in the figures. When appropriate, effect sizes (ES: partial η2) are provided. Repeated measures analysis of variance (RM-ANOVA) procedures were performed for within subject’s factors for Stimulation Condition (2-levels: anodal tDCS vs. sham tDCS) and Time (2 levels: Pre-Stim vs. Post-Stim). It should be noted that this experiment was initially designed as a pilot study to generate effect sizes on the effect of anodal tDCS on acutely enhancing muscle strength in the elderly. However, as noted in the results below, the effect size was negligible, and the senior author (BC Clark) decided to cessate this experiment. Because we, and many others, believe that negative results are an integral part of scientific progress to ensure a balanced presentation of data,20 herein, we present the results of a negative findings experiment.
RESULTS
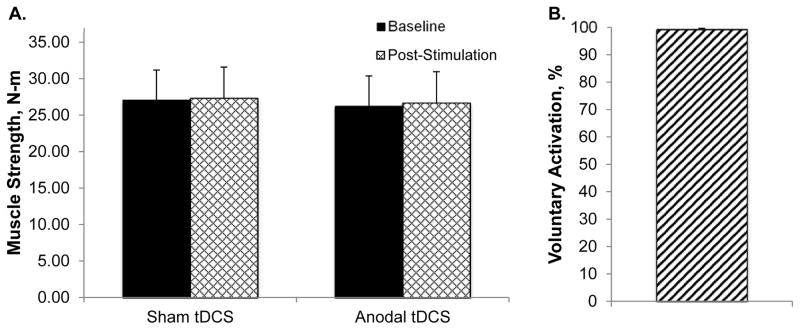
Anodal tDCS did not significantly alter muscle strength in the non-dominant arm when averaged across all trials (stimulation condition x time interaction p=0.87) or the peak trial (stimulation condition x time interaction p=0.81; Figure 1A). Similarly, there was no effect of anodal tDCS on EMG amplitude when averaged across all trials (stimulation condition x time interaction p=0.88) or the peak trial (stimulation condition x time interaction p=0.69). The effect sizes for anodal tDCS for all analyses were negligible (η2≤0.01). On average, the study participants demonstrated voluntary activation levels of 99.3 (1.8)%. Only 1 participant demonstrated a voluntary activation level less than 99%.
DISCUSSION
We sought to test the hypothesis that a single session of anodal transcranial direct current stimulation (tDCS) of the motor cortex would increase elbow flexion muscle strength and EMG amplitude in the very old. Our hypothesis was steeped in a conceptual framework of muscle weakness in the elderly being mechanistically associated with reduced motor cortex excitability 9 that resulted in impairments in voluntary (neural) activation.9 Numerous studies indicate that anodal tDCS transiently increases motor cortical excitability.10,12,13,21 Thus, we postulated that anodal tDCS would increase motor cortical excitability, which in turn, would enhance voluntary activation, muscle strength, and EMG amplitude. Contrary to our hypothesis, we observed no impairment in elbow flexor voluntary activation capacity and no effect of anodal tDCS. Below we discuss our findings within the context of the extant literature and note limitations of current work.
In recent years, numerous studies have investigated the effects of tDCS on motor function in older adults.22–26 Virtually all of these have focused on examining the question of whether tDCS can enhance fine motor skill performance, learning, and retention.27 A recent meta-analysis indeed reported a robust, medium effect size (standardized mean difference effect size=0.65) for the effect of tDCS on motor task domain performance in older adults.28 It has also been reported that tDCS enhances the time to task failure of a sustained, submaximal contraction in older adults.18 To our knowledge there is only 1 other study that has examined whether tDCS altered muscle strength values in aged individuals without an overt neurological impairment.29 This study by Marquez et al. also used a cross-over, double blind, sham controlled design to determine if anodal tDCS altered key grip and pinch grip strength in 34 individuals greater than 40 years old (mean age 61).29 They observed no effect on strength. However, there are studies that have reported that tDCS enhances muscle strength30–33 (contrary findings: 34–36). It is possible these discrepancies are related to the population tested (e.g., healthy adults vs. chronic stroke patients) or the muscle group studied (e.g., leg extensors vs. pinch grip vs. elbow flexors). Our findings suggest that anodal tDCS does not increase elbow flexor muscle strength in the very old.
It is possible that our finding of no effect of tDCS on muscle strength in the very old is due to a ceiling effect in voluntary activation capacity. We, and others, have previously reported that many older adults exhibit impairments in voluntary activation capacity.9,37,38 However, these impairments appear to be muscle group specific (for review see 39). For instance, data suggest that the leg extensors are more prone to aging related changes in voluntary activation compared to the dorsiflexors (for review see 39). In this study, we chose to examine the elbow flexors because we have previously reported that anodal tDCS increases motor evoked potential amplitude of the elbow flexors12 and also enhances muscle performance (time to task failure) in both younger12 and older adults.18 Additionally, several studies have suggested that older adults exhibit impairments in voluntary activation of the elbow flexors,40,41 although it should be noted that this is not a universal finding.42 We did not expect to observe such exceptionally high levels of voluntary activation amongst the very old (10 of 11 study participants exhibited levels > 99%), especially in the non-dominant arm, which was chosen for this study to control for potential differences in the level of use of the dominant arm. This observation, which is perhaps the most interesting finding of this study, indicates that the vast majority of our very old study participants were able to near fully recruit and optimally fire their elbow flexor motor units. Thus, within our abovementioned conceptual framework, anodal tDCS would not be expected to exert a positive effect on muscle strength due to a ceiling effect. Indeed, our findings are consistent with the previous study in young adults, where no significant improvement in elbow flexor maximum strength was observed after a 10 min anodal tDCS treatment.35 These authors suggested that the tDCS treatment did not improve muscle strength because of ceiling effects. To our knowledge, this is the first study in which the effectiveness of an anodal tDCS treatment was evaluated in conjunction with assessment of individuals’ voluntary activation level. Whether anodal tDCS would exert a positive effect and support our initial hypothesis in another muscle group, such as the leg extensors, that more commonly exhibits impairments in voluntary activation in older adults is a question that is still to be addressed.
There are several limitations to the present study that should be recognized. First, hindsight indicates that the muscle group examined likely resulted in a ceiling effect, as observed voluntary activation measures were incredibly high (>99%). It is unlikely that this was an issue with the target population per se, as we did obtain measures of leg extensor voluntary activation in 10 of the 11 study participants whose data are presented in this report as part of a larger study, and observed that the leg extensors exhibited a significantly lower degree of voluntary activation in comparison to the elbow flexors (leg extensor voluntary activation: 89.5 (6.9)%).43 Our rationale for choosing the elbow flexors was based on stronger evidence for anodal tDCS increasing motor cortical excitability of an upper extremity muscle group as opposed to a lower extremity muscle group.44 However, in retrospect, the muscle group examined likely confounded the ability to truly test our hypothesis. A second limitation of this study is that we did not obtain measures of motor cortical excitability. Measuring cortical excitability before and after tDCS would have allowed us to determine how much changes in excitability was induced by 20 min of tDCS.
In summary, we expected to observe impairment in elbow flexion voluntary activation capacity in the very old and hypothesized that a single session of anodal tDCS would acutely increase elbow flexion muscle strength and EMG amplitude. Contrary to our hypothesis, we observed no impairment in elbow flexor voluntary activation capacity in the very old and no effect of anodal tDCS. Whether anodal tDCS would exert a positive effect and support our initial hypothesis in another muscle group that does exhibit impairments in voluntary activation in older adults is a question that is still to be addressed.
Figure 2.
Transcranial direct current stimulation (tDCS) did not alter peak muscle strength (Figure 1A). It should be noted that voluntary activation capacity was very high among the study participants (99.3(1.8)%; Figure 1B).
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG044424. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Louie GH, Ward MM. Sex disparities in self-reported physical functioning: true differences, reporting bias, or incomplete adjustment for confounding? J Am Geriatr Soc. 2010;58(6):1117–1122. doi: 10.1111/j.1532-5415.2010.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55(3):451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Manini TM, Clark BC. Dynapenia and Aging: An Update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen A, Siejka S, Schulzer M, Calne D. Age-dependent decline in motor evoked potential (MEP) amplitude: with a comment on changes in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1991;81(3):209–215. doi: 10.1016/0168-5597(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 6.McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45(9):671–678. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99(4):1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kossev AR, Schrader C, Dauper J, Dengler R, Rollnik JD. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333(2):83–86. doi: 10.1016/s0304-3940(02)00986-2. [DOI] [PubMed] [Google Scholar]
- 9.Clark BC, Taylor JL, Hong SL, Law TD, Russ DW. Weaker seniors exhibit motor cortex hypoexcitability and impairments in voluntary activation. J Gerontol A Biol Sci Med Sci. 2015;70(9):1112–1119. doi: 10.1093/gerona/glv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Simul. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 12.Williams PS, Hoffman RL, Clark BC. Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS one. 2013;8(12):e81418. doi: 10.1371/journal.pone.0081418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitsche MA, Roth A, Kuo MF, et al. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosc. 2007;27(14):3807–3812. doi: 10.1523/JNEUROSCI.5348-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disability and Rehabilitation. 1995;17(5):217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 18.Oki K, Mahato NK, Nakazawa M, et al. Preliminary evidence that excitatory transcranial direct current stimulation extends time to task failure of a sustained, submaximal muscular contraction in older adults. J Gerontol A Biol Sci Med Sci. 2016;71(8):1109–1112. doi: 10.1093/gerona/glw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russ DW, Clark BC, Krause J, Hagerman FC. Development of a Neuromuscular Electrical Stimulation Protocol for Sprint Training. Med Sci Sports Exerc. 2012;44(9):1810–1819. doi: 10.1249/MSS.0b013e31825423f1. [DOI] [PubMed] [Google Scholar]
- 20.Matosin N, Frank E, Engel M, Lum JS, Newell KA. Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis Models Mech. 2014;7(2):171–173. doi: 10.1242/dmm.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsche MA, Doemkes S, Karakose T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski E, Hoff M, Rjosk V, et al. Anodal transcranial direct current stimulation does not facilitate dynamic balance task learning in healthy old adults. Front Human Neurosci. 2017;11:16. doi: 10.3389/fnhum.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiyama H, Hinder MR, Barzideh A, et al. Preconditioning tDCS facilitates subsequent tDCS effect on skill acquisition in older adults. Neurobiol Aging. 2017;51:31–42. doi: 10.1016/j.neurobiolaging.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Rumpf JJ, Wegscheider M, Hinselmann K, et al. Enhancement of motor consolidation by post-training transcranial direct current stimulation in older people. Neurobiol Aging. 2017;49:1–8. doi: 10.1016/j.neurobiolaging.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Dumel G, Bourassa ME, Desjardins M, et al. Multisession anodal tDCS protocol improves motor system function in an aging population. Neural Plasticity. 2016;2016:5961362. doi: 10.1155/2016/5961362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cog Neurosci. 2016;28(2):275–281. doi: 10.1162/jocn_a_00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perceval G, Floel A, Meinzer M. Can transcranial direct current stimulation counteract age-associated functional impairment? Neurosci Biobehav Rev. 2016;65:157–172. doi: 10.1016/j.neubiorev.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Summers JJ, Kang N, Cauraugh JH. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta- analysis. Ageing Research Rev. 2016;25:42–54. doi: 10.1016/j.arr.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Marquez J, Conley A, Karayanidis F, Lagopoulos J, Parsons M. Anodal direct current stimulation in the healthy aged: Effects determined by the hemisphere stimulated. Restor Neurol Neurosci. 2015;33(4):509–519. doi: 10.3233/RNN-140490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washabaugh EP, Santos L, Claflin ES, Krishnan C. Low-level intermittent quadriceps activity during transcranial direct current stimulation facilitates knee extensor force-generating capacity. Neurosci. 2016;329:93–97. doi: 10.1016/j.neuroscience.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimul. 2014;7(3):443–450. doi: 10.1016/j.brs.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196(3):459–465. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka S, Takeda K, Otaka Y, et al. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil Neural Repair. 2011;25(6):565–569. doi: 10.1177/1545968311402091. [DOI] [PubMed] [Google Scholar]
- 34.Murray LM, Edwards DJ, Ruffini G, et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil. 2015;96(4 Suppl):S114–121. doi: 10.1016/j.apmr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kan B, Dundas JE, Nosaka K. Effect of transcranial direct current stimulation on elbow flexor maximal voluntary isometric strength and endurance. Appl Physiol Nutr Metabol. 2013;38(7):734–739. doi: 10.1139/apnm-2012-0412. [DOI] [PubMed] [Google Scholar]
- 36.Au-Yeung SS, Wang J, Chen Y, Chua E. Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. Am J Phys Med Rehabil. 2014;93(12):1057–1064. doi: 10.1097/PHM.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 37.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27(1):99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- 38.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22(7):831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4(3):192–199. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93(2):457–462. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- 41.Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24(1):98–106. doi: 10.1002/1097-4598(200101)24:1<98::aid-mus11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 42.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91(3):1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- 43.Clark LA, Amano S, Clift R, et al. Octogenarians and nonagenarians exhibit impairments in leg extensor voluntary activation, but not elbow flexor voluntary activation. J Frailty Aging. 2016;5(Supplement 1: P29):63. [Google Scholar]
- 44.Madhavan S, Sriraman A, Freels S. Reliability and variability of tDCS induced changes in the lower limb motor cortex. Brain Sci. 2016;6(3) doi: 10.3390/brainsci6030026. [DOI] [PMC free article] [PubMed] [Google Scholar]