Abstract
We examined visual recognition memory and executive functioning (spatial working memory, spatial planning, rule learning, and attention shifting) in 12-year-olds (n =150) who participated in the Bucharest Early Intervention Project, a randomized controlled trial of foster care for institutionally reared children. Similar to prior reports at 8 years of age, institutionally reared children showed significant deficits in visual recognition memory and spatial working memory. Deficits in attention shifting and rule learning were also apparent at this time point. These data suggest that early experiences continue to shape the development of memory, learning, and executive functioning processes in pre-adolescence, which may explain broader cognitive and learning difficulties commonly associated with severe early life neglect.
Keywords: memory, executive function, psychosocial deprivation, early intervention, early adversity
Psychosocial deprivation experienced by children reared in institutions is a well-known risk factor for various cognitive delays and academic problems (Beckett et al., 2007; Nelson, Fox, & Zeanah, 2014; van Ijzendoorn, Juffer, & Poelhuis, 2005). Alterations in more basic neurodevelopmental processes involving memory and executive functions may contribute to these difficulties. Although memory and executive functioning problems have been observed among institutionally reared children during early and middle childhood (Bauer, Hanson, Pierson, Davidson, & Pollak, 2009; Bos, Fox, Zeanah, & Nelson, 2009; McDermott et al., 2013; McDermott, Westerlund, Zeanah, Nelson, & Fox, 2012; Pollak et al., 2010), the extent to which these deficits persist after middle childhood is less clear. Visual spatial memory and executive functioning become increasingly important for effective learning and academic success as children enter adolescence (Best, Miller, & Naglieri, 2011; Rourke & Finlayson, 1978). Therefore, understanding whether these problems persist as institutionally reared children approach adolescence is critical.
Both human and animal studies have shown that exposure to early adverse contexts affects the development of visual spatial memory and executive functioning (Blair, 2016). For example, maternal separation in rodent pups has been associated with long-term deficits in spatial memory and learning (Meaney, Aitken, van Berkel, Bhatnagar, & Sapolsky, 1988; Oitzl, Workel, Fluttert, Frosch, & De Kloet, 2000). Rodents and non-human primates reared in isolation have shown deficits in executive functioning, specifically in areas involving cognitive flexibility (McLean et al., 2010) and spatial working memory (Beauchamp, Gluck, Fouty, & Lewis, 1991; Capitanio & Mason, 2000; Martin, Spicer, Lewis, Gluck, & Cork, 1991). Animal work points to alterations in frontal and temporal brain regions that subserve memory and executive functioning (Ivy et al., 2010; Mathew et al., 2003; McEwen, 2005; Sanchez, Hearn, Do, Rilling, & Herndon, 1998; Sanchez, Ladd, & Plotsky, 2001) and there is some evidence that these neural regions are affected in institutionally reared as well (Chugani et al., 2001; Eluvathingal et al., 2006).
The broad construct of executive functioning, generally defined as skills necessary for purposeful and goal-directed behavior, is often subdivided into discrete abilities, which typically include inhibitory control, working memory, sustained attention, planning, initiation and cognitive flexibility (Niendam et al., 2012). Parent and teacher ratings suggest that institutional rearing leads to executive functioning problems in early childhood (between age 4 to 7; Jacobs, Miller, & Tirella, 2010), specifically affecting inhibitory control (Bruce, Tarullo, & Gunnar, 2009). These problems have also been noted once institutionally reached children reach middle childhood (10 to 11 years of age; Groza, Ryan, & Thomas, 2008; Merz & McCall, 2011). Longer time spent in the neglecting environment has been predictive of poorer parent or teacher-reported executive functioning in numerous studies, particularly for children adopted after 6, 18, and 36 months of age (Groza et al., 2008; Jacobs et al., 2010; Merz & McCall, 2011; Merz, McCall, & Groza, 2013).
In addition to parent or teacher-reported concerns, post-institutionalized, adopted children have shown poorer performance on standardized memory and executive functioning tasks when compared with non-neglected children. In terms of memory, institutionally reared, adopted children have shown difficulties on tasks involving visual spatial memory and learning (Bauer et al., 2009; Pollak et al., 2010). In terms of executive functioning, children have shown problems across several sub-domains, including inhibitory control, working memory, planning, initiation, and cognitive flexibility (Bauer et al., 2009; Bruce et al., 2009; Colvert et al., 2008; Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012; Loman et al., 2013; Merz, McCall, Wright, & Luna, 2013; Pollak et al., 2010). These behavioral differences have been reported during early childhood, around 3 years of age (Hostinar et al., 2012) and in middle and late childhood, between 8 and 14 years of age (Bauer et al., 2009; Colvert et al., 2008; Loman et al., 2013; Pollak et al., 2010).
Visual recognition memory and learning, and executive functioning involving spatial working memory, spatial planning, and inhibitory control have previously been examined in the Bucharest Early Intervention Program (BEIP), the only randomized controlled trial (RCT) to examine whether foster care can support more optimal developmental trajectories in institutionally reared children. Unlike other studies involving post-institutionalized, adopted children, the randomized design of the BEIP controls for potential selection factors that may influence their likelihood of adoption into family settings. As part of the BEIP, infants and toddlers reared in institutions in Bucharest, Romania, were randomly assigned to receive care as usual (where they remained in the institution; the care as usual group) or were removed from the institution and placed in high quality foster care (the foster care group). The development of these children has been followed longitudinally and compared to a group of children matched on age and gender, who were reared in their biological families in the local community (the never institutionalized group).
During early childhood (4 to 5 years of age), children in the BEIP were assessed on a core domain of executive functioning, inhibitory control, an early emerging skill that involves inhibiting a pre-potent response. At this assessment, institutionally reared children in the care as usual and foster care groups showed poorer performance when compared to the never institutionalized group (Nelson et al., 2014). Once they reached middle childhood (8 to 10 years of age), inhibitory control was re-assessed with two tasks, a go/no-go task and a flanker task. Although no intervention effect was observed in the flanker task, (requiring a response to target stimuli flanked by distracting stimuli), relative to the care as usual group, children placed in foster care showed better performance on the go/no-go task, which required a response to target stimuli on some trials, and inhibition of a response to non-target stimuli on other trials (McDermott et al., 2013; McDermott et al., 2012).
Broader domains of memory and executive functioning were also assessed using the Cambridge Neurodevelopmental Test and Assessment Battery (CANTAB) at this assessment. Select subtests of the CANTAB were administered to assess visual recognition memory and learning, spatial working memory, and spatial planning. Consistent with previous investigations that administered the CANTAB to post-institutionalized adopted youth (Bauer et al., 2009; Pollak et al., 2010), children in the care as usual and foster care groups showed poorer performance on all assessed domains of memory and executive functions, and there was no significant intervention effect for any domain (Bos et al., 2009).
In summary, data converges to suggest that visual recognition memory, spatial working memory, and spatial planning deficits are common in institutionally reared children in the BEIP. Further, there is only modest support for the potential for remediation in executive functioning, and this remediation has been limited to one form of inhibitory control. It is currently unknown whether memory and executive functioning problems persist as children enter adolescence. Given that adolescence is a significant period of cognitive development (especially in working memory, inhibitory control, planning, and goal-directed behavior; Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001; Brocki & Bohlin, 2004) and in the development of underlying circuitries that support these higher level processes (Giedd et al., 1996; Huttenlocher & Dabholkar, 1997), detecting potential delays as children approach adolescence seems critical.
The current study examined visual recognition memory and executive functioning (involving spatial working memory, spatial planning, attention set shifting, and rule learning) in 12-year-old children who participated in the BEIP. This is a follow up from the previous investigation that examined these domains of memory and executive functioning problems when children were 8 to 10 years. We expected that institutionally reared children would continue to show deficits in memory and executive function domains and explored whether entry into foster care would support remediation in these domains once children reached 12 years of age. Given the longitudinal design of this study, we also examined potential group differences in the rates of improvement of these memory and executive functioning domains. For all analyses, we questioned whether problems in these various domains could be explained by concurrent deficits in IQ (Almas, Degnan, Nelson, Zeanah, & Fox, under review). Finally, we explored whether the duration of the early psychosocial deprivation and stability of the post-institutional family environment would help explain the variability in performance on memory and executive function tasks.
Method
Participants
Participants in this study were Romanian children enrolled in the Bucharest Early Intervention Project (BEIP), a longitudinal, randomized controlled trial of foster care for institutionally reared children. Institutionally reared children were recruited from six institutions in Bucharest, Romania. The institutions were characterized by overly regimented schedules and a dearth of social, cognitive, and linguistic input typically provided in the first years of life. They also experienced a lack of stable caregiving due to rotating shifts and high child-to-caregiver ratios. At the baseline assessment, children ranged in age from 5 to 31 months of age, with a mean age of 20 months at the start of the study. Prior to enrollment, children in the BEIP were assessed for the exclusionary criteria, which included the presence of genetic syndromes, fetal alcohol syndrome, and micro- or macrocephaly.
Following the baseline assessment, half of the children were randomly assigned the foster care condition (the foster care group) and were compared to children who remained in the institution (the care as usual group). As part of the RCT, foster care was designed to provide a responsive family environment to the previously institutionally reared children. To achieve this goal, foster parents received substantial training on the specialized needs of the children placed into their care. Social workers supported the development of high quality relationships between the foster caregivers and children during regular visits with families. Foster parents were also helped to respond to the emotional and behavioral needs of the child in their care.
At the baseline assessment, children and families in a comparison group (i.e. the never-institutionalized group) were recruited from pediatric clinics in Bucharest, and were matched to the sample of institutionalized children by sex and age (see Nelson et al., 2014; Zeanah et al., 2003 for a more detailed description of the sample). These children were also followed longitudinally, and compared to children in the care as usual and foster care group at each assessment. Follow up assessments took place once children reached 30 months, 42 months, and 54 months of age. At that point in the study, the trial concluded, but follow-up assessments continued once children reached 8 and 12 years of age. When the RCT concluded, the foster care network was turned over to local child protection authorities. The support provided to foster families was reduced from that originally provided during the active trial. Given the reduced support, some foster parents requested the removal of the child from their home. In these cases, some children re-united with their original biological families and others were placed into another foster home. Further, throughout the study, a “non-interference” policy was adopted so that children in the care as usual group were supported in transitioning to a family environment, if the opportunity arose. Therefore, by the time children reached 12 years of age, many children experienced one or more transitions in their caregiving environment since the onset of the study. See the CONSORT diagram for additional details on placement status at the 12 year assessment.
For the current analyses, data were drawn from an assessment that took place when children were 12 years of age (M = 12.7, range = 11.1 to 14.4 years). At this assessment, there were 150 children enrolled in the study, including 49 in the care as usual group (51% were male, n = 25), 50 in the foster care group (50% were male, n = 25) and 51 in the never institutionalized group (43.1% were male, n = 22).
Measures
CANTAB
Participants completed a touch screen-based, automated neuropsychological battery (Cambridge Neuropsychological Test and Automated Battery; CANTAB; Cambridge Cognition, Cambridge, UK). The CANTAB focuses primarily on measuring functions of the temporal and prefrontal cortices, with tests falling into various domains that assess attention, memory and executive functioning. The CANTAB has been extensively validated for children in this age group (Luciana & Nelson, 1998, 2002). Five subtests of the CANTAB were administered at the 12 year assessment. The Delayed Matching to Sample and Paired Associates Learning tests were administered to assess visual recognition memory and learning. The Spatial Working Memory, Stockings of Cambridge, and Intra-Dimensional/Extra-Dimensional Set Shifting tests were administered to assess spatial working memory, planning, attention set shifting, and rule learning. All subtests, with the exception of the Intra-Dimensional/Extra-Dimensional Set Shifting subtest, were administered at the prior assessment, when children were 8 years of age.
Delayed matching to sample (DMS)
This subtest assesses attention and short term visual memory. The subject is shown a pattern and is asked to choose which pattern, out of four more patterns, exactly matches the original pattern. In some of the trials, the four choices are presented with the original pattern simultaneously. In others, the original pattern is obscured before the choices appear, or there is a brief delay between these steps. Outcome measures include accuracy and response latency. This task reflects the functioning of the medial temporal lobe with input from the frontal lobe (Sahakian et al., 1988).
Paired associates learning (PAL)
This subtest assesses visual episodic memory and new learning. In this task, subjects are required to remember patterns associated with different locations on the screen. A series of boxes is displayed. Boxes are opened one by one in random order, with some boxes revealing a pattern once opened. After all boxes have opened and closed, a pattern appears on the middle of the screen. The subject must identify the location where this pattern was located earlier. If the subject does not identify each location correctly, the trial is repeated. Once all trials are correctly completed, the task proceeds to the next set, in which an increasing number of boxes and patterns is displayed. Stages completed, number of trials, and the total number of errors are recorded. Performance on this task has been associated with medial temporal lobe functions (Aizenstein et al., 2000).
Stockings of Cambridge (SOC)
This is a spatial planning task based on the Tower of London (Shallice, 1982), in which the subject must copy a pattern displayed on the screen by moving colored circles one at a time, using the fewest number of moves possible. A key outcome measure is the number of problems that are solved in the minimum number of moves. Other outcome measures include response latency time and mean moves made. Performance on this task is also correlated with frontal lobe functioning (Baker et al., 1996; Owen, Downes, Sahakian, Polkey, & Robbins, 1990).
Spatial working memory (SWM)
This subtest assesses spatial working memory or abilities to continually update information about spatial locations in memory. The subject is asked to search through boxes to find a hidden token. S/he is told that once a token in a given box has been found, that specific box will not contain any tokens in the future. Outcome measures include the total number of errors committed (reflecting number of failures in the updating process) and a composite strategy score, reflecting the subject’s ability to search through available items in an organized method. This task involves recruitment of the dorsal/ventral prefrontal cortex and the ascending catecholamine systems (Luciana & Nelson, 1998; Owen et al., 1990; Owen, Evans, & Petrides, 1996; Owen, Morris, Sahakian, Polkey, & Robbins, 1996; Owen, Sahakian, Semple, Polkey, & Robbins, 1995).
Intra-extra dimensional set shifting (ID/ED)
This subtest measures attention shifting, discrimination, and reversal learning. As part of this task, subjects view a set of stimuli consisting of color-filled shapes and/or white lines that appear on the screen. The subject must first learn the correct rule (i.e. shapes with white lines are “correct”), by receiving feedback after selecting stimuli at random. After six correct responses, the rule is considered “learned” and subjects move to the next level. The rule may apply to the next set (i.e. shapes with white lines are still correct). If so, subjects are required to exhibit an intra-dimensional shift in attention (i.e. shift attention to a separate set of stimuli, but classify them along the same dimension as in the previous trial). On other trials, the rule changes (i.e. color-filled shapes are now correct). Here, subjects complete an extra-dimensional shift in attention (i.e. shift attention to a different set of stimuli, and classify them along a different dimension). The task consists of nine stages. Subjects progress through each stage by demonstrating six consecutive correct responses before reaching 50 trials, thereby satisfying a criterion of learning for that stage. Outcome measures include the total number of errors adjusted for the number of stages completed, the number of extra-dimensional shift errors, and the number of intra-dimensional shift errors. Prefrontal regions, specifically involving the dorsolateral and orbitofrontal regions, have been implicated in “intra-dimensional” and “extra-dimensional” set shifting during this task (Luciana & Nelson, 1998; Owen, Roberts, Polkey, Sahakian, & Robbins, 1991).
IQ
At 12 years of age, IQ was assessed with the Wechsler Intelligence Scale for Children, WISC-IV (Wechsler, 2003). Ten subtests were administered to assess four domains of IQ. These included verbal comprehension, perceptual reasoning, working memory, and processing speed. A composite full scale IQ (FSIQ) score was also calculated and used as a covariate in all analyses. The WISC was administered by trained and reliable psychologists in Bucharest, Romania.
Data Analyses
For each subtest, two sets of data analyses were conducted. In the first, the ever institutionalized group, composed of children in both the care as usual and foster care groups, was compared to the never institutionalized group. The second set of analyses used an intent-to-treat approach and examined whether early intervention status supported remediation in visual recognition memory, spatial working memory, and executive functioning. For these analyses, the care as usual group was compared with the foster care group. Outcome measures included performance on memory and executive functioning at the current assessment and also in the rate of change in memory and executive functioning between the two subtests administered at the prior assessment (when children were 8–10 years of age) and the current assessment. For all analyses, follow up analyses explored whether observed group differences were due to variability in IQ. Finally, we explored whether additional factors, such as duration of neglect, timing of intervention, or placement stability modulated outcomes observed in preadolescence.
Prior to analyses, data on each CANTAB subtest were inspected for extreme outlying values; values that exceeded three times the interquartile range were winsorized. This included three values from children in the care as usual group and four values from children in the foster care group. Whether males and females differed in their CANTAB performance was also examined. No significant gender differences emerged; therefore, gender was not included as a covariate in analyses.
Linear regression models were used to examine the association between institutional rearing and various domains of memory and executive functioning at 12 years of age. See table 2 for correlations between primary variables. Given the large number of analyses, for each of the two sets of regression analyses (the first that compared ever institutionalized and never institutionalized and the second that compared care as usual versus foster care), results were considered significant if they survived a bonferonni corrected p value of .006 (correcting for 9 comparisons). For each CANTAB domain, exploratory, follow up analyses were then conducted on additional subscales to determine if effects were driven by trial type or level of difficulty. Repeated measures analyses of variance (ANOVA) was used to examine group differences in the change in memory and executive functioning across assessments. Again, for each set of analyses, p values were also considered significant at the corrected level of .006. Linear regression was used to explore whether timing of the intervention, duration of the neglect, and number of placement transitions modulated performance on memory and executive functioning tasks.
Table 2.
Correlations between subtests on the CANTAB
| CANTAB Subtest | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| DMS pct correct all delays | 1 | – | – | – | – | – | – | – | – |
| DMS prob error given error | −.70** | 1 | – | – | – | – | – | – | – |
| DMS mean corr latency | .11 | −.09 | 1 | – | – | – | – | – | – |
| PAL mean errors to success | −.43** | .26** | –.08 | 1 | – | – | – | – | – |
| PAL Mean Trials to success | −.45** | .25** | –.08 | .94** | 1 | – | – | – | – |
| SOC prob solved in min moves | .20* | −.13 | .01 | −.27** | −.30** | 1 | – | – | – |
| SWM Total errors | −.46** | .28** | −.14 | .24** | .30** | −.43** | 1 | – | – |
| SWM Strategy | −.30** | .24** | −.11 | .14 | .17* | −.26** | .65** | 1 | – |
| ID/ED Total errors | −.29** | .12 | −.01 | .22** | .27** | −.06 | .20* | .16* | 1 |
DMS: Delayed Matching to Sample; PAL: Paired Associates Learning; SOC: Stockings of Cambridge; SWM: Spatial Working Memory; ID/ED: Intra-dimensional, Extra-dimensional set shifting;
: p < .001;
: p < .05.
For all models, residual values were inspected for normality. In the case of non-normally distributed residual values, a log10 transform was applied to the dependent variable, and models were re-run with transformed values. For all models, results with transformed data did not differ from those using raw data; therefore, results with transformed values are reported below. Birth weight was included as a covariate in all models as a control for potential pre- or peri-natal factors that may influence the development of memory or executive function.
For all analyses, follow up models examined whether general cognitive functioning, as measured by IQ, would affect any subgroup difference revealed in CANTAB functioning. Some domains of IQ measured by the WISC-IV (i.e. the Working Memory Index (WMI)), are considered more reliant on executive functioning skills than others (i.e. the Processing Speed Index (PSI) and the Perceptual Reasoning Index (PRI)). Further, children in both the foster care and care as usual groups showed deficits on the WMI domain at the 12 year assessment (Almas et al., under review). Therefore, we compared results when adding an IQ covariate that contained the working memory domain (i.e. the FSIQ), with an IQ covariate that did not include the working memory domain (i.e., the PSI and PRI). Results did not differ when we included the FSIQ versus the PSI or PRI score as covariates. Therefore, we only report results that used the FSIQ score as a covariate. Table 3 shows descriptive values of subtest scores across each group, with and without covariates.
Table 3.
Visual Memory and Learning and Executive Functioning Performance on the CANTAB
| CANTAB Subtests | Domain | CAUG M(SD) | FCG M(SD) | NIG M(SD) | Effect Covarying for BW | Effect Covarying for BW and IQ |
|---|---|---|---|---|---|---|
|
| ||||||
| Delayed Matching to Sample | Total percent correct (all delays) | 70.55(13.32) | 71.66(14.24) | 81.04(11.47) | EIG < NIG | ns |
| Probability of error following prev. error | .19(.14) | .19(.17) | .09(.12) | EIG > NIG | ns | |
| Mean correct | 3993.51 | 3578.36 | 3582.71 | EIG > NIG | ns | |
| Latency (all delays) | (1263.81) | (1082.15) | (1036.53) | |||
| Paired Associates Learning | Mean number of errors per stage | 1.66 (1.84) | 1.55(1.31) | .83(.85) | EIG > NIG | ns |
| Mean number of trials per stage | 1.51 (.32) | 1.50(.38) | 1.33(.26) | ns | ns | |
| Number of stages completed | 7.94(.24) | 7.92(.39) | 8.00(.00) | ns | ns | |
| Stockings of Cambridge | Number of probs. solved in min. number of moves | 6.52(1.72) | 7.34(1.71) | 7.94(1.48) | ns | ns |
| Spatial Working Memory | Total number of errors | 54.67(16.47) | 48.70(17.24) | 30.96(19.56) | EIG > NIG | ns |
| Strategy | 37.73(3.28) | 37.42(3.51) | 34.27(5.49) | EIG > NIG | ns | |
| ID/ED Set Shifting | Total errors (adj.) | 37.96(19.92) | 43.68(19.18) | 31.67(21.50) | EIG > NIG | ns |
| Stages Completed | 8.02(.93) | 7.92(.96) | 8.24(.92) | ns | ns | |
CAUG = care as usual group; FCG = foster care group; NIG = never institutionalized group; BW = birth weight; ID = Intra-dimensional; ED = Extra-dimensional.
Results
Visual Memory and Learning
Delayed matching to sample
Group differences at 12 years
Performance on the DMS subtest varied as a function of institutional rearing. The ever institutionalized group scored a lower percentage of correct responses (β = .315, p < .001), which survived correction for multiple comparisons. Exploratory follow-up analyses, considered significant at p = .05, were conducted to determine if effects were driven by performance on more difficult trials involving delays in stimulus presentation that engaged working memory. Associations remained significant across all trial types, including when the sample and choice patterns were presented simultaneously, or after a 0ms delay, and when increased working memory was required to complete the task, due to a 4000ms, or 12000ms delay in stimuli presentation (all p vals < .05). Relative to the never institutionalized group, the ever institutionalized group was also more likely to make an incorrect response after making an error on the previous trial (indicating reduced performance monitoring, β = −.297, p = .001), which survived correction. However, the association between institutional rearing histories and performance on both of these scales (percent of correct responses, and probability of making an incorrect response following a previous error), at any level of difficulty, was no longer significant once FSIQ was included as a covariate (p values ranged from .503 to .563).
The ever institutionalized group showed longer mean latencies on correct responses overall (β = −.286, p = .002), which survived correction. Exploratory follow-up analyses, considered significant at p = .05, were conducted on different trial types to determine if effects were driven by more difficult trials that engaged working memory via stimulus delays. The ever institutionalized group showed longer latencies when the sample and choice stimuli were presented simultaneously, (β = −.306, p = .001), but not when there was a 0ms delay (β = −.106, p = .254), 4000ms delay (β = −.155, p = .094), or 12000ms delay (β = −.067, p = .468).
When controlling for FSIQ and after correction for multiple comparisons, institutional rearing was not significantly associated with the mean latency for correct responses for trials collapsed across delay intervals (β = −.302, p = .019). Exploratory analyses examined whether group differences might emerge as tasks demands grew more difficult and required more working memory. With FSIQ as a covariate, significant group differences were observed when sample and choice stimuli were presented simultaneously (β = −.291, p = .009), and after a 400ms delay (β = −.278, p = .014), or 1200ms delay (β = −.218, p = .05), but not at a 0ms delay (β = −.192, p = .09).
Intervention Effects
For the intent-to-treat analyses, comparisons between children in the foster care group versus children in the care as usual group revealed no significant group differences on any subscale of the DMS, with and without IQ as a covariate (all p values > correction threshold of .006); thus, no intervention effect was observed for this domain.
Change from 8 to 12 years
On the DMS subtest, after correcting for multiple comparisons, there was no significant change in the percentage of correct responses from the 8 to 12 year assessment for the full sample, F(1,122) = 4.87, p = .029, or in rates of change of never institutionalized versus ever institutionalized groups (p value > .006). There was also no significant change in performance monitoring; groups did not differ in the likelihood that children made an error after an incorrect response. Latency of responses also did not change over time for the full sample or subgroups (all p values > correction threshold of .006).
In terms of the intent-to-treat comparisons, children in the foster care and care as usual groups did not significantly differ in the change in percentage of correct responses, probability in making an error after an error, or in their response latency from the 8 to 12 year assessment, with and without IQ as a covariate; thus revealing no significant effect of the intervention (all p values > correction threshold of .006).
Paired associates learning
Group differences at 12 years
Children with histories of institutional rearing showed poorer performance on the PAL subset, relative to the never institutionalized group. The ever institutionalized group made significantly more errors prior to successfully completing a stage (β = −.254, p = .005), which survived correction, but they did not take significantly more trials to complete each stage, after correction for multiple comparisons, (β = −.198, p = .034). The number of stages completed did not differ between the ever and never institutionalized groups; after controlling for FSIQ, the association between institutional rearing status and performance on any of the PAL subscales was no longer significant (p values ranged from .432 to .805).
Intervention Effects
Intent-to-treat comparisons revealed that relative to the care as usual group, children in the foster care group did not significantly differ in their performance on any domain of the PAL, with and without IQ included as a covariate; and thus, no intervention effect emerged (all p values > correction threshold of .006).
Change from 8 to 12 years
There was no significant change in the number of trials taken to complete each stage, the total number of stages completed, or the number of errors made prior to successfully completing each stage from the 8 year to 12 year assessment for the full sample or any subgroup comparisons (ever institutionalized versus never institutionalized, or care as usual versus foster care), all p-values > correction threshold of .006.
Executive Function
Stockings of Cambridge
Group differences at 12 years
After controlling for multiple comparisons, the number of problems solved in the minimum number of moves did not significantly differ between children with and without histories of institutional rearing (β = .224, p = .013). Total thinking time for all problem types did not differ across groups. When FSIQ was entered as a covariate, there was also no significant group difference in performance on the subscales of this task (p values ranged from .653 to .742).
Intervention Effects
After controlling for multiple comparisons, there were no significant differences in the number of problems solved in the minimum number of moves (β = .248, p = .014), or in the thinking time for each problem level for children in the foster care group versus care as usual group (all p values > correction threshold of .006), indicating no significant intervention effect for this domain.
Change from 8 to 12 years
The number of problems completed in the minimum number of moves and thinking time allocated to each problem did not significantly change over time for the full sample or for either subgroup, all p values > correction threshold of .006.
Spatial working memory
Group differences at 12 years
Children in the ever institutionalized group made more total errors than children in the never institutionalized group (β = −.341, p < .001), which survived correction. The ever institutionalized group also showed significantly worse strategy scores (i.e., the extent to which a repetitive/inefficient search pattern was used, determined from the number of search sequences that initiated with a previously opened box) relative to the never institutionalized group (β = −.327, p < .001), which also survived correction. After controlling for FSIQ, the associations between institutional rearing and performance on the spatial working memory task subscales were no longer significant (p values ranged from .227 to .287).
Intervention Effects
With regard to the intent-to-treat comparisons, children in the foster care group did not significantly differ in the number of errors made or in their strategy scores, relative to the care as usual group, with and without IQ as a covariate; thus, indicating no significant effect of the intervention, all p values > correction threshold of .006.
Change from 8 to 12 years
Both children in the ever and never institutionalized group showed significant decreases in the number of total errors made over time, F(1,122) = 12.35, p = .001, which survived correction. Children in the never institutionalized group showed significantly greater decline in the number of total errors made relative to children in the ever institutionalized group, F(1,122) = 10.80, p = .001, which also survived correction (see figure 1). Exploratory analyses, considered significant at p < .05, revealed that these associations were driven specifically by performance on the more difficult 8-move problems, F(1,122) = 15.00, p < .001. After controlling for FSIQ, group differences in changes over time were not significant (all p values > correction threshold of .006). Intent-to-treat comparisons showed that children in the foster care and care as usual groups did not significantly differ in the change in the number of errors made over time with and without IQ as a covariate (all p values > correction threshold of .006); thus, there was no significant effect of the intervention for this domain.
Figure 1.
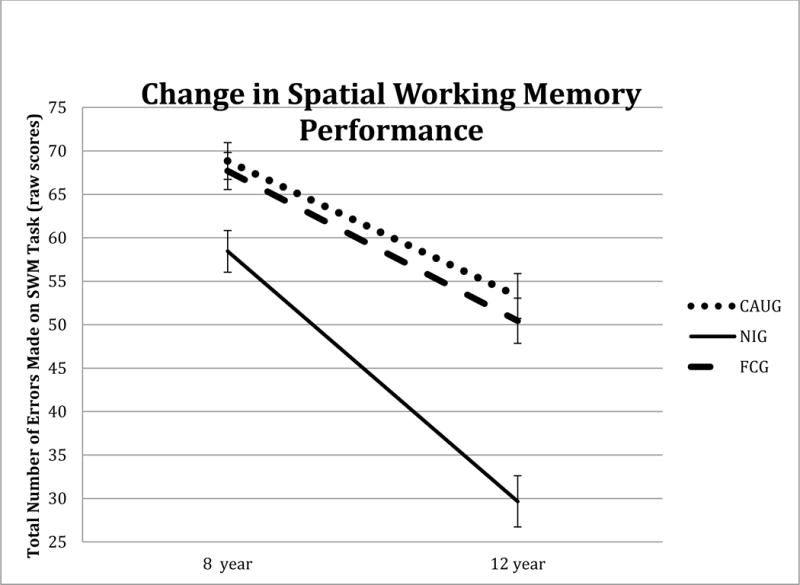
Change in Spatial Working Memory Performance across the 8 to 12 year assessment for children in the care as usual (CAUG), foster care group (FCG) and never institutionalized group (NIG).
Intra/extra dimensional set shifting
Group differences at 12 years
The ever institutionalized group showed poorer performance on this task when compared with the never institutionalized group. The ever institutionalized group committed more total errors adjusted for the number of stages completed, (β = −.251, p = .006), which survived correction. This association was no longer significant when FSIQ was included as a covariate (p values ranged from .274 to .992). Exploratory analyses, considered significant at p < .05, were conducted to see if overall group differences were driven by trial type. The ever institutionalized group did not differ in the number of intra- or extra-dimensional errors, or in the number of stages completed.
Intervention Effects
Intent-to-treat comparisons revealed no significant difference in the total number of errors made by the foster care group versus the care as usual group, with and without IQ entered as a covariate (all p values > correction threshold of .006).
Effects of intervention timing, stability, and duration of neglect
Intervention timing
We also examined whether the timing of the intervention, determined by the age at which children were placed into foster care, was associated with CANTAB performance. For these analyses, the child’s age at the time of assignment into foster care was correlated with performance on the primary subscales of each task. Consistent with previous observations when children were 8 years of age, age at the time of foster care placement was not significantly associated with performance on any domain of the CANTAB when children reached 12 years of age.
Foster care stability
At the 12-year assessment, a significant proportion of children originally placed into foster care had transitioned to another home. Specifically, of the 56 children originally assigned to the foster care group at the 12-year assessment, only 29 remained in their original placement. Using a series of linear regressions, we tested whether the performance of children who remained in their original foster placement differed from those that transitioned out of their original placement. We also tested whether foster placement stability interacted with age of placement to explain performance; we expected that children who were assigned to foster care at earliest ages who also remained in their original placements, would show significantly better performance than children who transitioned out of foster care and/or were placed at later ages. Results of this comparison revealed no main effect of placement stability or interaction between placement stability and age of foster care placement on CANTAB performance.
Duration of neglect
At the time of the 12-year assessment, only 20 children in the care as usual group remained in the institutional setting. Using linear regression, we explored whether the duration of neglect, as well as the number of placement transitions, experienced up until the 12-year assessment predicted CANTAB performance. However, no significant main effects or interactions between these variables emerged.
Discussion
This study examined links between institutional rearing and the development of visual recognition memory and learning and executive functioning (including spatial working memory, spatial planning, attention set shifting, and rule learning) during preadolescence. Children with histories of institutional rearing were compared to non-institutionally reared children on executive functioning and memory performance once they reached early adolescence. We also examined whether placement into foster care during early childhood supported long term remediation in executive function and memory. Results suggest that institutionally reared children in this study continued to show poorer performance on tasks requiring visual recognition memory, spatial working memory, attention set shifting and rule learning. As shown previously, there were minimal effects of the intervention in supporting long-term remediation of these functions. Collectively, these results suggest that institutional rearing continues to affect specific domains of memory and executive functioning as children approach adolescence.
Performance on the PAL and DMS at this assessment was consistent with results reported when children were in middle childhood (Bos et al., 2009). As reported previously, institutionally reared children generally performed more poorly on these tasks relative to never-institutionally reared children, and there was no significant intervention effect. Longitudinal analyses indicated that all children improved in their performance on this task over time, and the rate of improvement did not significantly differ as a function of institutional rearing. Performance on the DMS and PAL involve a range of neurocognitive functions, involving visual recognition memory and learning, attention, short term memory (for trials with longer delays), and performance monitoring. Performance on these tasks have been linked with medial temporal lobe functioning, particularly involving the hippocampus, with performance on the DMS also involving some input from the frontal lobe (Moscovitch, 1994; Robbins et al., 1998; Sahakian et al., 1988). Results here may provide indirect evidence for alterations in the development of fronto-temporal circuitry that results from institutional rearing.
Consistent with two prior studies involving institutionally reared children (Bauer et al., 2009; Pollak et al., 2010), children in the BEIP continued to show delays in spatial working memory at 12 years of age. In order to successfully complete the spatial working memory task, children were required to retain spatial information, manipulate information in working memory, and use a strategy to organize their search for targets. Therefore, delays on this task could indicate difficulties in working memory and/or employment of strategic approaches to solve complex problems. There was no evidence for remediation in spatial working memory for children randomly assigned to foster care, which is consistent with previous data reported when children were in middle childhood (Bos et al., 2009). Examination of trajectories over time revealed that all children improved in their spatial memory skills; however the rate of improvement was significantly lower for children with histories of institutional rearing. This is concerning in that it suggests that performance gaps in spatial working memory between institutionally reared and non institutionally reared children grow larger over time. Performance on the spatial working memory task has been shown to be sensitive to frontal lobe dysfunction, specifically involving the ventrolateral and dorsolateral cortices (Luna et al., 2002; Owen et al., 1990; Owen, Evans, et al., 1996; Owen, Morris, et al., 1996). Poorer performance in spatial working memory among institutionally reared children may reflect alterations in the development of these underlying neural structures.
Although not examined at the 8-year assessment, rule learning and attention shifting, assessed with the ID/ED task, was examined once children reached 12 years of age. On average, children successfully completed all stages of this task, and there was no significant difference in the number of stages completed across groups. However, children with histories of institutional rearing committed more errors relative to non-institutionally reared children, suggesting that they required more feedback in order to successfully shift their attention to new sets of intra-dimensional or extra-dimensional stimuli.
In summary, during pre-adolescence, institutionally reared children in the BEIP showed significant difficulty completing tasks requiring visual spatial recognition memory, spatial working memory, attention set shifting, and rule learning. Group differences in performance were no longer apparent after controlling for IQ. This suggests that institutional rearing has profound effects on general cognitive functioning, and on the more specific subdomains of memory and executive functioning that subserve broader domains of cognitive functioning. The identification of these specific cognitive deficits may help explain high rates of academic and learning problems, commonly associated with institutional rearing.
Early caregiving experiences, involving encouraging children’s self regulatory skills, are considered a primary driver of neurocognitive development, including the development of memory and executive function (Bernier, Carlson, & Whipple, 2010; Carlson, 2009). A core hypothesis of this study concerns the extent to which entry into a responsive family environment could support recovery in key cognitive functions. The majority of children were around two years of age when placed into foster care; few children were placed into foster care prior to 6 months of age. Therefore, future work should examine whether removal from institutional during the first months of life might support more optimal patterns of development. Further, mere entry into a responsive family environment may not be sufficient to support the development of these more specialized cognitive skills. Additional forms of intervention, such as those that specifically target executive function and memory development, may be required for improvements to be observed.
Findings from this study should be interpreted in the context of several methodological issues. First, comparisons between the institutionally reared and non-institutionally reared children in this study rely on a correlational design. Unlike prior work, which has demonstrated associations between the duration of neglect or timing of the intervention and executive functioning (Audet & Le Mare, 2010; Merz, McCall, Wright, et al., 2013), timing was not a significant predictor of performance in this sample. However, given the sample size, some of our analyses may have been underpowered to detect effects; replication with a larger sample will be critical. It is also possible that additional prenatal risk factors, not captured by our proxy variable (birth weight), might contribute to the various memory and executive functioning problems observed in this sample. While more systematic control for these risk factors is needed, these data are often difficult to acquire, given that children are often placed into the institution shortly after birth. Despite limitations, the results from this study extend knowledge on how early life neglect influences the long-term development of memory and executive functioning. They are among the first to examine various memory and executive functioning problems in pre-adolescence, a point in development where these skills become critical for effective learning and academic success.
Figure 2.
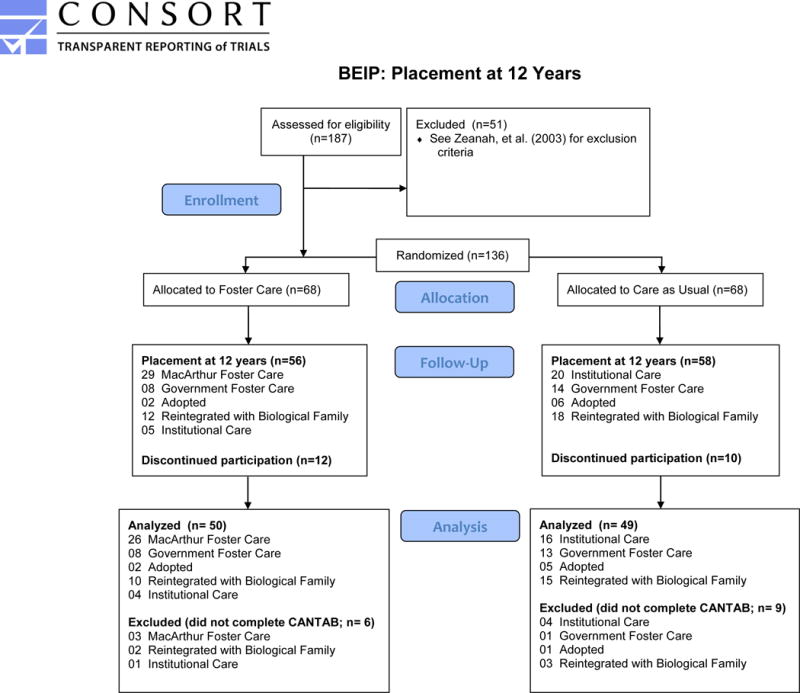
CONSORT diagram.
Table 1.
Demographic Characteristics of Children in the BEIP at the 12-year assessment
| Child Characteristics | CAUG | FCG | NIG |
|---|---|---|---|
| Age in years (SD) | 12.62 (.5525) | 12.65 (.54) | 12.77 (.45) |
| Ethnicity (%) | |||
| Romanian | 22 (44.9%) | 27 (54%) | 47 (92.2%) |
| Rroma (Gypsy) | 20 (40.8%) | 15 (30%) | 2 (3.9%) |
| Unknown | 66 (12.2%) | 7 (14%) | 49 (96.1%) |
| Other | 11 (2%) | 1 (2%) | 2 (3.9%) |
| Gender (%) | |||
| Female | 24 (49%) | 25 (50%) | 29 (56.9%) |
| Male | 25 (51%) | 25 (50%) | 22 (43.1%) |
Acknowledgments
Preparation of this manuscript was supported by the John D. and Catherine T. MacArthur Foundation, the Binder Family Foundation, the Help the Children of Romania, Inc foundation, and NIMH (MH091363) to C.A. Nelson.
References
- Aizenstein HJ, MacDonald AW, Stenger VA, Nebes RD, Larson JK, Ursu S, Carter CS. Complementary category learning systems identified using event-related functional MRI. Journal of Cognitive Neuroscience. 2000;12(6):977–987. doi: 10.1162/08989290051137512. [DOI] [PubMed] [Google Scholar]
- Almas A, Degnan K, Nelson CA, Zeanah CH, Fox NA. IQ at age 12 following a history of institutional care: Findings from the Bucharest Early Intervention Project. doi: 10.1037/dev0000167. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20(1):385–406. doi: 10.1207/s15326942dn2001_5. [DOI] [PubMed] [Google Scholar]
- Audet K, Le Mare L. Mitigating effects of the adoptive caregiving environment on inattention/overactivity in children adopted from Romanian orphanages. International Journal of Behavioral Development. 2010;35(2):107–115. doi: 10.1177/0165025410373313. [DOI] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34(6):515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2009;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp AJ, Gluck JP, Fouty HE, Lewis MH. Associative processes in differentially reared rhesus monkeys (Macaca mulatta): blocking. Developmental Psychobiology. 1991;24(3):175–189. doi: 10.1002/dev.420240304. [DOI] [PubMed] [Google Scholar]
- Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Sonuga-Barke EJ. Scholastic attainment following severe early institutional deprivation: a study of children adopted from Romania. Journal of Abnormal Child Psychology. 2007;35(6):1063–1073. doi: 10.1007/s10802-007-9155-y. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From External Regulation to Self-Regulation: Early Parenting Precursors of Young Children’s Executive Functioning. Child Development. 2010;81(1):326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Naglieri JA. Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learning and Individual Differences. 2011;21(4):327–336. doi: 10.1016/j.lindif.2011.01.007. http://dx.doi.org/10.1016/j.lindif.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. Developmental Science and Executive Function. Current Directions in Psychological Science. 2016;25(1):3–7. doi: 10.1177/0963721415622634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, Nelson CA. Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3:16. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Developmental Neuropsychology. 2004;26(2):571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Development and Psychopathology. 2009;21(1):157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA. Cognitive style: problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate substitute mothers. Journal of Comparative Psychology. 2000;114(2):115–125. doi: 10.1037/0735-7036.114.2.115. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Social origins of executive function development. New Directions for Child and Adolescent Development. 2009;2009(123):87–98. doi: 10.1002/cd.237. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Kreppner J, Beckett C, Castle J, Groothues C, Sonuga-Barke EJ. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: findings from the English and Romanian adoptees study. Journal of Abnormal Child Psychology. 2008;36(7):1057–1068. doi: 10.1007/s10802-008-9232-x. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Groza V, Ryan SD, Thomas S. Institutionalization, Romanian Adoptions and Executive Functioning. Child and Adolescent Social Work Journal. 2008;25(3):185–204. doi: 10.1007/s10560-008-0120-6. [DOI] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, Gunnar MR. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17208–17212. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience. 2010;30(39):13005–13015. doi: 10.1523/jneurosci.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, Miller LC, Tirella LG. Developmental and behavioral performance of internationally adopted preschoolers: a pilot study. Child Psychiatry and Human Development. 2010;41(1):15–29. doi: 10.1007/s10578-009-0149-6. [DOI] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Westerlund A, Pollak SD, Nelson CA, Gunnar MR. The effect of early deprivation on executive attention in middle childhood. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2013;54(1):37–45. doi: 10.1111/j.1469-7610.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developmental Neuropsychology. 2002;22(3):595–624. doi: 10.1207/s15326942dn2203_3. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59(6):834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. Journal of Neuroscience. 1991;11(11):3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Coplan JD. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biological Psychiatry. 2003;54(7):727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Frontiers of Human Neuroscience. 2013;7:167. doi: 10.3389/fnhum.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2(Supplement 1):S59–66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism – Clinical and Experimental. 2005;54(5):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J. Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. Journal of Psychopharmacology. 2010;24(1):57–63. doi: 10.1177/0269881108093842. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239(4841 Pt 1):766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Merz EC, McCall RB. Parent ratings of executive functioning in children adopted from psychosocially depriving institutions. Journal of Child Psychology and Psychiatry. 2011;52(5):537–546. doi: 10.1111/j.1469-7610.2010.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, McCall RB, Groza V. Parent-reported executive functioning in postinstitutionalized children: a follow-up study. Journal of Clinical Child and Adolescent Psychology. 2013;42(5):726–733. doi: 10.1080/15374416.2013.764826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, McCall RB, Wright AJ, Luna B. Inhibitory control and working memory in post-institutionalized children. Journal of Abnormal Child Psychology. 2013;41(6):879–890. doi: 10.1007/s10802-013-9737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1994;8(4):524–534. doi: 10.1037/0894-4105.8.4.524. [DOI] [Google Scholar]
- Nelson CA, Fox NA, Zeanah CH. Romania’s abandoned children: Deprivation, brain development and the struggle for recovery. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive Affective and Behavioral Neuroscience. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Workel JO, Fluttert M, Frosch F, De Kloet ER. Maternal deprivation affects behaviour from youth to senescence: amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. European Journal of Neuroscience. 2000;12(10):3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cerebral Cortex. 1996;6(1):31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119(Pt 5):1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29(10):993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33(1):1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Gunnar MR. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PMA. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Journal of the International Neuropsychological Society. 1998;4(05):474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Finlayson MAJ. Neuropsychological significance of variations in patterns of academic performance: Verbal and visual-spatial abilities. Journal of Abnormal Child Psychology. 1978;6(1):121–133. doi: 10.1007/bf00915788. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812(1–2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Juffer F, Poelhuis CW. Adoption and cognitive development: a meta-analytic comparison of adopted and nonadopted children’s IQ and school performance. Psychological Bulletin. 2005;131(2):301–316. doi: 10.1037/0033-2909.131.2.301. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-IV technical and interpretive manual. San Antonio: TX: Psychological Corporation; 2003. [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15(4):885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]


