Abstract
Contents
Persistent Müllerian duct syndrome (PMDS) is a sex-limited disorder in which males develop portions of the female reproductive tract. Important consequences of PMDS are cryptorchidism and its sequelae of infertility and increased risk for testicular cancer. Anti-Müllerian hormone (AMH) and its receptor (AMHR2) induce the regression of the Müllerian ducts in male embryos. In Miniature Schnauzer dogs the genetic basis has been identified as an autosomal recessive nonsense mutation in AMHR2, but the allele frequency of the mutation is unknown. Thus, the primary objective of this study was to estimate the prevalence of the AMHR2 mutation in North American Miniature Schnauzers, in order to ascertain the value of genetic testing in this breed. An additional objective was to determine whether mutations in AMH or AMHR2 were responsible for PMDS in a Belgian Malinois; this would aid development of a genetic test for the Belgian Malinois breed. Genomic DNA from 216 Miniature Schnauzers (including one known PMDS case) was genotyped for the AMHR2 mutation, and DNA from a single PMDS-affected Belgian Malinois was sequenced for all coding exons of AMH and AMHR2. The Miniature Schnauzer cohort had an AMHR2 mutation allele frequency of 0.16 and a carrier genotypic frequency of 0.27. The genetic basis for PMDS in the Belgian Malinois was not determined, as no coding or splicing mutations were identified in either AMH or AMHR2. These findings support a benefit to AMHR2 mutation testing Miniature Schnauzers used for breeding or with cryptorchidism.
1. Introduction
Persistent Müllerian duct syndrome (PMDS) is a reproductive disorder in which male animals develop parts of the female reproductive tract. In 1976, three cases of male pseudohermaphroditism and cryptorchidism in Miniature Schnauzers were reported (Brown, Burek, & McEntee, 1976). This was the first report of PMDS in dogs. Over the next 40 years, multiple case reports have been published describing clinical consequences of PMDS. Affected males can have oviducts, a uterus, uterine body, cervix, and even a cranial vagina that enters into the prostate (Kuiper, Wagner, Drögemüller, & Distl, 2004; Matsuu et al., 2009; Nickel, Ubbink, van der Gaag, & van Sluijs, 1992; Vegter, 2010). An important consequence of this syndrome is bilateral or unilateral cryptorchidism, which often occurs in PMDS-affected dogs and causes infertility as well as increased risk for testicular tumors (Meyers-Wallen, Donahoe, Ueno, Manganaro, & Patterson, 1989). PMDS can be missed as the underlying cause of cryptorchidism because external genitalia of affected dogs frequently look normal.
Anti-Müllerian hormone (coded by the gene AMH, located on CFA20, and comprised of 5 exons; NCBI Gene ID: 485072) and theanti-Müllerian hormone type II receptor (coded by the gene AMHR2, located on CFA27, and comprised of 11 exons; NCBI Gene ID: 486506), are involved in the regression of the Müllerian ducts in male embryos and are therefore essential in mammalian sex determination. During embryonic development, there is a sexually indifferent stage where both males and females have Müllerian ducts, the precursors of the female reproductive tract. Once testis differentiation occurs in males, there is a critical window where anti-Müllerian hormone is secreted by the testes, binds to AMHR2, and triggers a signaling pathway that results in regression of the Müllerian ducts (Banco, Veronesi, Giudice, Rota, & Grieco, 2012; Meyers-Wallen et al., 1991; Pretzer, 2008). The genetic basis for PMDS in Miniature Schnauzers is a sex-limited autosomal recessive C to T transition (c.241C>T; p.R81*) nonsense mutation in the third exon of the AMHR2 gene (Wu et al., 2009). As a result, AMHR2 is not expressed, and the Müllerian ducts fail to regress in affected males. Females with this mutation develop normal female genitalia. Though a diagnostic test for this mutation has been available in the United States since 2009 (Pujar & Meyers-Wallen, 2009), there are no reports that detail the prevalence of the mutation in the Miniature Schnauzer breed. In addition, it is unknown if PMDS cases of other breeds have the same mutation. Information about prevalence and occurrence of PMDS-associated mutations in dog breeds is crucial to determining the value of genetic testing and would aid development of new breed-specific genetic tests. Accordingly, these genetic tests could guide future breeding decisions to decrease PMDS in affected dog breeds.
The primary aim of the present study was to screen a large (200+ dog) cohort of Miniature Schnauzers in order to establish the prevalence of the c.241C>T AMHR2 mutation in that breed. An additional aim was to sequence DNA from a Belgian Malinois PMDS case (Lim et al., 2015) for all coding exons of AMH and AMHR2 to determine if this dog had the c.241C>T AMHR2 mutation or a unique mutation.
2. Materials and Methods
2.1 Miniature Schnauzer cohort
Genomic DNA samples from 216 Miniature Schnauzer (83 males and 133 females) were randomly chosen from all banked Miniature Schnauzer samples available in the Canine Genetics Laboratory at the University of Minnesota. These DNA samples had been extracted from EDTA whole blood (191 dogs) or cheek swabs (25 dogs) using a commercially available kit (Qiagen Gentra Puregene Blood Kit). The samples were previously obtained at the University of Minnesota’s Veterinary Medical Center (171 dogs) or were submitted by outside veterinary clinics or breeders in North America (45 dogs) through patient recruitment for other genetic studies, which were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (protocols 1207-A17243 and 1509-33019A). All dog owners gave informed written consent for participation. Pedigrees were available for 131/216 (61%) of the Miniature Schnauzers in this cohort, and amongst those, more than 60 different extended families were represented (not related within four generations); the cohort did contain 9 known first degree relative pairs (parent-offspring or full siblings).
2.2 Allele-specific mutation assay for c.241C>T
Primers were designed using Primer3 software (http://biotools.umassmed.edu/bioapps/primer3_www.cgi) to target the known Miniature Schnauzer mutation site (c.241C>T) in exon 3 of AMHR2. To be allele-specific, two versions of the forward-primer were designed so that one would target only the normal allele and one the mutant allele, which was dependent on the last nucleotide of the 3′-end of the primer (shown in red text in Table 1). The same reverse primer was used for both forward primers with a product size of 153 base pairs.
Table 1.
Primer sequences for allele-specific assay
| Primer | Sequence |
|---|---|
| Normal allele forward primer | CCC ACC CTA TCA GGA TGC C |
| Mutant allele forward primer | CCC ACC CTA TCA GGA TGC T |
| Reverse primer | AGG CAG ATG GCT GTA ATT GG |
Each dog’s DNA was subjected to two PCR reactions, one with each forward primer. For each reaction, 0.15 μL of Apex Hot Start Taq DNA polymerase (5U/μL) was used, along with its included buffer II (10X Combination Reaction Buffer Tris-HCl, pH8.7; Balanced KCl/(NH4)2SO4; 1% Tween 20). Since these buffers are MgCl2-free, 0.3 μL of 50 mM MgCl2 was added to boost amplification yield. PCRs were carried out under standard conditions, with the following adaptations: equivalent amounts of forward and reverse primers were utilized from the allele-specific set, an additional set of internal control primers that produced a differently-sized product of 390 base pairs was included (Fig. 1), and bovine serum albumin (New England Biolabs, 0.15μL of 20mg/mL per reaction) was added to improve primer specificity. The PCR conditions were: initial denaturation of 95 ºC for 2 minutes, followed by 35 cycles of denaturation (95 °C for 30 sec), annealing (62 °C for 30 sec), and extension (72 °C for 60 sec), with a final extension (72 ºC, for 60 sec). After amplification, the products were confirmed (presence and size) by electrophoresis in a 2% agarose gel stained with ethidium bromide (0.3ug/mL), using 100 base pair size standard ladder and visualized under UV light.
Figure 1. Gel Image of Allele Specific Assay Testing for Miniature Schnauzer Mutation c.241C>T; p.R81*.
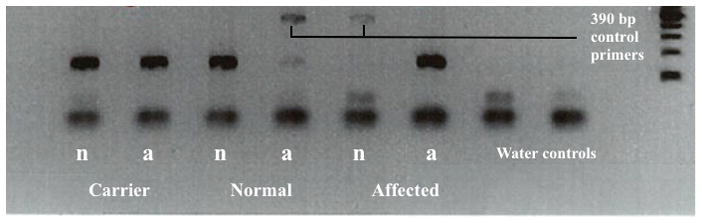
This gel electrophoresis image demonstrates the allele specific assay results with three dogs of known genotype status. Each dog has two lanes, in which the normal and mutant AMHR2 alleles are separately tested for their presence. The first (left) column for each dog contains primers for the normal allele (indicated with “n”), and the second (right) column for each dog contains primers for the mutant allele (indicated with “a”). From left to right: a carrier dog (presence of both the normal and mutant allele, e.g., heterozygote), a normal/wild-type dog (homozygous for the normal allele), and an affected dog (homozygous for the mutant allele). Where no target allele was present, an additional primer for a 390 base pair product acted as an internal PCR control. The last two columns are template-free water controls for detecting any PCR-mixture contamination.
2.3 Allele-frequency in Miniature Schnauzers
The Miniature Schnauzer cohort contained a known male PMDS case and his dam. These two dogs were Sanger sequenced for the c.241C>T AMHR mutation and served as ‘case’ and ‘carrier’ comparison PCR controls in this study. One additional Miniature Schnauzer was verified by Sanger sequencing to be clear of the disease allele and served as the ‘normal’ PCR comparison control (Fig. 2). Each dog’s DNA was tested separately with the normal allele forward primer and the disease allele forward primer, and the reaction was verified with the internal control pair of primers. Each tested batch included DNA from the three comparison control dogs (‘case’, ‘carrier’ and ‘normal’) and one template-free water control to check for contamination of the PCR mixture. A chi square test was calculated using all genotypes, to check for deviation from Hardy-Weinberg equilibrium (www.husdyr.kvl.dk/htm/kc/popgen/genetik/applets/kitest.htm). A p-value of 0.05 or less was considered a deviation from Hardy-Weinberg equilibrium.
Figure 2. DNA Sequence Chromatographs Confirming Miniature Schnauzer Mutation c.241C>T; p.R81*.
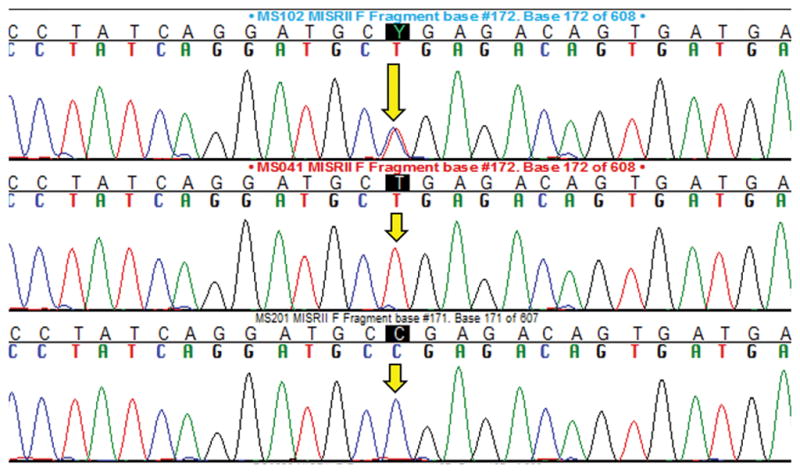
The three chromatographs demonstrate each genotype from a known/control dog, with the yellow arrows indicating the nucleotide of interest. Top = heterozygous (carrier dog), Middle = homozygous for the mutant nonsense allele (PMDS affected dog), Bottom = homozygous for the normal/reference allele (clear dog).
2.4 Belgian Malinois PMDS case
A paraffin-embedded tissue block of uterus masculinus (persistent Müllerian duct) was obtained from an 8 and a half year old intact male Belgian Malinois diagnosed with PMDS at Purdue University’s Veterinary Teaching Hospital (Lim et al., 2015). Genomic DNA was extracted from the block using the Hemo-De version of the Qiagen Puregene DNA purification kit (for paraffin-embedded tissues), following manufacturer’s instructions. This DNA was first tested for the known Miniature Schnauzer c.241C>T AMHR2 nonsense mutation using the allele specific assay designed in the present study.
The Belgian Malinois DNA was subsequently sequenced for all coding exons and ≥ 50 base pairs of flanking intronic sequences of both AMH and AMHR2; reference sequences were obtained via Ensembl (www.ensembl.org) and the University of California, Santa Cruz Genome Browser (https://genome.ucsc.edu). Primers were designed using Primer3 software (http://biotools.umassmed.edu/bioapps/primer3_www.cgi), and all primer sequences are provided in Table 2. Any exons exceeding 300 base pairs were subdivided into more than one sequencing reaction because the DNA from this paraffin-embedded sample was somewhat degraded. On review of protein alignments across multiple species (https://www.ncbi.nlm.nih.gov/homologene), it was discovered that the reference nucleotide sequence for exon 5 of AMH was incompletely annotated due to a gap in the canine reference genome (CanFam 3.1, chr20:56786329–56786911); the missing nucleotides and correct annotation were determined by sequencing a control dog. The correct annotation has been uploaded to GenBank (accession number KY769473). Since DNA quality from formalin-fixed paraffin-embedded tissues tends to be lower and because there were some GC-rich areas to be sequenced, amplification was performed using a ‘slowdown’ PCR program (Frey, Bachmann, Peters, & Siffert, 2008) with the only modification to the published protocol being that, rather than dropping the annealing temperature one degree Celsius every three cycles, the annealing temperature was dropped one third of a degree Celsius every cycle. In addition, the PCR amplification of exon 2 of AMHR2 and exon 5 of AMH required additives (dimethyl sulfoxide (DMSO) at 3% v/v and betaine (4μL of 5M stock) due to high GC content. After amplification, the products were confirmed (presence and size) by electrophoresis on agarose gels. Products with correct sizes were sent to the University of Minnesota Genomics Center for Sanger sequencing and results were viewed in the Sequencher program (v4.9, GeneCodes, Ann Arbor, MI). Both forward and reverse sequences were obtained for each exon.
Table 2.
Primer sequences for AMH and AMHR2 exons
| Gene | Exon | Forward Primer | Reverse Primer | Product Size |
|---|---|---|---|---|
| AMH | ||||
| AMH Ex 1a | CAAGGTCACATCCCAGGAG | CTCCCTTGTCCAGGGTCAC | 320 | |
| AMH Ex 1b | CCCATATAAGCCGGAGCAG | CGAGGAAGGTGTGCTCGTAG | 322 | |
| AMH Ex 1c | CCGGAGGTGAGGTCTCG | CACCTACCTTCCTCCAGGTG | 373 | |
| AMH Ex 1d | CCTGGTGACCCTGGACAA | GCTTAGCACCCCATCTGGA | 334 | |
| AMH Ex 1e | GCTACGAGCACACCTTCCTC | CCCTAGCCAGGAACGTAAGG | 353 | |
| AMH Ex 2 | GTCCCCTAGGCTCCCAGTC | CCTCTCACTTGCACACGTTC | 355 | |
| AMH Ex 3 | CCGGTTGGAATCCTGAAG | GTCATCCGCGTGAAGCAG | 359 | |
| AMH Ex 4 | ACGGTAGGCGCTCAAAGAT | CTACCCCCACCCCCAAC | 289 | |
| AMH Ex 4 b | CCCCGAGCACTAAGCTGTG | GTCTCCAGGAAGGGGTCTG | 330 | |
| AMH Ex 4–5 | TGGACCTCGTGCCCTTC | TTGTTGGCCTGGTAGGTCTC | 758 | |
| AMH Ex 5a | GCTGCTGCTGCTCAAGG | CCTCCGACAGGCTGATGA | 355 | |
| AMH Ex 5b | ACCTACCAGGCCAACAACTG | CTGCTGCTTGCTGCTCTTTA | 308 | |
| AMH Ex 5 d | TGGACCTCGTGCCCTTC | CGACACAGGAGCGCCT | 302 | |
| AMH Ex 5g | TGGACCTCGTGCCCTTC | GGTGGCAGCAGCAGCA | 344 | |
| AMH Ex 5 h | ACCCCGCGACACAGGA | GGCAGAGGGCGAGCAG | 243 | |
| AMH Ex 5 i | CCCTGCTGCTGCTGCT | CAGGTCCACGCTCAGCTC | 392 | |
| AMHR2 | ||||
| AMHR2 Ex 1 F | ACCCACAGTGCCCTATATCTG | ACCCACCCCACTCATTCTTC | 246 | |
| AMHR2 Ex 2 F | CCTGTTTTGTCTCTCCCTTGG | TTTCTCCTCCTCTCCCACTG | 362 | |
| AMHR2 Ex 3 F | GGGGGTTATCCTCTCTGTTCC | TAGGGTGAGAACCAGCCTTG | 356 | |
| AMHR2 Ex 4 F | GTGGGTCCAGAAGCAAGTTC | GTCAGGGGAAGCTGGAGAG | 252 | |
| AMHR2 Ex 5 F | TCTAGAGCCTGAGCCCACTG | ATTACCTGGCGGAAACACAG | 339 | |
| AMHR2 Ex 6a F | AGCTGTGTTTCTCCCAGGTG | TGCAGTTCCAGTACCAGCAG | 349 | |
| AMHR2 Ex 6b F | TGTGTTTCCGCCAGGTAATC | AGCGGATGAATAAGCACGAG | 337 | |
| AMHR2 Ex 6c F | AGCTGTGTTTCTCCCAGGTG | AGCGGATGAATAAGCACGAG | 448 | |
| AMHR2 Ex 7 F | GGGAGATGAGAGCGAGGTC | CCAATTCTTTCCCTTCAAGC | 301 | |
| AMHR2 Ex 8 F | TACGCTGCTGTGTCTGGTTC | TAGGTACTGCCGCACTGTTG | 368 | |
| AMHR2 Ex 9 F | TACGGAGTTTGGGACATTGC | GCAGCAGGAGGTAGGTGGAC | 341 | |
| AMHR2 Ex 10 F | TGTCCACCTACCTCCTGCTG | GGCTGGTCCCAGTAGACAAG | 324 | |
| AMHR2 Ex 11 F | TGTTGAAAACAGGGTAGTGGTG | TAACTGCGCGTTTACAGGTG | 382 | |
| AMHR2 Ex 11b F | AGGACTGTTGGGATGCAGAC | CATCCGACCACCAGATTTATG | 302 | |
We additionally undertook sex verification to confirm that this dog was male. Its genomic DNA was tested for the presence of the sex-determining region Y (SRY), an established method of sex determination (Drobnic, 2006). The following previously-published (Priat, Jiang, Renier, Andre, & Galibert, 1999) primer pair, giving a 132 base pair product, was used: forward 5′-GAACGCATTCTTGGTGTGGTCTC-3′ and reverse 5′-GGCCATTTTTCGGCTTCTGTAAG-3′. Standard PCR reaction conditions were used. As positive and negative controls, DNA from three other male dogs and one female dog were included and tested under the same conditions.
3. Results
3.1 Allele-specific assay
The allele-specific assay designed for this study worked well as a definitive diagnostic test for the known Miniature Schnauzer mutation. Figure 1 demonstrates the assay with three known/control dogs, one for each genotype, on gel electrophoresis and, Figure 2 demonstrates the DNA sequence chromatographs for these three genotypes.
3.2 Allele-frequency in Miniature Schnauzers
Two hundred and sixteen Miniature Schnauzers were genotyped for the c.241C>T AMHR2 mutation, including 83 males and 133 females. Genotype frequencies are displayed in Figure 3. Four dogs, two females and two males, were homozygous for the mutation (4 of 216 dogs, or 1.9%). As expected, the two homozygous female dogs were clinically normal. One of the homozygous males was a confirmed PMDS affected male dog; he had a history of bilateral cryptorchidism, and a uterus masculinus was discovered and removed during an open abdominal cryptorchidectomy. The other homozygous male was neutered and had no reported history of PMDS. He had undergone abdominal imaging on multiple occasions, and reports were available for one set of abdominal radiographs and four abdominal ultrasounds. There were no comments about a uterus masculinus or unidentified structure in the abdomen. Medical records were not available from the time of his neuter, and it is unknown whether or not he was cryptorchid. He went into cardiopulmonary arrest at 12 years of age after presenting for cluster seizures; a necropsy was not performed. In addition to the 4 dogs homozygous for the c.241C>T AMHR2 mutation, 59 heterozygous dogs were observed (59 of 216 dogs, or 27.3%), and 153 dogs were homozygous for the reference allele (70.8%). Overall, the tested Miniature Schnauzer population had an allele frequency of 0.16 for the c.241C>T AMHR2 mutation. The chi square test for deviation from Hardy-Weinberg equilibrium resulted in a X2 test statistic of 0.39 (one degree of freedom), and a p-value of 0.16, indicating that this locus does not differ from Hardy-Weinberg equilibrium expectations within our cohort.
Figure 3. Genotype frequency of the c.241C>T AMHR2 mutation in Miniature Schnauzers.
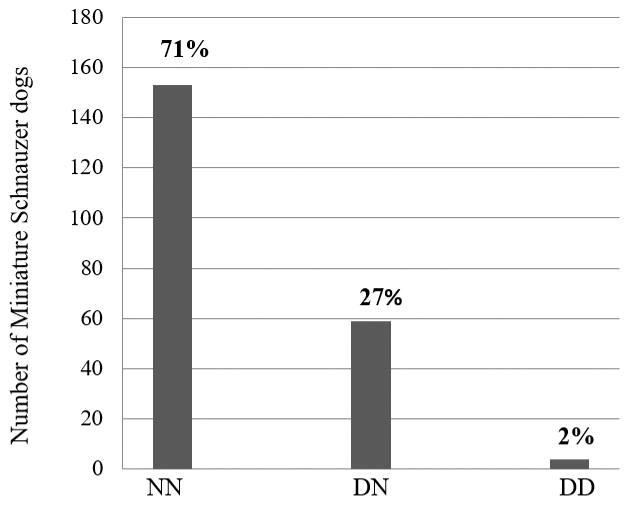
Dogs homozygous for the reference allele are indicated as NN, heterozygous for the mutant allele as DN, and homozygous for the mutant allele as DD.
3.3 Belgian Malinois PMDS case
The Belgian Malinois PMDS case DNA was successfully sequenced for all exons and flanking intronic sequences of both AMH and AMHR2 genes. He did not have the c.241C>T AMHR2 mutation (i.e., he was homozygous for the reference allele). No exonic mutations and no splicing mutations in the proximate flanking intronic sequence were found. The Belgian Malinois PMDS case was then confirmed to be SRY-positive and thus genetically male.
4. Discussion
The results of the present study indicate for the first time that the c.241C>T AMHR2 mutation is not rare in Miniature Schnauzers with a mutant allele frequency of 0.16 and carrier/heterozygous genotype frequency of 0.27 in the study population. It is important to note that this cohort was comprised primarily from dogs residing in Minnesota and its surrounding states. As such, it represents only a subset of the total North American Miniature Schnauzer population, and the true frequency in the entire breed could vary from the numbers observed. However, with 216 dogs (equating to 432 chromosomes tested) and 60 extended families represented (not related within 4 generations), this population is still expected to be an adequate representation of the breed.
An estimated 50% of male dogs affected by PMDS are cryptorchid (Meyers-Wallen, 2009). Bilaterally cryptorchid males are sterile, and unilaterally cryptorchid males have subfertility. Cryptorchidism also increases the risk for testicular tumors. Other pathologies reported in male dogs with PMDS include pyometra, hydrometra, urinary tract infections, and prostatitis (Lim et al., 2015; Marshall et al., 1982; Matsuu et al., 2009). Therefore, it is important to avoid the production of PMDS affected puppies. Males that are cryptorchid will be naturally removed from the breeding pool, but non-cryptorchid males and all females homozygous for the AMHR2 mutation are expected to be subclinical. Thus, molecular diagnostic tools are essential for detection of both homozygous and heterozygous individuals. As such, both the prevalence of the mutant allele and the pathobiology of PMDS support a benefit of testing all Miniature Schnauzers used for breeding, those suffering from cryptorchidism, and any male dogs with imaging or gross findings suspicious for PMDS.
A Belgian Malinois PMDS case was investigated in this study and did not have the c.241C>T AMHR2 mutation. Furthermore, no coding mutations were identified in the total exonic AMH or AMHR2 sequence analyzed. Regions that were not yet sequenced and could harbor a causal mutation include the 5′ and 3′ untranslated regions, regulatory regions, and residual intronic sequences of both AMH and AMHR2. Despite the fact that the Belgian Malinois showed distinct features of PMDS and was confirmed to be an SRY-positive male, there is still a small chance the phenotype diagnosis could be incorrect. There are other genes which code for upstream or downstream products in the AMH transduction pathway, but the typical corresponding phenotypes differ significantly from that observed in the Belgian Malinois (Josso, Belville, di Clemente, & Picard, 2005; Matsushita, 2013). In humans, approximately 15% of described PMDS cases have no identified mutations in AMH or AMHR2 explaining their phenotype; this demonstrates the complexity of identifying genetic causes for PMDS (Nishi et al., 2012; Wongprasert, Somanunt, De Filippo, Picard, & Pitukcheewanont, 2013). Nevertheless, more research needs to be done in the Belgian Malinois and other breeds known to have PMDS cases, including the Papillion, Basset Hound, Leonberger, Doberman Pincher, and English Cocker Spaniel (Lim et al., 2015; Nickel et al., 1992; Whyte et al., 2009).
In summary, we report a high frequency, at 16%, of the c.241C>T AMHR2 mutant allele in a cohort of 216 Miniature Schnauzers. This substantial prevalence of the mutant allele endorses the value of genetic testing in dogs used for breeding or those with clinical signs associated with PMDS. Finding causal variants in the Belgian Malinois and other dog breeds affected by PMDS should be the subject of further research, since this would provide the opportunity to develop more breed-specialized tests. Results of genetic diagnostic testing for PMDS would inform breeding decisions and thereby reduce the incidence of the syndrome and its associated health problems.
Acknowledgments
FUNDING SOURCES
Partial funding for E.F. was provided by the Office of the Director, National Institute of Health (NIH) under award number K01-OD019912. Funding for M.M.S. was provided by Merial® via the 2016 Merial® Veterinary Scholars Program.
Footnotes
Authorship Statement:
E.F. conceived and designed the experiments; M.M.S. and K.M.M performed the experiments; M.M.S., K.J.E., K.M.M., and E.F. analyzed the data; C.K.L and E.F contributed materials; M.M.S., K.J.E., and E.F. wrote the paper with input from K.M.M., C.K.L., and P.A.J.L.; all authors reviewed and approved the final manuscript
The study was conducted at the College of Veterinary Medicine, University of Minnesota, St. Paul, MN, USA.
CONFLICT OF INTEREST STATEMENT
Genetic testing for PMDS is offered through the University of Minnesota Canine Genetics Laboratory, and proceeds from the test are used to fund ongoing research in the laboratory.
AUTHOR CONTRIBUTIONS
E.F. conceived and designed the experiments; M.M.S and K.M.M. performed the experiments; M.M.S., K.J.E., K.M.M., and E.F. analyzed the data; C.K.L. and E.F. contributed materials; M.M.S., K.J.E., and E.F. wrote the paper with input from K.M.M., C.K.L., and P.A.J.L.; all authors reviewed and approved the final manuscript.
References
- Banco B, Veronesi MC, Giudice C, Rota A, Grieco V. Immunohistochemical evaluation of the expression of anti-Müllerian hormone in mature, immature and neoplastic canine sertoli cells. Journal of Comparative Pathology. 2012;146:18–23. doi: 10.1016/j.jcpa.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Brown TT, Burek JD, McEntee K. Male pseudohermaphroditism, cryptorchism, and sertoli cell neoplasia in three miniature schnauzers. Journal of the American Veterinary Medical Association. 1976;169:821–825. [PubMed] [Google Scholar]
- Drobnic K. A new primer set in a SRY gene for sex identification. International Congress Series. 2006;1288:268–270. [Google Scholar]
- Frey U, Bachmann H, Peters J, Siffert W. PCR-amplification of GC-rich regions: ‘Slowdown PCR’. Nature Protocols. 2008;3:1312–1317. doi: 10.1038/nprot.2008.112. [DOI] [PubMed] [Google Scholar]
- Josso N, Belville C, di Clemente N, Picard J. AMH and AMH receptor defects in persistent müllerian duct syndrome. Human Reproduction Update. 2005;11:351–356. doi: 10.1093/humupd/dmi014. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kwon H, Byun H, Yeom D, Choi J, Kim J, Shim H. Surveyor assay to diagnose persistent Müllerian duct syndrome in miniature Schnauzers. Journal of Veterinary Science. 2016 doi: 10.4142/jvs.2017.18.4.547. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper H, Wagner F, Drögemüller C, Distl O. Persistent Mullerian duct syndrome causing male pseudohermaphroditism in a mixed-breed dog. Veterinary Record. 2004;155:400–401. doi: 10.1136/vr.155.13.400. [DOI] [PubMed] [Google Scholar]
- Lim CK, Heng HG, Hui TY, Thompson CA, Childress MO, Adams LG. Ultrasonographic features of uterus masculinus in six dogs. Veterinary Radiology & Ultrasound. 2015;56:77–83. doi: 10.1111/vru.12189. [DOI] [PubMed] [Google Scholar]
- Marshall LS, Oehlert ML, Haskins ME, Selden JR, Patterson DF. Persistent Müllerian duct syndrome in miniature schnauzers. Journal of the American Veterinary Medical Association. 1982;181:798–801. [PubMed] [Google Scholar]
- Matsushita M. A novel SOX9 H169Q mutation in a family with overlapping phenotype of mild campomelic dysplasia and small patella syndrome. American Journal of Medical Genetics. Part A. 2013;161:2528–2534. doi: 10.1002/ajmg.a.36134. [DOI] [PubMed] [Google Scholar]
- Matsuu A, Hashizume T, Kanda T, Nagano M, Sugiyama A, Okamoto Y, Hikasa Y. A case of persistent Müllerian duct syndrome with sertoli cell tumor and hydrometra in a dog. Journal of Veterinary Medical Science. 2009;71:379–381. doi: 10.1292/jvms.71.379. [DOI] [PubMed] [Google Scholar]
- Meyers-Wallen VN, Manganaro TF, Kuroda T, Concannon PW, MacLaughlin DT, Donahoe PK. The critical period for mullerian duct regression in the dog embryo. Biology of Reproduction. 1991;45:626–633. doi: 10.1095/biolreprod45.4.626. [DOI] [PubMed] [Google Scholar]
- Meyers-Wallen VN, Donahoe PK, Ueno S, Manganaro TF, Patterson DF. Müllerian inhibiting substance is present in testes of dogs with persistent müllerian duct syndrome. Biology of Reproduction. 1989;41:881–888. doi: 10.1095/biolreprod41.5.881. [DOI] [PubMed] [Google Scholar]
- Meyers-Wallen VN. Review and update: genomic and molecular advances in sex determination and differential in small animals. Reproduction in Domestic Animals. 2009;44:40–46. doi: 10.1111/j.1439-0531.2009.01433.x. [DOI] [PubMed] [Google Scholar]
- Nickel RF, Ubbink G, van der Gaag I, van Sluijs FJ. Persistent müllerian duct syndrome in the basset hound. Tijdschrift Voor Diergeneeskunde. 1992;117(Suppl 1):31S–31S. [PubMed] [Google Scholar]
- Nishi M, Domenice S, Maciel Guerra A, Zaba Neto A, da Silva Marcia Alessandra Cavalaro Pereira, Costa EMF, … de Mendonca B. Analysis of anti-Müllerian hormone (AMH) and its receptor (AMHR2) genes in patients with persistent Müllerian duct syndrome. Arquivos Brasileiros De Endocrinologia E Metabologia. 2012;56:473–478. doi: 10.1590/s0004-27302012000800002. [DOI] [PubMed] [Google Scholar]
- Pretzer SD. Canine embryonic and fetal development: A review. Theriogenology. 2008;70(3):300–303. doi: 10.1016/j.theriogenology.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Priat C, Jiang ZH, Renier C, Andre C, Galibert F. Characterization of 463 Type I markers suitable for dog genome mapping. Mammalian Genome. 1999;10(8):803–813. doi: 10.1007/s003359901095. [DOI] [PubMed] [Google Scholar]
- Pujar S, Meyers-Wallen VN. A molecular diagnostic test for persistent Müllerian duct syndrome in miniature schnauzer dogs. Sexual Development. 2009;3(6):326–328. doi: 10.1159/000273264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegter A. Persistent Mullerian duct syndrome in a Miniature Schnauzer dog with signs of feminization and a sertoli cell tumour. Reproduction in Domestic Animals. 2010;45:447–452. doi: 10.1111/j.1439-0531.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- Whyte A, Monteagudo L, Díaz Otero A, Lebrero ME, Tejedor MT, Falceto MV, Whyte J, Gallego M. Malformations of the epididymis, incomplete regression of the mesonephric tubules and hyperplasia of leydig cells in canine persistence of Müllerian duct syndrome. Animal Reproduction Science. 2009;115:328–333. doi: 10.1016/j.anireprosci.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Wongprasert H, Somanunt S, De Filippo R, Picard JY, Pitukcheewanont P. A novel mutation of anti-Mullerian hormone gene in Persistent Mullerian Duct Syndrome presented with bilateral cryptorchidism: A case report. Journal of Pediatric Urology. 2013;9:e147–e149. doi: 10.1016/j.jpurol.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Wu X, Wan S, Pujar S, Haskins ME, Schlafer DH, Lee MM, Meyers-Wallen VN. A single base pair mutation encoding a premature stop codon in the MIS type II receptor is responsible for canine persistent Müllerian duct syndrome. Journal of Andrology. 2009;30:46–56. doi: 10.2164/jandrol.108.005736. [DOI] [PMC free article] [PubMed] [Google Scholar]


