Abstract
Background
Although syphilis rates have been relatively high in Italy for over 15 years, no data on the molecular types of Treponema pallidum subsp. pallidum (T. pallidum) circulating in this country are yet available. Likewise, no data exist on how widespread is resistance to macrolide or tetracycline antibiotics in these strains. Such data would however promote comprehensive studies on the molecular epidemiology of syphilis infections in Italy and inform future interventions aiming at syphilis control in this and other European countries.
Goals and Study Design
Swabs from oral, genital, cutaneous, or anal lesions were obtained from 60 syphilis patients attending dermatology clinics in Milan, Turin, Genoa, and Bologna. Molecular typing of T. pallidum DNA was performed to provide a snapshot of the genetic diversity of strains circulating in Northern Italy. Samples were also screened for mutations conferring resistance to macrolides and tetracyclines.
Results
T. pallidum DNA was detected in 88.3% of the specimens (53/60) analyzed. Complete and partial T. pallidum typing data were obtained for 77.3% (41/53) and 15.0% (8/53) of samples, respectively, while four samples could not be typed despite T. pallidum DNA being detected. The highest strain type heterogeneity was seen in samples from Bologna and Milan, followed by Genoa. Minimal diversity was detected in samples from Turin, in spite of the highest number of typeable samples collected there. Resistance to macrolides was detected in 94.3% (50/53) of the strains, but no known mutations associated with tetracycline resistance were found.
Conclusions
Genetic diversity among T. pallidum strains circulating in Northern Italy varies significantly among geographical areas regardless of physical distance. Resistance to macrolides is widespread.
INTRODUCTION
Far from being a disease of the past, syphilis is still a source of concern for global health, with an estimated burden of ~36 million cases worldwide and a global incidence of over 11 million new cases every year (1). Although the vast majority of syphilis cases still occur in developing nations, also developed countries such as the United States, Canada, and China, and many European nations have experienced a resurgence in syphilis incidence in the last years (2–5). in many Northern and Western European countries syphilis incidence has been increasing approximately since 1996 (5), following a period of steady decline during the first half of the 1990’s (5). Italy has been no exception to this general trend, even though the number of reported cases has increased significantly only after 2000 (6). According to the latest data from the European CDC syphilis rates in Europe reached 5.1 cases per 100,000 population in 2014. In this context, however, Italy stood out as one of the very few European nations with the lowest syphilis rates (<3 per 100,000 population) compared to countries such as Spain, United Kingdom, Germany, Lithuania, Malta, and Iceland (>7 cases per 100,000 population), or Ireland, Latvia, Luxembourg, Norway, Finland, Romania, Bulgaria, Czech Republic, Denmark, and Slovakia, were the reported rate was 3–6 cases per 100,000 population (7). Noteworthy is also that syphilis rates in Italy have been low for over 20 years, even during the years of resurgence of the disease (6).
Molecular tools for typing of T. pallidum strains have been increasingly adopted in countries with resurgent syphilis, including many European ones (8), to better understand T. pallidum acquisition and transmission dynamics. No such studies are however yet available from Italy. Given the reported low incidence of the infection, it would be important to know which strain types are circulating in Italy. This and future studies could support that these strains are particularly virulent and more capable of persisting within a population. In parallel, the knowledge of how widespread is antibiotic resistance in circulating T. pallidum strains could help circumvent the public health threat posed by treatment-resistant syphilis. Altogether, these data could inform and guide intervention programmes that aim at eliminating syphilis in Italy and other European countries.
The goal of our study was to type T. pallidum strains circulating in four Italian cities (Turin, Milan, Bologna and Genoa) to evaluate the level of genetic heterogeneity in neighboring geographical areas. In the regions where these centers are located (Piedmont, Lombardy, Emilia-Romagna, and Liguria) the most recently reported syphilis rates were 5.5 (2013), 7 (2014), and 9.1 (2013) cases per 100,000 population, respectively (9, 10) (D’Antuono M. and Gaspari V., personal communication). No recent data were already available for the Ligurai region. In addition, we evaluated the rate of resistance to macrolides in these strains. We also screened for mutations known to confer resistance to doxycycline and tetracycline antibiotics in general. Doxycycline was in fact used in alternative to beta-lactam antibiotics to treat eight patients from the Turin cohort, and was also administered to all patients from the Genoa cohort according to the enhanced syphilis treatment protocol introduced by Drago et al. (11).
MATERIALS AND METHODS
Sample collection
Swabs from genital, oral, or anal lesions were collected anonymously and exclusively from early syphilis patients attending the Dermatology Clinics of the San Martino Hospital in Genoa, the University of Turin, the Ospedale Maggiore in Milan, and the S. Orsola Hospital of the University of Bologna from approximately December 2016 to March 2017. The only exclusion criterion for sample collection was an existing record of antibiotic therapy initiated within 30 days from the patient visit. At the moment of sample collection, demographics (age, gender, sexual orientation and, when possible, travel history and history of previous syphilis infection) and clinical data (non-treponemal and treponemal test results, HIV status, and neurological and ocular involvement when available) were also gathered. For sample collection, the lesion area was gently squeezed to imbibe the swabs with exudate. The swabs were then placed in sterile microcentrifuge tubes containing 1 ml of 1X lysis buffer (10 mM Tris-HCl, 0.1 M EDTA, and 0.5% SDS) suitable for DNA extraction. The swab shafts were subsequently cut to leave the swab in the buffer, and the samples and frozen at −80°C until DNA extraction. Sample collection was authorized by the Human Subject Committee of each collecting institution (Protocol code: PR033REG2016 for the Universities of Turin and Genoa, Protocol N.2103/2016 for the University of Bologna, and Protocol Code TREPO2016 for the University of Milan) and informed consent was obtained from each patient. Specimens were then sent as de-identified samples in dry ice to the University of Washington for T. pallidum typing and detection of antibiotic resistance mutations. The University of Washington Institutional Review Board determined this investigation not to be human subject research.
DNA extraction and strain typing
Frozen samples were thawed at room temperature and vortexed before processing. DNA extraction was performed from 200 μl of sample suspension using the QIAamp DNA mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Aliquots of each extracted sample were made to avoid DNA damage due to repeated thawing and freezing cycles.
Lack of PCR inhibitors was checked by amplification of a fragment of the human β-globin gene using sense and antisense primers 5′-caacttcatccacgttcacc and 5′-gaagagccaaggacaggta, respectively. Amplifications were carried on in a 50 μl final volume using 5 μl of DNA template and 2.5 units of GoTaq polymerase (Promega, Madison, WI). Final concentrations of MgCl2, dNTPs, and each primer were 1.5 mM, 200 μM, and 0.32 μM, respectively. Cycling conditions were initial denaturation at 95°C for 4 minutes, followed 95°C for 1 min, 60°C for 1 min and 72°C for 1 min for a total of 40 cycles. Final extension was at 72°C for 5 min. Samples that failed to amplify were re-extracted and β-globin amplification re-attempted. If a second negative result for β-globin amplification was obtained, the sample was not further analyzed and DNA extraction re-attempted. The enhanced T. pallidum strain typing method was performed as described by Marra et al. (12). DNA extracted from the Nichols laboratory strain of T. pallidum was used as positive control for all the typing reactions. Duplicate samples collected from a subset of patients served as internal control. A no-template control (NTC) was obtained by extracting a fresh aliquot of lysis buffer along with the clinical samples.
Detection of genetic resistance to macrolides and tetracyclines
Genetic resistance to macrolides in T. pallidum is associated with either the A2058G or A2059G mutation on the 23S rRNA gene (13). Samples were screened for both mutations as previously described (13). As positive controls for the A2058G and A2059G mutations, we used DNA from the SS14 and UW254 strains, respectively. T. pallidum Nichols strain DNA (known to lack such mutations) was used as negative control. Genetic resistance to tetracyclines has been associated with mutations at position 965–968 (AGA) and 1058 (G) in H. pylori and E. coli 16S rRNA genes (14, 15). In T. pallidum the cognate nucleotides in these positions are TGA and G, respectively. Detection of these mutations was performed by amplification and sequencing as described by Xiao et al. (16).
RESULTS
In approximately four months, a total of 60 samples were collected from early syphilis patients attending the Dermatology Clinics of the San Martino Hospital in Genoa (N=5), the University of Turin (N=29), the Ospedale Maggiore in Milan (N=11), and the S. Orsola Hospital of the University of Bologna (N=15). Demographics (Table 1) showed that 59/60 patients were male and 52 of them (88.1%) were MSM. Of the 60 patients, 23 (38.3%) were living with HIV.
Table 1.
Characteristics of the syphilis-infected participants (60) involved in this study
| Milan (11 participants) | Turin (29 participants) | Bologna (15 participants) | Genoa (5 participants) | |
|---|---|---|---|---|
|
| ||||
| Male (N, %) | 10 (90.9) | 29 (100) | 15 (100) | 5 (100) |
| Mean Age (range) | 40 (18–70) | 36 (20–54) | 40.2 (23–67) | 33.6 (23–49) |
|
| ||||
| Sexual orientation | ||||
| MSM (N, %) | 8 (80) | 27 (93.1) | 13 (86.6) | 3 (60) |
| Bisexual (N, %) | None | None | 1 (6.6) | None |
| Heterosexual (N, %) | 2 (20) | 2 (6.9) | 1 (6.6) | 2 (40) |
|
| ||||
| HIV status | ||||
| Positive (N, %) | 3 (27.3) | 11 (37.9) | 6 (40) | 1 (20) |
| Negative (N, %) | 8 (72.7) | 18 (62.1) | 9 (60) | 4 (80) |
|
| ||||
| Syphilis stage | ||||
| Primary (N, %) | 7 (63.7) | 13 (44.9) | 14 (93.4) | 4 (80) |
| Secondary (N, %) | 4 (36.3) | 16 (55.1) | 1 (6.6) | 1 (20) |
|
| ||||
| Lesion location | ||||
| Genital (N, %) | 7 (63.7) | 11 (37.9) | 14 (93.4) | 4 (80) |
| Anal (N, %) | 4 (36.3) | 10 (34.4) | None | None |
| Oral (N, %) | None | 6 (20.6) | 1 (6.6) | None |
| Cutaneous (N, %) | None | 2 (6.9) | None | 1 (20) |
|
| ||||
| VDRL or RPR median titer (IQR) | VDRL, 16 (2–256) | VDRL, 8 (4–64)1 RPR, 16 (2–64)2 |
VDRL, 4 (1–16) | All VDRL + No titer available |
|
| ||||
| TPPA or TPHA median titer (IQR) | TPHA, 803 | TPPA, 640 (80–20480) | TPHA, 40 (4–128) | TPHA, 5120 (2560–10240) |
|
| ||||
| Neurological/ocular involvement | Not determined | Absent in all patients | Not determined | Not determined |
VDRL was performed on 4 patients of this cohort
RPR was performed for 25 patients of this cohort
No further dilution was tested
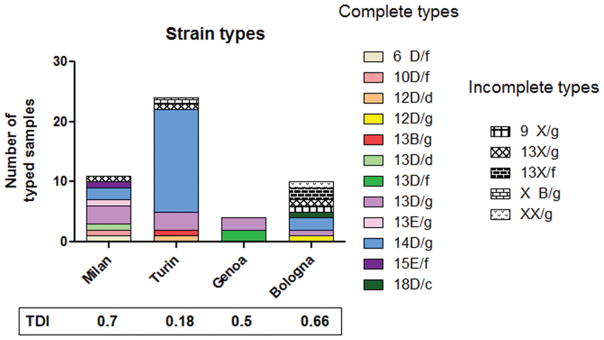
T. pallidum DNA was detected from 53 (88.3%) of these 60 samples. However, only 41 samples (77.3%) were fully typeable with the arp, tprE/G/J and tp0548 assays, while 8 samples (15.0%) could only be partially typed. Four additional samples failed to provide information about the typing targets, although amplification of T. pallidum 23S and 16S rRNA genes for detection of macrolide and tetracycline resistance was positive, due to the higher sensitivity of these methods that use a nested approach compared to the typing PCR reactions. Overall, at least 13 different strain types were detected (Fig. 1 and Table 2) in this population, including 12 strain types from the fully typeable samples and one partially typeable strain (9 X/g, Fig. 1 and Table 3), which carried a unique number of arp repeats. The most prevalent strain type among the 41 fully typed isolates was 14D/g, detected in 40.8% of the samples, followed by 13D/g and 13D/f detected in 18.3% and 4.0% of the cases, respectively. All other strain types were detected only once (2.0%) in the population (Fig. 1 and Table 2). Duplicate samples from the same patients always yielded the same strain type. Differences in strain type distribution were evident among the collecting centers. A type diversity index (TDI), calculated as the ratio between the number of different types detected and the number of fully typeable samples collected by each institution was highest in Bologna (0.8), followed by Milan (0.7) and Genoa (0.5). On the contrary, despite of the highest number of samples collected for this study, Turin had the lowest TDI (0.18), with only four different strain types (mostly 14D/g, Fig. 1 and Table 2) detected from 22 fully typeable samples.
Fig. 1.
Distribution of T. pallidum strain types. Fully typed isolates are color-coded; partially typed isolates are represented by a pattern on a white background. Missing information for partially typed isolates is replaced by an upper or lower case “x”. Type Diversity Index (TDI = Number of different types detected/Number of typed samples) is reported for each collecting institution.
Table 2.
Strain type and antibiotic resistance/sensitivity distribution of 49 T. pallidum-positive samples fully (41) or partially (8) typeable by the arp, tprE/G/J, and tp0548 assays, and 53 samples with detectable T. pallidum DNA for the 23S rRNA genes.
| Milan N (%) |
Turin N (%) |
Bologna N (%) |
Genoa N (%) |
Previous syphilis and travel history1 | |
|---|---|---|---|---|---|
|
| |||||
| Complete types | |||||
| 6 D/f | 1 (10) | ||||
| 10D/f | 1 (10) | ||||
| 12D/d | 1 (4.5) | ||||
| 12D/g | 1 (20) | ||||
| 13B/g | 1 (4.5) | PS &TH | |||
| 13D/d | 1 (10) | TH | |||
| 13D/f | 2 (50) | TH2 | |||
| 13D/g | 3 (30) | 3 (4.5) | 1 (20) | 2 (50) | PS3 |
| 13E/g | 1 (10) | ||||
| 14D/g | 2 (20) | 17 (77.2) | 2 (40) | PS or TH4 | |
| 15E/f | 1 (10) | ||||
| 18D/c | 1 (20) | ||||
| Partial types | |||||
| 9 X/g | 1 | ||||
| 13X/g | 1 | 1 | 1 | ||
| 13X/f | 2 | ||||
| X B/g | 1 | ||||
| X X/g | 1 | PS & TH | |||
|
| |||||
| Macrolide S5 strains | 1 (9.1) | 2 (7.7) | None | None | |
| Macrolide R6 strains | 10 (90.9) | 24 (92.3) | 12 (100) | 4 (100) | |
|
|
|||||
| 23S rRNA A2058G7 | 9 (90) | 24 (00) | 12 (100) | 4 (100) | |
| 23S rRNA A2059G7 | 1 (10) | 0 | 0 | 0 | |
|
|
|||||
| Tetracycline S5 strains | 11 (100) | 26 (100) | 12 (100) | 4 (100) | |
| Tetracycline R6 strains | 0 | 0 | 0 | 0 | |
PS = strain isolated from patients with previous syphilis history, TH = History of travel and sex abroad
One patient only
One patient from Milan, one from Turin, and one from Bologna
All patients from Turin; Four had previous infections, and four others had history of travel and sex abroad
Sensitive
Resitant
Mutations associated to Macrolide resistance
Resistance to macrolides was detected in 50/53 (94.3%) of the isolates due to the presence of the A2058G mutation in all but one sample (isolated in Milan, type 15E/f) that carried the A2059G mutation. Neither mutation was detected in three isolates (one from Milan, and two from Turin). No mutations associated with resistance to doxycycline were found on the 16S rRNA genes.
DISCUSSION
According to the latest data from the European CDC and Italian Ministry of Health, Italy is one of the few countries with the lowest rates of syphilis in Europe (6, 7). Such evidence prompted us to collect samples from syphilis patients in Italy to type T. pallidum strains. In addition to characterizing the types of T. pallidum currently circulating in Italy, the goals of this study were also to compare such strains with those reported in neighboring countries, and to compare the level of genetic heterogeneity among T. pallidum strains in geographically close areas of Northern Italy. We hope that our study will encourage an increasing number of STI investigators in Italy to complement their epidemiological data on syphilis with molecular typing of T. pallidum to better understand syphilis transmission dynamics and the possible link between clinical manifestations and specific strain types.
In our population 13 different strain types were identified. Such diversity is not due to ambiguity in our results, given that the analysis of duplicate samples from the same patient always yielded the same strain type. Also, our inability to fully type or detect T. pallidum DNA in ~32% of the samples should not be of concern, given that our success rate is comparable to that reported by others that used this typing system (12, 17), and is likely due to scarcity of treponemal DNA in the patient sample. High strain type heterogeneity, like that seen in our samples, has been suggested to be unique to areas where syphilis is endemic (18, 19). However, both our data and previous T. pallidum typing studies (8) suggest that detection of high heterogeneity may be related to the use the typing method by Marra et al. (12) that is more discriminatory than the previously used typing method from the CDC (19).
The most common strain type identified in our study regardless of the origin of the sample was 14D/g, followed by 13 D/g (representing 51.2% and 21.9% of the fully-typeable samples, respectively). Several reports have shown that the 14D/g or the 14D strain type (the latter type from studies that preceded the adoption of the current typing method) is the predominant type in many European and non-European cities and countries (e.g. Denmark, Scotland, Czech Republic, Paris, London, Lisbon, Canada, United States, and South Africa) (8), suggesting that this might be a strain with marked virulence. Our data, however, do not speak at all about how strain distribution evolved over time. Marra et al. (12) have demonstrated the introduction and loss of some strain types in Seattle from 1999 through 2008, and significant changes in type distribution over time. In their study, the 14D/f strain was predominant in Seattle from 2001 to 2004, to be then replaced by the 14D/g type (12). Our work, therefore, provides a snapshot of the syphilis types circulating now in Northern Italy, and again should encourage further monitoring to evaluate if and when new strains will appear and replace the existing ones. Some of the least represented strain types in our group were previously reported in studies from China (6D/f, 10D/f, 12D/d, 13D/f, and 18D/c) (16, 20) and Seattle (13D/d) (8). To our knowledge, the 13B/g, 13D/g, 13E/g, 15E/f, and 9X/g strain types were never described before, although both 13B and 13E strains were found in Australia (21).
An important finding of this work is that there can be significant differences in type heterogeneity within close geographical areas. The TDI was highest in Bologna, followed by Milan and Genoa, while Turin had the lowest TDI, despite the Turin clinic provided the highest number of typeable samples for this study. Although it is possible that the highest TDI value reported for the Bologna samples might have been affected by the small number of typeable samples, the difference in type heterogeneity between the samples collected in Milan and Turin, which are only 80 miles apart, is substantiated by larger sample sizes. In both cities, most of the samples were collected from self-identified MSM patients, indicating localized and restricted transmission networks. The reason for the syphilis epidemics among MSM has been recently attributed to increased sexual risk behavior justified by the evidence that pre-exposure prophylaxis (PrEP) protects from contracting HIV (22) or, in our specific case, on the improved effect of antiretroviral therapy on HIV, given that PrEP is not available in Italy. Even assuming that a core group of MSM may be responsible for the currently circulating syphilis strains, this would not explain the differences in diversity seen between T. pallidum strains in Turin and Milan, since the majority of samples collected in both centers came from MSM. Additional information that might shed light on these differences include whether distinct specimens came from epidemiologically linked cases through partner notification, which would contribute to a falsely low appearance of the degree of genetic diversity, or perhaps collected from patients with travel history that might have “imported” novel types into a previously homogeneous background, thus increasing its heterogeneity. Additionally, history of previous syphilis infection could be an important factor to take into account, given that sufficient pre-existing immunity against a strain could perhaps favor re-infection with a different one. For example, one of the patients carrying a never-seen before type (13B/g) was known to have been previously infected with syphilis, and to have traveled to and had sexual encounters in a neighboring foreign country (Table 2). Also the patient carrying the 13D/d strain type reported that the infection might have occurred abroad. Another patient carrying a never-seen before strain (12D/d), however, did not report knowledge of past infections or any recent travel (Table 2). In our cohort, however, travel history and history of previous infection did not contribute significantly to the diversity seen in Bologna or Milan.
Aside from epidemiological studies, a valuable typing system for a microbial pathogen should also be able to predict specific outcomes of an infection characterized by protean clinical manifestations. Such predictive value could further justify the adoption of a typing system in routine STD surveillance programmes or in clinical laboratories at the moment of diagnosis. Significant links between strain type and unique clinical manifestations in syphilis have been elusive thus far, and only a few studies have provided insight to this end. It was shown for example that in the rabbit model of syphilis, animals infected with 14A/a and 14D/f treponemal isolates had the greatest degree of neuroinvasion (23). Another study suggested that the 14D/g and 8D/g strain types might be cause ocular syphilis (24), although no statistical significance was however achieved in this study due to the limited number of samples analyzed.
Although we used the current method of choice for T. pallidum typing, this protocol requires trained personnel, and is remarkably time-consuming. Alternative multi-locus system for typing (MLST) method based exclusively on sequencing of variable loci are currently under scrutiny (25–27), particularly after a report that showed unexpected differences in strain type when the typing protocol used here was applied to specimens collected from the same patient but from different anatomical sources (25). Confidence in the enhanced typing protocol, however, was strengthened by both Pillay et al. (19) and Marra et al. (12), who showed that a strain type was stable with repeated rabbit passages of the Nichols, Sea81-4 and Chicago isolates of T. pallidum, respectively. Devising a less complex MLST method is nonetheless desirable, and should be facilitated by the increasing number of T. pallidum genomes available to perform comparative genomics and select suitable gene candidates.
Genetic resistance to macrolides was detected in virtually all samples. This result was not surprising given that macrolide resistance was already reported to be widespread in other European countries, as well as the United States, China, and Australia (28). Furthermore macrolides have been reported by the Italian Medicines Agency to be the second most prescribed group of antibiotics in Italy since at least 2002, with a DDD/1000 ab die of 3.66 (29). Not surprisingly, given the high prevalence of macrolide resistance reported by different studies and the increasing trend in macrolide resistance in T. pallidum clinical isolates, macrolide treatment for syphilis is no longer recommended in Italy. The presence of mutations in the 16S rRNA gene that could decrease susceptibility to doxycycline (14, 15) has not been yet extensively explored in T. pallidum, with the exception of one study that, however, failed to detect such mutations in over 400 specimens (16). Similarly, no such mutations were found in our samples. This is likely due to the limited use of doxycycline in syphilis patients so far, even though several patients in our cohort were treated with it. Like for macrolides, however, resistance to doxycycline could arise very quickly if this antibiotic was to be used more frequently.
In conclusion, even though this report follows several others that focused on T. pallidum typing in European countries, it reiterates the importance of typing syphilis strains to generate a global database that would facilitate the identification of new and emerging types, the monitoring of how prevalence and distribution of types changes overtime within a population, and the detection of strains associated with outbreaks. At the same time, continued research efforts are necessary to understand the link between a particular T. pallidum type, specific disease manifestations and pathogen virulence.
Acknowledgments
Source of funding: this work was supported by the NIH Grant 1U01AI115497 (to LG),
The authors wish to thank Bess Charmie Godornes for providing her arp-based DNA ladder for typing, Dr. Christina Marra for providing the UW254 strain DNA for macrolide resistance analysis, and Mark C. Fernandez for his constructive criticisms on the manuscript.
References
- 1.WHO. Prevalence and incidence of selected sexually transmitted infections Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. World Health Organization; Geneva: 2011. [Google Scholar]
- 2.WHO. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organization; 2001. [Google Scholar]
- 3.Tucker JD, Chen XS, Peeling RW. Syphilis and social upheaval in China. N Engl J Med. 2010;362(18):1658–61. doi: 10.1056/NEJMp0911149. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Sexually Transmitted Disease Surveillance 2009. 2010. [Google Scholar]
- 5.Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255–63. doi: 10.1136/sti.2004.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salfa MC, Regine V, Ferri M, Suligoi B. Le Infezioni Sessulmente Trasmesse: Aggiornamento dei Dati dei Due Sistemi di Sorveglianza Sentinella Attivi in Italia al 31 Dicembre 2013. Not Ist Super Sanità. 2015;28(2):3–22. [Google Scholar]
- 7.ECDC. 2016 https://ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2016-2014-data.
- 8.Tipple C, Taylor GP. Syphilis testing, typing, and treatment follow-up: a new era for an old disease. Curr Opin Infect Dis. 2015;28(1):53–60. doi: 10.1097/QCO.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 9.SEREMI. INFEZIONI SESSUALMENTE TRASMESSE IN PIEMONTE Rapporto 2013. 2015. 2015. [Google Scholar]
- 10.Cereda D, Senatore S, Rosa A, Mentasti M, Gramegna M. Sorveglianza delle malattie infettive in Lombardia. 2015 [Google Scholar]
- 11.Drago F, Ciccarese G, Broccolo F, et al. A new enhanced antibiotic treatment for early and late syphilis. J Glob Antimicrob Resist. 2016;5:64–6. doi: 10.1016/j.jgar.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Marra CM, Sahi SK, Tantalo LC, et al. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis. 2010;202(9):1380–8. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimes M, Sahi SK, Godornes BC, et al. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex Transm Dis. 2012;39(12):954–8. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen F, Starosta AL, Arenz S, Sohmen D, Donhofer A, Wilson DN. Tetracycline antibiotics and resistance mechanisms. Biol Chem. 2014;395(5):559–75. doi: 10.1515/hsz-2013-0292. [DOI] [PubMed] [Google Scholar]
- 15.Pringle M, Fellstrom C, Johansson KE. Decreased susceptibility to doxycycline associated with a 16S rRNA gene mutation in Brachyspira hyodysenteriae. Vet Microbiol. 2007;123(1–3):245–8. doi: 10.1016/j.vetmic.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y, Liu S, Liu Z, et al. Molecular Subtyping and Surveillance of Resistance Genes In Treponema pallidum DNA From Patients With Secondary and Latent Syphilis in Hunan, China. Sex Transm Dis. 2016;43(5):310–6. doi: 10.1097/OLQ.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 17.Grange PA, Allix-Beguec C, Chanal J, et al. Molecular subtyping of Treponema pallidum in Paris, France. Sex Transm Dis. 2013;40(8):641–4. doi: 10.1097/OLQ.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 18.Muller EE, Paz-Bailey G, Lewis DA. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex Transm Infect. 2012;88(6):470–4. doi: 10.1136/sextrans-2011-050322. [DOI] [PubMed] [Google Scholar]
- 19.Pillay A, Liu H, Chen CY, et al. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis. 1998;25(8):408–14. doi: 10.1097/00007435-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Martin IE, Gu W, Yang Y, Tsang RS. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49(4):515–21. doi: 10.1086/600878. [DOI] [PubMed] [Google Scholar]
- 21.Azzato F, Ryan N, Fyfe J, Leslie DE. Molecular subtyping of Treponema pallidum during a local syphilis epidemic in men who have sex with men in Melbourne, Australia. J Clin Microbiol. 2012;50(6):1895–9. doi: 10.1128/JCM.00083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS. 2016;30(14):2251–2. doi: 10.1097/QAD.0000000000001185. [DOI] [PubMed] [Google Scholar]
- 23.Tantalo LC, Lukehart SA, Marra CM. Treponema pallidum strain-specific differences in neuroinvasion and clinical phenotype in a rabbit model. J Infect Dis. 2005;191(1):75–80. doi: 10.1086/426510. [DOI] [PubMed] [Google Scholar]
- 24.Oliver S, Sahi SK, Tantalo LC, et al. Molecular Typing of Treponema pallidum in Ocular Syphilis. Sex Transm Dis. 2016;43(8):524–7. doi: 10.1097/OLQ.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikalova L, Pospisilova P, Woznicova V, Kuklova I, Zakoucka H, Smajs D. Comparison of CDC and sequence-based molecular typing of syphilis treponemes: tpr and arp loci are variable in multiple samples from the same patient. BMC Microbiol. 2013;13:178. doi: 10.1186/1471-2180-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillova L, Petrosova H, Mikalova L, et al. Molecular typing of Treponema pallidum in the Czech Republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52(10):3693–700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flasarova M, Pospisilova P, Mikalova L, et al. Sequencing-based molecular typing of Treponema pallidum strains in the Czech Republic: all identified genotypes are related to the sequence of the SS14 strain. Acta Derm Venereol. 2012;92(6):669–74. doi: 10.2340/00015555-1335. [DOI] [PubMed] [Google Scholar]
- 28.Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121(12):4584–92. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AIFA. 2016 http://www.aifa.gov.it/content/luso-dei-farmaci-italia-rapporto-osmed-2016.