Abstract
Background & Aims
Few studies have examined the association between coffee intake and survival after diagnosis of colorectal cancer (CRC). We performed a prospective study to investigate the association between coffee intake after a diagnosis of CRC and mortality.
Methods
We collected data from the Nurses’ Health Study (1984–2012) and Health Professionals Follow-up Study (1986–2012), following 1599 patients diagnosed with stage 1 or 2 CRC. CRC was reported on questionnaires and ascertained by review of medical records and pathology reports; intake of food and beverages was determined from responses to semi-quantitative food frequency questionnaires. Participants were asked how often during the previous year that they consumed coffee, with 1 cup as the standard portion size. The first questionnaire response collected at least 6 months but not more than 4 years after diagnosis was used for assessment of post-diagnostic intake (median time from diagnosis to the dietary assessment, 2.2 years). The last sFFQ prior to diagnosis was used to assess pre-diagnostic dietary intake.
Results
During a median of 7.8 years of follow-up, we documented 803 deaths, of which 188 were due to CRC. In the multivariable adjusted models, compared with nondrinkers, patients who consumed at least 4 cups of coffee per day had a 52% lower risk of CRC-specific death (hazard ratio [HR] 0.48; 95% CI, 0.28–0.83; P for trend =.003) and 30% reduced risk of all-cause death (HR, 0.70; 95% CI, 0.54–0.91; P for trend <.001). High intake of caffeinated and decaffeinated coffee (2 or more cups/day) was associated with lower risk of CRC-specific mortality and all-cause mortality. When coffee intake before vs after CRC diagnosis were examined, compared with patients consistently consuming low amounts (less than 2 cups/day), those who maintained a high intake (2 or more cups/day) had a significantly lower risk of CRC-specific death (multivariable HR, 0.63; 95% CI, 0.44–0.89) and death from any cause (multivariable HR, 0.71; 95% CI, 0.60–0.85).
Conclusions
In an analysis data from the Nurses’ Health Study and Health Professionals Follow-up Study, we associated intake of caffeinated and decaffeinated coffee after diagnosis of CRC with lower risk of CRC-specific death and overall death. Studies are needed to determine the mechanisms by which coffee might reduce CRC progression.
Keywords: colon cancer, rectal cancer, post-diagnostic coffee intake, diet
According to a report by the American Cancer Society, colorectal cancer (CRC) is the second leading cause of cancer death in the U.S., with a projection of approximately 50,260 individuals dying from the disease in 2017.1 Owing to improved diagnoses and advances in treatment, more than 1.4 million Americans are living with CRC, of which 65% survive more than 5 years and 58% live over 10 years.2 The prolonged survival of CRC prompts patients to seek information regarding dietary modifications that may benefit the prognosis; however, evidence about factors that may improve CRC survival is still limited.3–5 A western dietary pattern, characterized by high intake of meat, fat, refined grains, sugar-sweetened beverages, and dessert, has been associated with increased risk of mortality among CRC patients,6,7 but only a few individual dietary factors have been linked to patients’ survival.8
Coffee is one of the most commonly consumed beverages in the world. Some evidence suggest a beneficial effect of coffee in lowering risk of mortality and several chronic diseases, such as type 2 diabetes and coronary heart disease;9–11 and therefore, the 2015 American Dietary Guidelines recommend daily 3–5, 8-oz cups of coffee intake.12 Recently, two large prospective cohort studies of Europeans and ethnically diverse Americans reported a consistent inverse association between high coffee consumption and lower mortality across different subgroups of country origin and ethnicity.13,14 The beneficial effect of coffee may be due to its anti-inflammatory and insulin-sensitizing properties.15,16 Given the important role of inflammation and insulin resistance in CRC, coffee has been suggested to protect against CRC. Furthermore, coffee contains various anti-carcinogenic substances including polyphenols, diterpenes, melanoidins, and antioxidants16 which may be beneficial in improving CRC survival by ameliorating systemic disturbances due to metabolic reprogramming of cancer or by promoting an anti-carcinogenic microenvironment that slows down tumor progression. However, the influence of coffee consumption on survival of patients with established CRC remains largely unknown.
To our knowledge, only one recent study has examined the relationship between post-diagnostic coffee intake and CRC survival, and found that higher coffee intake was associated with reduced risk of cancer recurrence and death in patients with stage III colon cancer.17 However, whether the findings could be generalized to patients with less advanced cancer remains unclear. Moreover, due to lack of pre-diagnostic data in the previous study, it remains unknown whether coffee consumption after diagnosis may confer additional benefits beyond those resulting from pre-diagnostic coffee intake. Therefore, we evaluated the prognostic influence of pre- and post-diagnostic coffee intake among 1,599 patients diagnosed with stage I to III CRC in two large prospective cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS).
Material and Methods
Study population
The NHS cohort was established in 1976 when 121,700 female registered nurses, aged 30–55 years, completed a questionnaire on medical history and lifestyle characteristics. The HPFS began in 1986 when 51,529 U.S. male health professionals, aged 40–75 years, responded to a similar baseline questionnaire. Every two years, a follow-up questionnaire was mailed to participants in both cohorts to collect updated information and identify newly diagnosed CRC and other diseases. The response rates for the NHS and HPFS exceeded 90%. Detailed description of the two cohorts can be found elsewhere.18,19 Since 1984 for the NHS and 1986 for the HPFS, a comprehensive semi-quantitative food frequency questionnaire (sFFQ) has been administered every four years. In the current analysis, the exclusion criteria were death or diagnosis of CRC before baseline (1984 for NHS and 1986 for HPFS), death before diagnosis of CRC, cases diagnosed after the end of follow-up, not having dietary assessment within 4 years of diagnosis, having missing data for pre-diagnostic or post-diagnostic coffee intake, and stage IV CRC (Supplementary Figure 1). After exclusion, we included 1,027 participants from the NHS and 572 participants from the HPFS who were diagnosed with stage I to III CRC during follow-up. This study was approved by the Institutional Review Board at the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Assessment of CRC cases and death
The diagnosis of CRC was first reported by the participants on a biennial follow-up questionnaire and subsequently ascertained by review of medical records and pathological reports by study physicians blinded to exposure data. The disease stage, histological findings, and tumor location were recorded during review.20 For participants who did not respond to our request for medical record collection, the National Death Index was searched to identify deaths and confirm any CRC diagnosis that contributed to death or was a secondary diagnosis.21 For participants who died from CRC, we sought permission from next-of-kin to review medical records. In a subset of CRC patients in the NHS (N=397), information regarding chemotherapy was collected using a supplemental questionnaire in which patients were asked whether they had received chemotherapy and, if yes, the types of chemotherapy received.
Assessment of coffee intake and other dietary factors
In 1984, a 116-item sFFQ was administered to the NHS participants to collect information on diet from the previous year. The dietary information was updated in 1986 and every four years thereafter using similar but expanded sFFQs. Since 1986 in the HPFS, the same sFFQ has been used to collect and update dietary information every four years. In the sFFQ, participants from both cohorts were asked how often, on average, during the previous year that they consumed coffee with one cup as the standard portion size. The consumptions of caffeinated coffee and decaffeinated coffee were assessed separately. Nine possible responses ranged from never or <1 time/month to ≥6 times/day. The nutrient intake was computed by first multiplying the frequency of consumption for each food by its nutrient content and then summing up nutrient contributions across all food items. Both caffeinated and decaffeinated coffee intake were assessed in the sFFQ, and the sum of the two was calculated as the consumption of total coffee. The validity and reproducibility of the sFFQ have been described in detail elsewhere.22,23 Briefly, in a previous validation study, coffee intake was assessed at baseline using the sFFQ and in four, 1-week dietary records collected over a one-year period.23 The correlation coefficient was 0.78 between coffee intake assessed by sFFQ and the average of the four dietary records.
To avoid the influence of active cancer treatment on dietary intake, the first sFFQ, collected at least 6 months but not more than 4 years after diagnosis, was used for assessment of post-diagnostic intake. The median time from diagnosis to the dietary assessment was 2.2 years. The last sFFQ prior to diagnosis was used to assess pre-diagnostic dietary intake. Pre-specified cutoffs were applied for total coffee intake (nondrinker, ≤ 1 cup/d, 2–3 cups/d, ≥ 4 cups/d), caffeinated coffee intake (nondrinker, ≤ 1 cup/d, ≥ 2 cups/d), and decaffeinated coffee intake (nondrinker, ≤ 1 cup/d, ≥ 2 cups/d).
The average glycemic index (GI) and glycemic load (GL) were derived based on available databases and publications.24–26 Briefly, the average GI reflected the glucose response for overall food intake and the average GL standardized the average GI by the amount of carbohydrate contained in each food. Analogous to the concept of GI and GL, the dietary insulin index (II) and insulinogenic load (IL) are metrics that reflect the insulin response after the meal relative to the reference food (white bread or glucose). Details regarding the assessment of these covariates are provided in the Supplementary Methods.
Assessment of covariates
In the NHS and HPFS, follow-up questionnaires were sent biennially to collect and update occurrence of diseases and a comprehensive assessment of lifestyle and demographic risk factors, including smoking status, multivitamin use, menopausal status (NHS only), body weight and height, and leisure-time physical activity. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2) to measure overall adiposity. For physical activity, we calculated energy expenditure as metabolic equivalent (MET) measured in hours per week. A series of validation studies have demonstrated the validity of these self-reported measures.27–31
Statistical analysis
Person-time for each participant was counted from the return date of the sFFQ used for post-diagnostic assessment to death or the end of the study period (June 1, 2012 for the NHS; January 31, 2012 for the HPFS), whichever occurred first. In the primary analysis, CRC mortality was the main endpoint, and deaths from other causes were censored. In the secondary analysis, all-cause mortality was treated as the outcome. Because no statistical significant interaction was found between men and women (P=0.06 for CRC-specific mortality and 0.07 for all-cause mortality, Supplementary Table 1), we pooled data from two cohorts for all survival analyses.
Cumulative incidence curves of CRC-specific and all-cause mortality were plotted for total coffee intake. To evaluate the association between post-diagnostic coffee intake and CRC survival, we used a Cox proportional hazard model with time since diagnosis as the time scale, accounting for left truncation due to variations between patients in the timing of post-diagnostic assessment. We calculated the hazard ratios (HRs) and 95% confidence intervals (CIs) of death, adjusting for age at diagnosis (continuous), cancer stage (I, II, III, and unspecified), grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum, and unspecified), and post-diagnostic lifestyle and dietary predictors including pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), regular use of aspirin and NSAIDs (defined as ≥2 standard tablets/week; yes or no), menopausal hormone use (never, current, past users; women only), total folate (quartiles), total fat (quartiles), total calcium (quartiles), total vitamin D (quartiles), and sugar-sweetened beverages (<1 serving/mo, 1–4 servings/mo, 2–6 servings/wk, ≥ 1 serving/d). The median value of coffee intake within each category of coffee intake was modelled as continuous variables to examine linear trend. The proportional hazards assumption was tested by including in the model the interaction terms between continuous terms of exposures (total coffee, caffeinated coffee, and decaffeinated coffee) and the follow-up time. We did not detect any statistically significant violation of this assumption.
To minimize the potential selection bias introduced by exclusion of patients from the study due to lack of post-diagnostic dietary data, we applied inverse probability weighting (IPW) method to all survival analyses related to post-diagnostic coffee intake, as previously described (Supplementary methods).32,33
In a secondary analysis for post-diagnostic coffee intake, we additionally adjusted for pre-diagnostic coffee intake. In addition, we also evaluated the joint associations of total coffee intake before and after diagnosis with mortality by classifying patients into four categories: maintaining < 2 cups/d, increasing intake from < 2 cups/d to ≥ 2 cups/d, decreasing from ≥ 2 cups/d to < 2 cups/d, and maintaining ≥ 2 cups/d. We also assessed the association of pre-diagnostic coffee intake with mortality adjusting for pre-diagnostic covariates. In this analysis, cancer stage, subsites, and grade were not adjusted for because they might be the intermediate factors on the causal pathway between pre-diagnostic coffee intake and mortality. To address potential reverse causation that patients in poor health may have avoided coffee intake, we conducted a sensitivity analysis by excluding patients who died within 3 years after diagnosis (N=64).
To determine whether the effects of coffee intake on CRC varied by cancer stage, we examined the associations among patients in early (stage I, II) and advanced (stage III) cancer stages separately. We combined stage I and II due to limited number of events within each of these stages and patients with unknown cancer stages were excluded from this stratified analysis. To assess the influence of unspecified stage (~12% in this study) on our study results, we performed a sensitivity analysis for the main associations between total post-diagnostic coffee intake and survival by imputing missing cancer stage with a Markov Chain Monte Carlo (MCMC)-based multiple imputation method or excluding participants with unspecified cancer stage.34 We also compared total coffee consumption between patients who received (42%) or did not receive chemotherapy among those who provided chemotherapy information.
Given the strong association of cigarette smoking with both coffee intake and CRC survival, a stratified analysis was performed by post-diagnostic smoking status (never vs ever smokers) to minimize the potential confounding effect by smoking.35 Additionally, we stratified by other clinicopathological factors, including age (< 65, ≥ 65 years), sex, alcohol consumption (<7g/d, ≥ 7g/d), BMI (<25kg/m2, ≥25kg/m2), physical activity (<7.5 MET-h/wk, ≥7.5 MET-h/wk), aspirin use (yes, no), glycemic index (<median, ≥median), glycemic load (<median, ≥median), insulinogenic load (<median, ≥median), dietary insulin index (<median, ≥median), and cancer site (proximal colon, distal colon, rectum). Proximal CRC was defined as tumor arising from the cecum, ascending colon, and transverse colon; distal CRC included tumors arising from the splenic flexure, descending colon, and sigmoid; and rectal cancer included tumors originated in the rectosigmoid and rectum. Linear terms of the continuous stratified variables were adjusted in the model to reduce residual confounding. The interaction was tested via a likelihood ratio test by comparing the model with and without the product terms between categorical stratified variable and continuous total coffee intake. To account for multiple comparisons in the stratified analysis according to 12 covariates, we employed the Bonferroni correction and adjusted the significance level to 0.05/12≈0.004. All the statistical tests were 2-sided and performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Basic characteristics of participants at diagnosis
Among 1,599 participants diagnosed with stage I to III CRC, we documented 803 total deaths and 188 CRC-specific deaths during a median of 7.8 years of follow-up. The median intake of total coffee was 1 cup/d for both NHS and HPFS. Baseline characteristics of study participants by frequency of post-diagnostic total coffee intake are shown in Table 1. Patients with higher total coffee intake after CRC diagnosis tended to be current smokers and aspirin users, consume higher intake of alcohol and total fat, and have distal colon cancers. Among a subset of patients in which we collected chemotherapy data, the median total coffee intake was 1 cup/d for patients who received and did not receive chemotherapy (n=232 and 145, respectively). In addition, participants with available post-diagnostic coffee data had similar characteristics to those without, except for a lower BMI, higher proportion of current smoker, and lower regular use of aspirin and multivitamin (Supplementary Table 2). Patients’ characteristics according to pre-diagnostic total coffee intake are shown in Supplementary Table 3. In general, the relationships between pre-diagnostic total coffee intake and baseline characteristics were similar to those observed for post-diagnostic total coffee intake. Patients who consumed more coffee prior to diagnosis tended to have lower intake of folate, calcium, and vitamin D, and a higher proportion of stage III CRC.
Table 1.
Baseline Characteristics of Patients with Colorectal Cancer According to Total Coffee Intake After Diagnosis
| Total coffee intake | ||||
|---|---|---|---|---|
|
| ||||
| Nondrinker (n=282) | ≤1 cup/d (n=567) | 2–3 cups/d (n=483) | ≥4 cups/d (n=267) | |
| Female, % | 64.9 | 61.8 | 65.1 | 67.3 |
| Age of diagnosis, year | 69.0(9.3) | 69.5(8.6) | 68.4(8.8) | 66.4(7.9) |
| Body mass index, kg/m2 | 26.2(5.3) | 26.4(4.7) | 26.3(4.8) | 25.9(4.4) |
| Physical activity, MET-h/wka | 19.2(23.6) | 18.3(23.9) | 19.8(25.1) | 18.9(22.0) |
| Pack-years of smoking | 11.6(21.0) | 15.4(20.8) | 18.5(23.0) | 21.8(26.1) |
| Current smoking, % | 5.2 | 3.9 | 7.0 | 10.3 |
| Multivitamin use, % | 58.3 | 62.6 | 59.6 | 62.5 |
| Regular aspirin use, %b | 30.2 | 29.8 | 36.9 | 40.1 |
| Menopausal hormone use, %c | 17.2 | 19.2 | 21.6 | 14.6 |
| Alcohol intake, g/d | 4.8(10.6) | 7.1(11.6) | 8.5(12.9) | 10.0(14.3) |
| Total fat intake, g/d | 29.3(7.0) | 29.8(6.9) | 30.1(6.4) | 31.4(6.4) |
| Folate intake, mcg/d | 710(388) | 696 (356) | 649(307) | 662(313) |
| Calcium intake, mg/d | 1184(612) | 1148(529) | 1194(569) | 1152(491) |
| Vitamin D intake, IU/d | 553 (432) | 535 (379) | 547(445) | 527(378) |
| Sugar-sweetened beverages, serving/d | 0.3(0.7) | 0.3(0.5) | 0.2(0.5) | 0.2(0.5) |
| Subsite | ||||
| - proximal colon, % | 45.2 | 40.4 | 42.4 | 40.5 |
| - distal colon, % | 27.1 | 32.3 | 31.9 | 34.0 |
| - rectum, % | 22.0 | 22.4 | 22.0 | 20.7 |
| - Unspecified, % | 5.7 | 4.9 | 3.6 | 4.8 |
| Grade | ||||
| - Well Differentiated, % | 13.4 | 16.0 | 14.1 | 14.8 |
| - Grade 2 Moderately Diff, % | 54.8 | 59.6 | 60.3 | 54.3 |
| - Grade 3 Poorly Diff, % | 14.9 | 10.0 | 13.2 | 12.3 |
| - Unspecified, % | 16.9 | 14.4 | 12.5 | 18.7 |
| Stage | ||||
| - stage I, % | 32.3 | 33.2 | 34.0 | 34.1 |
| - stage II, % | 29.9 | 30.9 | 31.1 | 30.6 |
| - stage III, % | 22.1 | 24.8 | 24.1 | 22.6 |
| - Unspecified, % | 15.7 | 11.0 | 10.8 | 12.7 |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Metabolic equivalent from recreational and leisure-time activities.
Regular user was defined as ≥2 standard (325 mg) tablets of aspirin per week.
Calculated among postmenopausal women.
Coffee intake after diagnosis and survival
Supplementary Figures 2 and 3 show the cumulative incidence curves for overall and CRC-specific deaths according to post-diagnostic total coffee intake. Table 2 shows that higher total coffee intake was associated with reduced risk for both CRC-specific and all-cause mortality. Compared with abstainers, participants who drank coffee for at least 4 cups per day had 52% lower risk of CRC-specific mortality (HR 0.48; 95% CI, 0.28–0.83; P for trend = 0.003) and 30% lower risk of all-cause mortality (HR 0.70; 95% CI: 0.54–0.91; P for trend < 0.001). Similar beneficial associations were observed for both caffeinated and decaffeinated coffee. The HRs of CRC-specific mortality comparing ≥ 2 cups/d with 0 cup/d were both 0.61 for caffeinated coffee (95% CI, 0.40–0.91, P for trend = 0.02) and decaffeinated coffee (95% CI, 0.38–0.97, P for trend = 0.04). The corresponding HRs (95% CI) for all-cause mortality were 0.75 (0.62–0.91, P for trend = 0.004) and 0.70 (0.55–0.87, P for trend = 0.002), respectively.
Table 2.
Associations Between Post-diagnostic Coffee Intake and Mortality
| Total coffee | |||||
|---|---|---|---|---|---|
| Non drinkers | ≤1cup/d | 2–3 cups/d | ≥4 cups/d | P trend | |
|
| |||||
| Colorectal cancer-specific mortality | |||||
| Events/total | 37/282 | 73/567 | 55/483 | 23/267 | |
| Age adjusted HR (95% CI) | 1 | 0.89(0.60–1.32) | 0.74(0.49–1.12) | 0.50(0.30–0.83) | 0.005 |
| Multivariable adjusted HR (95% CI)a | 1 | 0.93(0.61–1.42) | 0.70(0.45–1.09) | 0.48(0.28–0.83) | 0.003 |
| All-cause mortality | |||||
| Events/total | 145/282 | 300/567 | 234/483 | 124/267 | |
| Age adjusted HR (95% CI) | 1 | 0.89(0.73–1.09) | 0.75(0.61–0.93) | 0.70(0.55–0.89) | <0.001 |
| Multivariable adjusted HR (95% CI)a | 1 | 0.92(0.74–1.13) | 0.76(0.61–0.94) | 0.70(0.54–0.91) | <0.001 |
|
| |||||
| Caffeinated coffee | |||||
| Non drinkers | ≤1cup/d | ≥2 cups/d | P trend | ||
|
|
|||||
| Colorectal cancer-specific mortality | |||||
| Events/total | 75/583 | 70/580 | 43/436 | ||
| Age adjusted HR (95% CI) | 1 | 0.87(0.63–1.21) | 0.67(0.46–0.98) | 0.04 | |
| Multivariable adjusted HR (95% CI)a | 1 | 0.83(0.59–1.18) | 0.61(0.40–0.91) | 0.02 | |
|
| |||||
| All-cause mortality | |||||
| Events/total | 303/583 | 303/580 | 197/436 | ||
| Age adjusted HR (95% CI) | 1 | 0.92(0.78–1.08) | 0.79(0.66–0.95) | 0.01 | |
| Multivariable adjusted HR (95% CI)a | 1 | 0.89(0.76–1.06) | 0.75(0.62–0.91) | 0.004 | |
| Decaffeinated coffee | |||||
| Non drinkers | ≤1cup/d | ≥2 cups/d | P trend | ||
|
|
|||||
| Colorectal cancer-specific mortality | |||||
| Events/total | 112/877 | 54/526 | 22/196 | ||
| Age adjusted HR (95% CI) | 1 | 0.73(0.53–0.99) | 0.59(0.37–0.94) | 0.03 | |
| Multivariable adjusted HR (95% CI)a | 1 | 0.74(0.53–1.03) | 0.61(0.38–0.97) | 0.04 | |
|
| |||||
| All-cause mortality | |||||
| Events/total | 447/877 | 255/526 | 101/196 | ||
| Age adjusted HR (95% CI) | 1 | 0.86(0.74–1.00) | 0.67(0.54–0.84) | <0.001 | |
| Multivariable adjusted HR (95% CI)a | 1 | 0.91(0.78–1.07) | 0.70(0.55–0.87) | 0.002 | |
Adjusting for age at diagnosis (continuous), cancer stage (I, II, III and unspecified), grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), post-diagnostic pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-ours/week), regular use of aspirin and NSAIDs (yes or no) and postmenopausal hormone use (women only: never, current, past users), folate (quartiles), total fat (quartiles), calcium (quartiles), vitamin D (quartiles) and sugar-sweetened beverages (<1 serving/mo, 1–4 servings/mo, 2–6 servings/wk, ≥ 1 serving/d)
We also assessed pre-diagnostic intake of total coffee and found an inverse association with CRC-specific mortality (HR 0.53; 95% CI, 0.32–0.88, for the ≥ 4 cups/d vs 0 cup/d; P for trend = 0.04), but not all-cause mortality (HR 0.90; 95% CI, 0.70–1.16, for the ≥ 4 cups/d vs 0 cup/d; P for trend = 0.16) (Supplementary Table 4). The correlation coefficient between pre- and post-diagnostic coffee intake was 0.63. Further adjusting for pre-diagnostic coffee intake attenuated, but did not eliminate, the associations for post-diagnostic coffee intake with mortality; comparing patients consuming ≥ 4 cups/d with nondrinkers, the HRs were 0.62 (95% CI, 0.32–1.17, for the P for trend = 0.03) for CRC-specific mortality and 0.76 (95% CI, 0.55–1.05; P for trend = 0.03) for all-cause mortality.
In the sensitivity analysis that excluded participants died within 3 years after diagnosis, the results were not materially changed (Supplementary Table 5). In the sensitivity analysis that evaluated the influence of missing data on cancer stage, we obtained similar results by either imputing the unspecified stage or excluding participants with unspecified stage (Supplementary Table 6).
Total coffee intake after diagnosis and survival by cancer stage
When stratified by cancer stage, the inverse associations between total coffee intake and mortality appeared to be stronger among patients with stage III than with stage I or II cancers (P for interaction = 0.07 for CRC-specific mortality and 0.02 for all-cause mortality) (Table 3). For patients with stage III CRC, each cup/d increase of total coffee intake was associated with 18% lower risk of CRC-specific mortality (HR 0.82; 95%CI, 0.68–1.00; P for trend = 0.01) and 20% lower risk of all-cause mortality (HR 0.80; 95%CI, 0.69–0.91; P for trend <0.0001). In contrast, no association was found for patients with stage I or II CRC with either CRC-specific mortality (HR 1.00; 95%CI, 0.86–1.16; P for trend= 0.80) or all-cause mortality (HR 0.97; 95%CI, 0.91–1.03; P for trend = 0.19).
Table 3.
Associations Between Post-diagnostic Coffee Intake and Mortality by cancer stage
| Non drinkers | ≤1cup/d | 2–3 cups/d | ≥4 cups/d | 1 cup/d increment | P trend | |
|---|---|---|---|---|---|---|
|
|
||||||
| Colorectal cancer-specific mortality | ||||||
| Stage I, II | ||||||
| Events/Total | 12/179 | 29/363 | 26/314 | 10/170 | ||
| Multivariable adjusted HR (95% CI)a | 1 | 1.04(0.52–2.07) | 1.28(0.64–2.58) | 0.71(0.30–1.69) | 1.00(0.86–1.16) | 0.80 |
| Stage III | ||||||
| Events/Total | 19/62 | 31/140 | 22/116 | 9/62 | ||
| Multivariable adjusted HR (95% CI)a | 1 | 0.53(0.27–1.07) | 0.41(0.20–0.86) | 0.33(0.13–0.82) | 0.82(0.68–1.00) | 0.01 |
| P for interaction | 0.07 | |||||
|
| ||||||
| Non drinkers | ≤1cup/d | 2–3 cups/d | ≥4 cups/d | 1 cup/d increment | P trend | |
|
|
||||||
| All-cause mortality | ||||||
| Stage I, II | ||||||
| Events/Total | 80/179 | 186/363 | 144/314 | 83/170 | ||
| Multivariable adjusted HR (95% CI)a | 1 | 0.96(0.73–1.25) | 0.84(0.64–1.12) | 0.87(0.63–1.20) | 0.97(0.91–1.03) | 0.19 |
| Stage III | ||||||
| Events/Total | 39/62 | 71/140 | 57/116 | 25/62 | ||
| Multivariable adjusted HR (95% CI)a | 1 | 0.60(0.38–0.94) | 0.36(0.21–0.60) | 0.31(0.18–0.56) | 0.80(0.69–0.91) | <0.0001 |
| P for interaction | 0.02 | |||||
Adjusting for age at diagnosis (continuous), grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), post-diagnostic pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-ours/week), regular use of aspirin and NSAIDs (yes or no) and postmenopausal hormone use (women only: never, current, past users), folate (quartiles), total fat (quartiles), calcium (quartiles), vitamin D (quartiles) and sugar-sweetened beverages (<1 serving/mo, 1–4 servings/mo, 2–6 servings/wk, ≥ 1 serving/d)
Joint association of coffee intake before and after diagnosis of CRC with subsequent survival
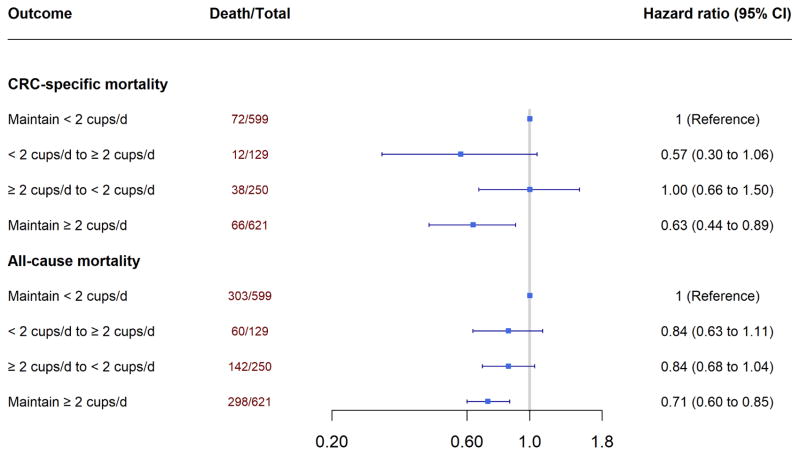
Figure 1 illustrates the joint association between total coffee intake before and after diagnosis and CRC survival. Participants who maintained higher total coffee intake after CRC diagnosis had a 37% (HR 0.63; 95% CI, 0.44–0.89) lower risk of CRC-specific mortality, compared with those having total coffee consumption consistently below 2 cups/d before and after diagnosis (Panel A). A slightly weaker association was observed for all-cause mortality (HR 0.71; 95% CI, 0.60–0.85, for the maintaining ≥ 2 cups/d vs maintaining < 2 cups/d) (Panel B).
Figure 1.
Joint Association of Total Coffee Intake Before and After Diagnosis and Mortality
Adjusting for age at diagnosis (continuous), cancer stage (I, II, III, and unspecified), grade of differentiation (1–3, and unspecified), subsite (proximal colon, distal colon, rectum, and unspecified), post-diagnostic pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), regular use of aspirin and NSAIDs (yes or no) and postmenopausal hormone use (women only: never, current, past users), folate (quartiles), total fat (quartiles), calcium (quartiles), vitamin D (quartiles) and sugar-sweetened beverages (<1 serving/mo, 1–4 servings/mo, 2–6 servings/wk, ≥ 1 serving/d)
X-axis is in logarithm scale
Total coffee intake and survival within subgroups
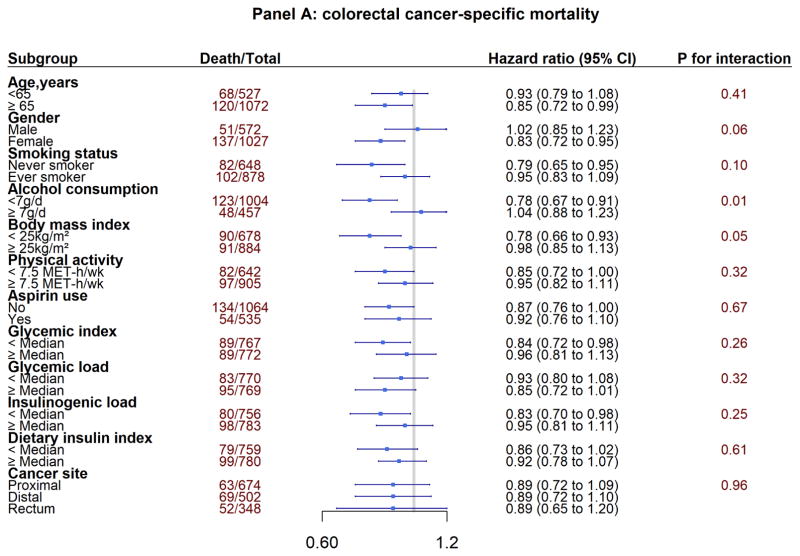
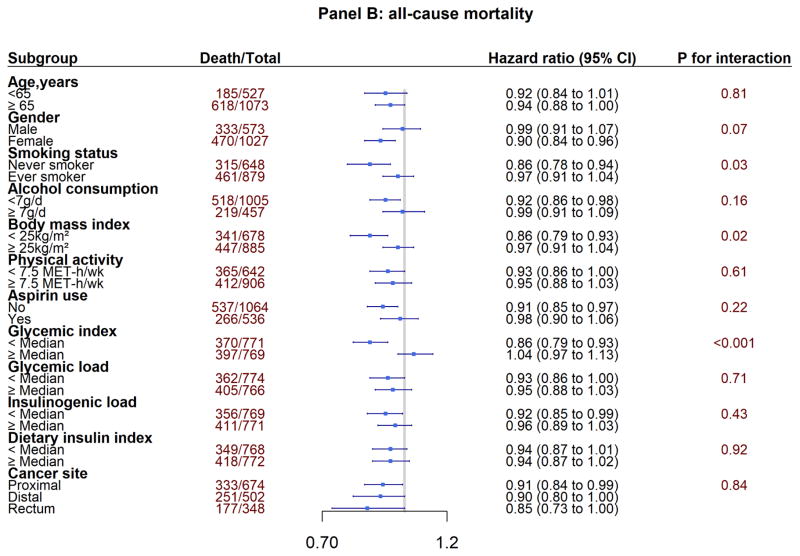
In the subgroup analyses, we explored whether the associations between post-diagnostic total coffee consumption and mortality differed by clinical and lifestyle factors (Figure 2). Under the corrected statistical significance threshold (α=0.004), we identified an interaction by glycemic index for all-cause mortality (P for interaction < 0.001). The inverse associations between total coffee intake and all-cause mortality appeared to be stronger among patients with lower post-diagnostic glycemic index. We did not find any different association by tumor subsite.
Figure 2.
Subgroup Analyses of Association Between Post-diagnosis Total Coffee Intake Colorectal Cancer Survival
Panel A: colorectal cancer-specific mortality
Panel B: all-cause mortality
Adjusting for the same covariates as in Table 2 and 3 except for the stratification variables.
P values for interactions were evaluated using likelihood ratio test.
X-axis is in logarithm scale
Discussion
The current findings within two large prospective cohort studies suggest that higher intake of coffee after diagnosis was associated with lower risk of CRC-specific mortality and all-cause mortality. The inverse associations were unlikely to be attributed to caffeine because high intake of both caffeinated and decaffeinated coffee was associated with reduced risk of mortality. The potential beneficial effects of coffee intake after diagnosis might not be entirely independent of pre-diagnostic coffee intake that also predicted lower risk of mortality. In addition, participants who had high total coffee consumption in both pre- and post-diagnostic periods had lower risk of mortality in comparison with participants having consistently lower coffee intake. These findings provide further evidence for the benefits of coffee intake on improvement of CRC prognosis.
Our findings were generally consistent with a previous study that showed an inverse association between higher coffee intake and CRC recurrence or mortality in patients with stage III colon cancer.17 Both studies found more than 50% reduced risk of CRC mortality among patients consuming at least 4 cups/d of coffee, compared with the nondrinkers. In our study, several novel findings merit discussion. In the joint analysis, although the observed inverse associations were primarily attributed to the post-diagnostic coffee intake for CRC-specific mortality, there was a potential synergic effect of both pre- and post-diagnostic coffee intake on overall survival. Compared to patients who had consumed less than 2 cups/d of coffee, those who drank ≥ 2 cups/d of coffee before or after diagnosis had a 16% lower risk of death, whereas patients who maintained ≥ 2 cups/d of coffee intake at both periods had a 29% lower risk for all-cause mortality. In addition, we found that additionally adjusting for pre-diagnostic coffee intake attenuated the inverse association between post-diagnostic intake and survival. Given the high correlation of pre- and post-diagnostic coffee intake (r=0.63), these results may suggest that both pre- and post-diagnostic coffee intake are associated with improved CRC survival.
We also found that the beneficial association of post-diagnostic coffee intake with survival appeared to be stronger among stage III patients than stage I or II patients. Although the exact mechanisms remain unclear, the findings are unlikely to be explained by reverse causality (i.e. patients with more severe disease tend to avoid stimulating beverages such as coffee) because total coffee intake did not vary by cancer stages (Table 1), and the HRs were virtually unchanged after excluding patients who died within 3 years after diagnosis. Given multiple comparisons for the stratified analysis and the modest p values for interactions by cancer stage, the possibilities of chance findings cannot be ruled out.
Observational studies investigating the association between coffee intake and CRC incidence produced mixed results, and the most recent meta-analysis of prospective cohort studies including NHS and HPFS concluded null associations.36,37 There are several explanations for the discrepancy between the current results and the null findings for the incidence of CRC. First, coffee may be more potent in suppressing tumor metastasis than preventing the occurrence of neoplasms. Recent experimental data suggest that the polyphenol caffeic acid found in coffee may inhibit colon cancer metastasis through targeting mitogen-activated protein kinases and T-cell-originated protein kinase.38 One study using a rodent model showed that kahweol, a coffee-specific diterpene, had the potential to inhibit metastasis by disruption of STAT3-mediated transcription of the pro-metastatic genes (e.g., MMP and VEGF).39 In addition, the coffee melanoidins have been reported to have the ability to inhibit matrix metalloproteases which play a central role in tumor growth and metastasis.40 Second, long-term coffee consumption may help the body maintain a relatively low-inflammation and high insulin sensitivity status. Both population-based and clinical studies suggested that higher coffee consumption was associated with lower level of C-peptide and inflammatory biomarkers (e.g., CRP, IL-18).41–43 Moreover, some studies have reported a stronger association of type 2 diabetes with CRC mortality than with CRC incidence.44,45 Therefore, considering that a hyperinsulinemia environment may be more pertinent to tumor progression than to tumor initiation, it is possible that the insulin-sensitizing effect of coffee may exert a particularly greater benefit for patients with established CRC than for the general population that is at average risk of developing CRC. Finally, coffee may improve CRC survival by preventing liver metastases, the major contributor of CRC-specific death.46 Coffee consumption has been shown to decrease levels of liver transaminases that are markers of liver injury. In addition, cafestol and kahweol, two bioactive constituents of coffee, can induce phase II enzyme activity, enhance hepatic glutathione levels, and decrease liver DNA adducts caused by chemical carcinogens in animal models.47–49 Furthermore, several cross-sectional and case-control studies have suggested that coffee may reduce the severity of non-alcoholic fatty liver disease owing to its anti-inflammatory and anti-fibrosis properties.50 Therefore, it is possible that high coffee consumption may help improve CRC survival by inhibiting the ability of metastases to seed the liver.
The strengths of the current study include the prospective design, relatively large sample size, inclusion of patients with stage I to III CRC, comprehensive assessment of pre- and post-diagnostic dietary and lifestyle factors, rigorous outcome assessment, and long-term follow-up. Several limitations are also worth discussion. First, because pre- and post-diagnostic coffee intake was highly correlated, we were unable to disentangle their independent effects on cancer prognosis. However, an inverse association of post-diagnostic coffee intake remained even after adjustment for pre-diagnostic coffee intake, suggesting that higher coffee intake after diagnosis may confer additional benefits beyond those from pre-diagnostic intake. Second, we were unable to examine the impact of coffee intake on cancer recurrence, because the recurrence data were not available in the two cohorts. Nonetheless, given the relatively short survival time (< 10 months) of metastatic CRC,51 CRC-specific mortality should be a reasonable surrogate outcome for cancer recurrence.4 Third, treatment data were not available for all CRC patients. However, in a subset of patients who provided chemotherapy data, we did not note any difference in total coffee consumption between those who received chemotherapy and those who did not. Finally, since our study population consisted of health professionals with high education level, the generalizability of our findings may be limited. However, we observed similar beneficial associations as previously reported in a clinical cohort,17 supporting generalizability of the findings.
In conclusion, our study suggested that higher consumption of coffee after diagnosis was associated with lower CRC-specific and all-cause mortality in patients with stage I to III CRC. Patients who maintain a high level of coffee consumption both before and after diagnosis may have particularly favorable survival. More prospective cohort studies in CRC patients are warranted to confirm our findings and further mechanistic studies are needed to better understand the potential role of higher coffee intake in improving CRC survival.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003, 5R01CA169141, 5R01CA118553 to C.S.F.; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726 to A.T.C.; R01 CA151993, R35 CA197735 to S.O.]; and by the grants from the Stand Up to Cancer (SU2C) Colorectal Cancer Dream (C.S.F.), the 2017 AACR-AstraZeneca Fellowship in Immuno-oncology Research (Grant Number 17-40-12-SONG, M.S.), the American Institute for Cancer Research (K.W.), the Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Author contributions
Study concept and design: YH, MS, ELG
Acquisition of data: ELG, FBH
Analysis and interpretation of data: YH, MS
Drafting of the manuscript: YH
Critical revision of the manuscript for important intellectual content: MS, ELG, MD, YC, KW, SAS-W, ATC, JAM, SO, CSF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Phipps AI, Robinson JR, Campbell PT, et al. Prediagnostic alcohol consumption and colorectal cancer survival: The Colon Cancer Family Registry. Cancer. 2017;123:1035–1043. doi: 10.1002/cncr.30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M, Zhang X, Meyerhardt JA, et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2016:1–7. doi: 10.1136/gutjnl-2016-311990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin d concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32:2430–9. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs MA, Sato K, Niedzwiecki D, et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance) PLoS One. 2014:9. doi: 10.1371/journal.pone.0099816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 8.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65:427–455. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding M, Satija A, Bhupathiraju SN, et al. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation. 2015;132:2305–2315. doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding M, Bhupathiraju SN, Satija A, et al. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Bhupathiraju SN, Chen M, et al. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-analysis. Diabetes Care. 2014;37:569–86. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USDA, DHHS. Dietary Guidelines for Americans, 2015–2020. 8. 2015. [Google Scholar]
- 13.Park S-Y, Freedman ND, Haiman CA, et al. Association of Coffee Consumption With Total and Cause-Specific Mortality Among Nonwhite Populations. Ann Intern Med. 2017 doi: 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunter M, Murphy N, Cross AJ, et al. Coffee Drinking and Mortality in 10 European Countries. Ann Intern Med. 2017;16:789–91. doi: 10.7326/L17-0689. [DOI] [PubMed] [Google Scholar]
- 15.Higdon JV, Frei B. Coffee and Health: A Review of Recent Human Research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 16.Alicandro G, Tavani A, La Vecchia C. Coffee and cancer risk: a summary overview. Eur J Cancer Prev. 2017 doi: 10.1097/CEJ.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 17.Guercio BJ, Sato K, Niedzwiecki D, et al. Coffee intake, recurrence, and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance) J Clin Oncol. 2015;33:3598–3607. doi: 10.1200/JCO.2015.61.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 19.Colditz Ga, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, TA AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual (7th edition) Am Jt Commitee Cancer. 2010;38:2011. [Google Scholar]
- 21.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 23.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 24.The University of Sydney. Glycemic index database. Available at: http://www.glycemicindex.com/
- 25.Higgins JA, Brand Miller JC, Denyer GS. Development of insulin resistance in the rat is dependent on the rate of glucose absorption from the diet. J Nutr. 1996;126:596–602. doi: 10.1093/jn/126.3.596. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins DJA, Wolever TMS, Taylor RH. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 27.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 28.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 29.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 31.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 32.Hamada T, Cao Y, Qian ZR, et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J Clin Oncol. 2017;35:1836–1844. doi: 10.1200/JCO.2016.70.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Herring AH, Zhou H, et al. A multiple imputation method for sensitivity analyses of time-to-event data with possibly informative censoring. J Biopharm Stat. 2014;24:229–253. doi: 10.1080/10543406.2013.860769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Ann Oncol. 2014;25:1517–1525. doi: 10.1093/annonc/mdu040. [DOI] [PubMed] [Google Scholar]
- 36.Vieira A, Chan D, Vingeliene S, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017 doi: 10.1093/annonc/mdx171. In press. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Albanes D, Beeson WL, et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: Pooled analysis of prospective cohort studies. J Natl Cancer Inst. 2010;102:771–783. doi: 10.1093/jnci/djq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang NJ, Lee KW, Kim BH, et al. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011;32:921–928. doi: 10.1093/carcin/bgr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HG, Hwang YP, Han EH, et al. The coffee diterpene kahweol inhibits metastasis by modulating expressions of MMPs and VEGF via STAT3 inactivation. Food Chem. 2012;133:1521–1529. [Google Scholar]
- 40.Moreira ASP, Nunes FM, Domingues MR, et al. Coffee melanoidins: structures, mechanisms of formation and potential health impacts. Food Funct. 2012;3:903–915. doi: 10.1039/c2fo30048f. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Garcia E, Van Dam RM, Qi L, et al. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Willett WC, Hankinson SE, et al. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care. 2005;28:1390–1396. doi: 10.2337/diacare.28.6.1390. [DOI] [PubMed] [Google Scholar]
- 43.Kempf K, Herder C, Erlund I. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 44.Deng L, Gui Z, Zhao L, et al. Diabetes mellitus and the incidence of colorectal cancer: An updated systematic review and meta-analysis. Dig Dis Sci. 2012;57:1576–1585. doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 45.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheth KR, Clary BM. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg. 2005;18:215–223. doi: 10.1055/s-2005-916282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber WW, Scharf G, Rossmanith W, et al. The coffee components kahweol and cafestol induce gamma-glutamylcysteine synthetase, the rate limiting enzyme of chemoprotective glutathione synthesis, in several organs of the rat. Arch Toxicol. 2002;75:685–694. doi: 10.1007/s00204-001-0295-5. [DOI] [PubMed] [Google Scholar]
- 48.Huber WW, Prustomersky S, Delbanco E, et al. Enhancement of the chemoprotective enzymes glucuronosyl transferase and glutathione transferase in specific organs of the rat by the coffee components kahweol and cafestol. Arch Toxicol. 2002;76:209–217. doi: 10.1007/s00204-002-0322-1. [DOI] [PubMed] [Google Scholar]
- 49.Cavin C, Holzhaeuser D, Scharf G, et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 2002;40:1155–1163. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Teoh NC, Chitturi S, et al. Coffee and non-alcoholic fatty liver disease: Brewing evidence for hepatoprotection? J Gastroenterol Hepatol. 2014;29:435–441. doi: 10.1111/jgh.12422. [DOI] [PubMed] [Google Scholar]
- 51.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.