Abstract
The inflammatory response may contribute to retinal pigment epithelial (RPE) dysfunction associated with the pathogenesis of age-related macular degeneration (AMD). We investigated whether the inflammatory response affects the expression of long coding RNAs (lncRNAs) in human RPE-derived ARPE-19 cells. This class of regulatory RNA molecules recently came to prominence due to their involvement in many pathophysiological processes. A proinflammatory cytokine mixture consisting of IFN-γ, IL-1β and TNF-α altered the expression several lncRNAs including BANCR in these cells. The cytokine responsible for increasing BANCR expression in ARPE-19 cells was found to be IFN-γ. BANCR expression induced by IFN-γ was suppressed when STAT1 phosphorylation was blocked by JAK inhibitor 1. Thus, proinflammatory cytokines could modulate the expression of lncRNAs in RPE cells and IFN-γ could upregulate the expression of BANCR by activating JAK-STAT1 signaling pathway.
Keywords: BRAF-activated non-coding RNA (BANCR), Long non-coding RNA (lncRNA), Interferon-γ, JAK-STAT1 signaling, Retinal pigment epithelium, Age-related macular degeneration
1. Introduction
Recent studies have shown that long non-coding RNAs (lncRNAs), non-protein coding transcripts with a minimum length of 200 nucleotides, may mediate many pathophysiological processes including inflammatory response [1, 2]. BRAF-activated non-coding RNA (BANCR, LINC00586), a 693-nucleotide long lncRNA transcribed from human chromosome 9, has been shown to regulate proliferation and migration of melanoma and other cancer cells [3–5]. This lncRNA is also reported to regulate epithelial-mesenchymal transition (EMT) [4, 5]. Furthermore, BANCR is highly expressed in retinoblastoma tissues and cells, and silencing its expression resulted in a decrease in retinoblastoma cell proliferation, migration and invasion [6].
Retinal pigment epithelium (RPE), a polarized monolayer of pigmented cells located adjacent to photoreceptor cells, is indispensable for visual function since it provides nutrients to the photoreceptor cells, regenerates the visual chromophore 11-cis-retinal and phagocytoses rod outer segment discs generated by circadian shedding [7]. Dysfunction of RPE resulting from abnormal inflammatory response may play a role in the pathology of age-related macular degeneration (AMD) [8, 9]. Proinflammatory cytokines secreted by infiltrating macrophages and lymphocytes may trigger retinal pigment epithelial (RPE) cell dysfunction. We have reported earlier that human RPE derived ARPE-19 cells respond to proinflammatory cytokines IFN-γ, TNF-α and IL-1β by increasing the expression of cytokines and chemokines [10]. The proinflammatory cytokines also decreased the expression of RPE characteristic genes and induced epithelial-mesenchymal transition (EMT)-like changes in these cells [11]. It is not yet known whether lncRNAs play a role in mediating the deleterious effect of IFN-γ, TNF-α and IL-1β on RPE cells. Therefore, we investigated whether these proinflammatory cytokines can affect the expression of lncRNAs like BANCR in the ARPE-19 cells. We observed that the inflammatory response altered the expression of several lncRNAs in these cells and that the activation of JAK-STAT1 signaling pathway by IFN-γ increased the expression of BANCR.
2. Materials and methods
2.1. Cell culture
Human ARPE-19 cells were cultured until they exhibited RPE characteristics as recently described from our laboratory [11–13]. Briefly the cells were grown in Dulbecco’s modified Eagle’s medium containing 4.5 g/L glucose, L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin and 1% fetal bovine serum at 37°C in a humidified environment of 5% CO2. The culture medium was replaced twice every week. The cells kept under these conditions for 4 months exhibited RPE characteristics such as pigmentation, epithelial morphology and expressed visual cycle genes. The cells were treated with the proinflammatory cytokine mixture consisting of IFN-γ (100 u/ml), IL-1β (10 ng/ml) and TNF-α (10 ng/ml) in the presence of serum for 4 days unless indicated otherwise. Human IL-1β was purchased from R&D Systems, Minneapolis, MN while TNF-α and IFN-γ were from Roche Applied Science, Indianapolis, IN. Activation of JAK-STAT1 signaling pathway was blocked by pre-incubating the cells with 1 μM of JAK inhibitor 1 (CAS 457081-03-7; 2-(1,1-dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one; Calbiochem, San Diego, CA.) for 30 min prior to treatment with IFN-γ (10 or 100 units/ml) for 20 h. A 10 mM stock solution of this compound in DMSO was diluted to the desired concentration with the culture medium immediately before use. The same amount of DMSO was also added to the control medium.
2.2. Real-time RT-PCR
Real-time RT-PCR analysis of the expression of lncRNA, mRNA and miRNA was performed using total RNA fractions isolated from ARPE-19 cells following the exposure to cytokines. An Applied Biosystems ViiA7 Real-Time PCR System (ThermoFisher Scientific) was employed and the gene expression was analyzed by the relative quantification (ΔΔCT) method. Expression of lncRNAs were analyzed using RT2 First Strand Kit, human lncFinder RT2 lncRNA PCR array, and RT2 SYBR Green ROX qPCR Mastermix (Qiagen Inc., Germantown, MD). An RT2 lncRNA qPCR Assay for BANCR was also used. Expression of mRNAs and miRNAs was analyzed using TaqMan reagents (Applied Biosystems, ThermoFisher Scientific). High-Capacity cDNA Reverse Transcription Kit, validated TaqMan Gene Expression Assays (RPE65, RLBP1, MITF, TRPM1 and TRPM2) and TaqMan Fast Advanced Master Mix were used for mRNA analysis. Human 18S rRNA and GAPDH served as endogenous controls. Expression of miRNAs was analyzed using TaqMan MicroRNA Reverse Transcription Kit, TaqMan MicroRNA assays (miR-204 and miR-211) and RNU48 (endogenous control).
2.3. Western blot Analysis
Western blot analysis of phospho-STAT1 was performed using extracts of ARPE-19 cells prepared with RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing protease and phosphatase inhibitors (Cell Signaling Technology, Inc., Danvers, MA). Cell extracts (25 μg protein) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were then blotted onto a nitrocellulose membrane using the iBLOT system (Invitrogen, Carlsbad, CA). Immunoreactive bands on blots were detected using Amersham ECL prime Western blot detection reagents and horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Pittsburgh, PA). A rabbit anti-phospho-STAT1 (Tyr701) antibody (Cell Signaling Technology SC 9171S, 1000-fold dilution) was used to detect phospo-STAT1. The blot was then stripped and reprobed with mouse anti-α-tubulin monoclonal antibody (Li-COR Biotechnology, Lincoln, NE).
3. Results and discussion
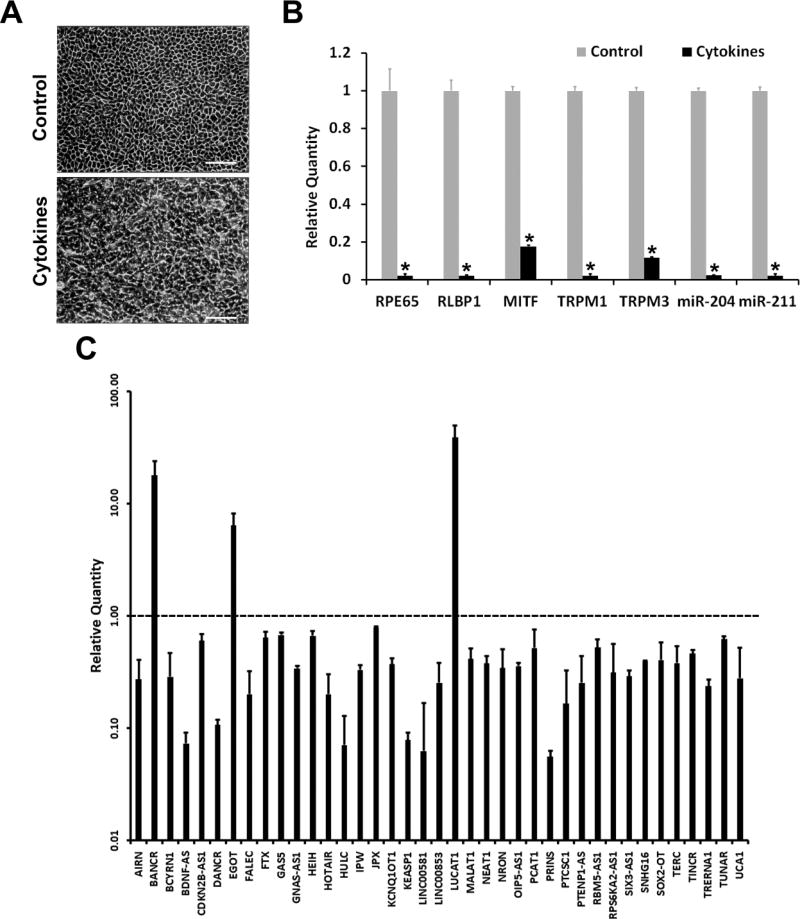
Human RPE-derived ARPE-19 cells cultured for 4 months differentiate into a RPE phenotype characterized by pigmentation, typical epithelial morphology, and visual cycle gene expression [12, 13]. Recently we reported that proinflammatory cytokines IFN-γ, IL-1β and TNF-α can drastically decrease the expression of genes critical for RPE function in these differentiated cells while disrupting their epithelial morphology [11]. Therefore, we employed this system to study the response of lncRNA expression to proinflammatory cytokines. Differentiated ARPE-19 cells were exposed to a cytokine mixture consisting of IFN-γ, IL-1β and TNF-α. The expression of IL8, CCL5 and CXCL10 transcripts was highly increased in the treated cells indicating that the cytokine treatment was effective (data not shown). Phase contrast microscopic analysis showed that the cells lost the typical epithelial morphology following the treatment, as expected (Fig. 1A). The treatment also markedly decreased the expression of RPE characteristic mRNAs (RPE65, RLBP1, MITF, TRPM1 and TRPM3) and miRNAs (miR-204 and miR-211) (Fig. 1B). The expression of a panel of 84 known human lncRNAs in control and treated cells were analyzed by real-time PCR. The proinflammatory cytokines altered the expression of many of the lncRNAs tested (Fig. 1C). The expression of 36 lncRNAs were decreased while those of BANCR, EGOT and LUCAT1 were increased several fold. We selected BANCR for further investigation since it is a well characterized lncRNA that has been shown to regulate cell proliferation, migration and EMT [3–5].
Fig. 1.
Proinflammatory cytokines alter the expression of lncRNAs in RPE cells. Differentiated ARPE-19 cells were treated for 4 days with a proinflammatory cytokine mixture consisting of IFN-γ (100 u/ml), TNF-α (10 ng/ml) and IL-1β (10 ng/ml). (A), Phase contrast microscopic images of control and treated cells shows that proinflammatory cytokines caused a disruption in the epithelial morphology of ARPE-19 cells. Photomicrographs were taken at a magnification of 100X; scale bar = 100 μm. (B) Real-time PCR analysis shows that proinflammatory cytokines decrease the expression of RPE characteristic genes and miRNAs in ARPE-19 cells. * = p < 0.05 when compared to control, n = 3. (C) Real-time PCR analysis shows that proinflammatory cytokines alter the expression of lncRNAs in ARPE-19 cells. Relative expression level of lncRNAs affected by the treatment are shown (p < 0.05 when compared to control; n = 3). Broken line indicates that the relative expression level of an lncRNA in the control (untreated cells) is 1.
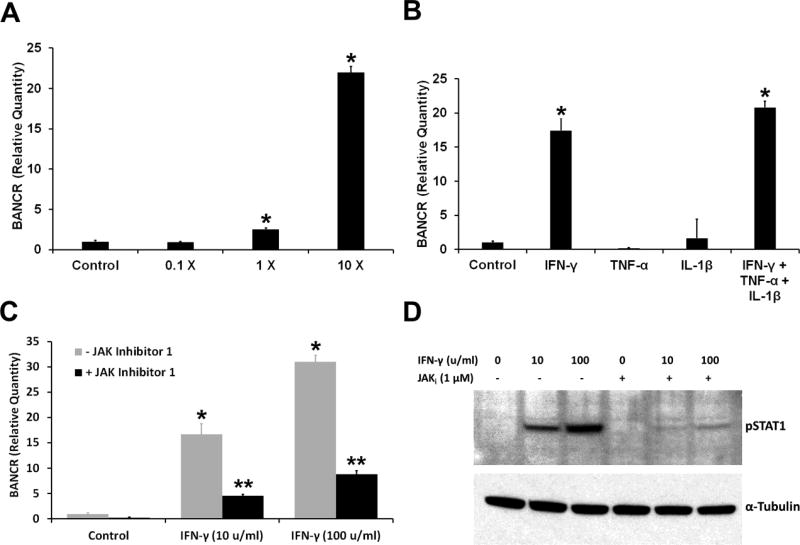
The expression of BANCR in ARPE-19 cells treated with IFN-γ, IL-1-1β and TNF-α was analyzed by real-time PCR. The validity of this assay was verified by sequencing the amplification product. The DNA sequence thus obtained showed 100% identity to the BANCR cDNA region expected to be amplified by the primers. BANCR expression showed a dependence on the concentration of the proinflammatory cytokines (Fig. 2A). The response of BANCR to each proinflammatory cytokine was also tested (Fig. 2B). IFN-γ by itself increased BANCR expression. However, IL-1β and TNF-α did not affect the expression of this lncRNA in ARPE-19 cells. Also, these two cytokines did not noticeably alter the effect of IFN-γ on BANCR expression. As IFN-γ elicits its action in RPE cells by activating the JAK-STAT1 signal transduction pathway [14], we investigated the role of this pathway in regulating BANCR expression. Western blot analysis was employed to detect phospho-STAT1, an indicator of STAT1 activation by IFN-γ. The duration of IFN-γ treatment was decreased from 4 days to 20 hours to facilitate the detection of phospho-STAT1 on Western blots. IFN-γ increased BANCR expression in ARPE-19 cells under this condition and JAK inhibitor 1, an inhibitor of Janus protein tyrosine kinases and a known blocker of JAK-STAT1 signaling, effectively suppressed this increase (Fig. 2C). JAK inhibitor 1 also markedly reduced phospho-STAT1 content in IFN-γ-treated cells, as expected. Thus, JAK-STAT1 signaling pathway appears to be involved in the induction of BANCR expression by IFN-γ.
Fig. 2.
IFN-γ regulates the expression of the lncRNA BANCR. (A) Proinflammatory cytokines increase the expression of BANCR in RPE cells. ARPE-19 cells were exposed to different concentrations of cytokine mixture for 4 days and BANCR expression analyzed by real-time PCR. 1X = IFN-γ (10 u/ml), TNF-α (1 ng/ml) and IL-1β (1 ng/ml). * = p < 0.05 when compared to control; n = 4. (B) IFN-γ by itself increases BANCR expression in RPE cells. The cells were treated with IFN-γ (100 u/ml), TNF-α (10 ng/ml) or IL-1β (10 ng/ml) for 4 days. A combination of all three cytokines was also used. BANCR expression was analyzed by real-time PCR. * = p < 0.05 when compared to control, n = 4. (C) JAK inhibitor 1 decreased BANCR expression induced by IFN-γ in RPE cells. ARPE-19 cells were treated with indicated concentration of IFN-γ for 20 hours with or without JAK inhibitor 1, and BANCR expression analyzed by real-time PCR. * = p < 0.05 when compared to control, ** = p < 0.05 when compared to - JAK inhibitor; n = 4. (D) JAK inhibitor decreased phospho-STAT1 (pSTAT1) content in ARPE-19 cells exposed to IFN-γ. The cells were treated with indicated concentration of IFN-γ for 20 hours in the presence or absence of JAK inhibitor 1, and the cell extracts were analyzed for pSTAT1 by western blotting. α-Tubulin was used as the gel loading control.
We have shown that the expression of many lncRNAs is altered during the interaction of human RPE (ARPE-19) cells with proinflammatory cytokines IFN-γ, IL-1β and TNF-α. The expression of the lncRNA BANCR in these cells is increased by IFN-γ and this increase is effectively blocked by inhibiting the JAK-STAT1 signaling pathway. BANCR is reported to regulate cell migration and EMT in cancer cells [3–5]. The role of BANCR in the inflammatory response of RPE cells remains to be elucidated. We have reported earlier that IFN-γ, IL-1β and TNF-α elicit EMT-like changes and decrease the expression of many RPE characteristic genes in ARPE-19 cells [11]. Here we have shown that these proinflammatory cytokines-induced dysfunctional changes in these cells are associated with an increase in the expression of BANCR. Therefore, BANCR, due to its ability to regulate EMT, may potentially serve as a link between inflammatory response and RPE dysfunction.
HIGHLIGHTS.
Proinflammatory cytokines (IFN-γ + IL-1β +TNF-α) altered the expression of lncRNAs in retinal pigment epithelial (RPE) cells.
IFN-γ, but not IL-β or TNF-α, increased the expression of the lncRNA BANCR.
Inhibition of JAK-STAT1 signaling pathway effectively blocked IFN-γ-induced BANCR expression.
Modulation of lncRNA expression by inflammatory response may play a role in RPE dysfunction implicated in the pathogenesis of age-related macular degeneration.
Acknowledgments
This study was supported by the Intramural Research Program of the National Eye Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35(9):408–19. doi: 10.1016/j.it.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012;22(6):1006–14. doi: 10.1101/gr.140061.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D, Sun Y. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol Lett. 2014;8(2):869–75. doi: 10.3892/ol.2014.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun M, Liu XH, Wang KM, Nie FQ, Kong R, Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, Chen JF, Zhang EB, De W, Wang ZX. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68. doi: 10.1186/1476-4598-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015;36(9):7205–11. doi: 10.1007/s13277-015-3413-3. [DOI] [PubMed] [Google Scholar]
- 7.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10(9):802–23. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438–51. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62(3):369–81. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutty RK, Samuel W, Abay R, Cherukuri A, Nagineni CN, Duncan T, Jaworski C, Vijayasarathy C, Redmond TM. Resveratrol attenuates CXCL11 expression induced by proinflammatory cytokines in retinal pigment epithelial cells. Cytokine. 2015;74(2):335–8. doi: 10.1016/j.cyto.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutty RK, Samuel W, Boyce K, Cherukuri A, Duncan T, Jaworski C, Nagineni CN, Redmond TM. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis. 2016;22:1156–68. [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 13.Samuel W, Jaworski C, Postnikova OA, Kutty RK, Duncan T, Tan LX, Poliakov E, Lakkaraju A, Redmond TM. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol Vis. 2017;23:60–89. [PMC free article] [PubMed] [Google Scholar]
- 14.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Hooks JJ, Redmond TM. Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem Biophys Res Commun. 2010;402(2):390–5. doi: 10.1016/j.bbrc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]