Abstract
Background & Aims
Survival times vary among patients with neuroendocrine tumors (NETs)—even among those with the same site, stage, and grade of primary tumor. This makes it difficult to select treatment for patients with unresectable NETs, because some patients can survive decades without treatment. 68Gallium-DOTATATE positron emission tomography with computed tomography (68Ga-DOTATATE PET/CT) is a sensitive imaging technique for detection of NETs. We investigated the prognostic accuracy of 68Ga-DOTATATE PET/CT analysis of tumor volume in patients with NETs.
Methods
We performed a prospective study of 184 patients with NETs (128 [69.6%] with metastases and 11 patients [6.0%] with locally advanced disease) at the National Institutes of Health Clinical Center from 2013 through 2017. All patients underwent 68Ga-DOTATATE PET/CT image analysis and total 68Ga-DOTATATE-Avid tumor volume (68Ga-DOTATATE TV) was determined. We also measured fasting serum chromogranin A, neuron-specific enolase, gastrin, glucagon, vasoactive intestinal peptide, pancreatic polypeptide, and 24-hour urinary 5-hydroxyindoleacetic acid levels in all patients. Disease progression was defined as a new lesion or a growth of a known lesion, during the interval between baseline 68Ga-DOTATATE PET/CT scan and follow-up imaging (14.0±6.1 months; range 1–35 months). The primary outcomes were progression-free survival (PFS) and disease-specific mortality during a median follow-up time of 18 months (range 4–35 months).
Results
We found an inverse correlation between quartiles of 68Ga-DOTATATE TV and PFS (P=.001) and disease-specific survival (P=.002). A 68Ga-DOTATATE TV of 7.0 mL or more was associated with higher odds of disease progression (hazard ratio, 3.0; P=.04). A 68Ga-DOTATATE TV of 35.8 mL or more was associated with increased risk of disease-specific death (hazard ratio, 10.6) in multivariable analysis (P=.01), as well as in subgroup analysis of patients with pancreatic NETs.
Conclusions
In a prospective study, we demonstrated the prognostic utility of 68Ga-DOTATATE TV in a large cohort of patients with NETs, in terms of PFS and disease-specific mortality.
Keywords: survival, radiology, tumor size, pancreas
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of malignancies arising from neuroendocrine cells that are dispersed throughout the human body. About two-thirds of NETs originate from the gastrointestinal tract and pancreas, 25% from the bronchopulmonary tract, and the remaining are from other sites.1 The incidence of NETs is increasing and is estimated to exceed 5 cases per 100,000 people per year.2 Although most NETs have an indolent course, a subset of patients with NETs have aggressive disease, and a substantial number of patients with NETs present with distant metastases at initial diagnosis.3–5
Many treatment options have been developed over the last decade for patients with locally advanced and metastatic NETs. Such new treatments include medical therapy with somatostatin analogs,6,7 everolimus,8,9 sunitinib,10,11 liver-directed therapies,12 and peptide receptor radionuclide therapy (PRRT).13 However, the optimal timing of treatment interventions for NETs is unknown, as the disease course of patients with locally advanced and metastatic NETs is highly variable, even when patients have the same tumor stage and grade. Thus, new clinical prognostic tools are required in order to select the population of patients that are at risk of disease-progression and disease-specific mortality. Such prognostic tools could determine which patients with NETs would benefit from treatment intervention, the type and timing of treatment, and whether the treatment-associated side effects are justified in light of the estimated life expectancy and their impact on quality of life.
Positron emission tomography (PET)/computed tomography (CT) imaging has been shown to improve the management of patients with both solid and hematological malignancies. For example, in patients with non-small cell lung cancer preoperative 18Fluoro-deoxy-glucose (18FDG) PET/CT scanning reduced the number of thoracotomies,14 and its use for surveillance of advanced head and neck cancer reduced the intervention rate.15 In patients with Hodgkin’s lymphoma, the use of 18FDG-PET/CT might avert further radiotherapy in patients with early disease16 and lead to reduced treatment toxicity among those with advanced disease.17 The clinical utility of measuring total ligand-avid tumor volume (TV) based on PET/CT scanning has been evaluated in patients with cancer in small cohort and/or retrospective studies. For example, 18FDG-PET/CT based volume measurements predicted shorter progression-free survival (PFS) in follicular lymphoma18 and breast cancer,19 and total 11C-Methionine-avid volume predicted PFS in high-grade glioma.20 Furthermore, 18FDG-PET/CT TV in multiple myeloma21, adrenocortical carcinoma22 and non-small cell lung cancer23 were associated with patient survival, as was 18F-fluoroethyl-tyrosine (18F-FET) PET/CT in patients with gliomas24. A large prospective study has shown that a high SUVmax (>3) derived from 18F-FDG PET/CT was independently associated with shorter PFS in patients with NETs.25 Furthermore, the combined use of 68Ga-DOTATATE and 18F-FDG PET/CT scans was found to be beneficial in the clinical management of patients with poorly differentiated NET.26
Radiolabeled somatostatin receptor (SSR)–binding molecules with PET/CT imaging are commonly used to stage patients with NETs.27 This imaging approach is highly sensitive for detecting sites of NETs because these tumors express SSR. Among the new-generation radiolabeled high-affinity SSR ligands (DOTATATE, DOTATOC, DOTANOC) developed and evaluated in studies, 68Ga-DOTATATE PET/CT imaging is one of the more sensitive and specific imaging modalities for detecting NETs.28 To our knowledge, no study has utilized this quantitative imaging measurement approach in patients with NETs who had 68Ga-DOTATATE PET/CT imaging to determine if it has any prognostic utility.
In this prospective study, we evaluated the prognostic utility of 68Ga-DOTATATE TV as a marker for PFS and disease-specific mortality in a large cohort of patients with NETs. In addition, we performed multivariable analyses of other clinical and biochemical variables associated with PFS and disease-specific mortality.
METHODS
Study Population
Patients known to have NETs based on imaging (CT, magnetic resonance imaging [MRI], and 18F-FDG PET) and biochemical evidence, and/or a pathologically confirmed NET, were enrolled in the study. This was a single center prospective study, conducted at the National Institutes of Health Clinical Center between the years 2013 and 2017 and focused on mostly patients with gastrointestinal and pancreatic NETs. The current analysis included only subjects with 68Ga-DOTATATE-avid disease and who had yearly follow up in order to assess survival rates.
NETs were classified according to the primary tumor location based on anatomical imaging, 68Ga-DOTATATE PET/CT localization, and/or pathological diagnosis. Primary tumor locations were subdivided into pancreatic NETs (PNETs) or small intestine NETs (SINETs), whereas subjects with NETs of gastric, duodenal, rectal, lung, or appendiceal subtypes were grouped as “other NET” due to their small numbers. Subjects with metastatic NETs, with pathological 68Ga-DOTATATE uptake but no clear primary lesion, were defined as NET of unknown primary. Tumor grade was determined according to the 2010 World Health Organization (WHO) classification.30 All patients underwent testing for fasting serum chromogranin A (CGA), neuron-specific enolase (NSE), gastrin, glucagon, vasoactive intestinal peptide (VIP), and pancreatic polypeptide (PP), as well as 24-hour urinary 5-hydroxyindoleacetic acid (5HIAA) levels.
The study was performed under an Investigational New Drug approval from the United States Food and Drug Administration, and was approved by the National Cancer Institute Institutional Review Board and the National Institutes of Health Radiation Safety Committee. Written informed consent was obtained from all study participants.
68Ga-DOTATATE PET/CT Studies
For 68Ga-DOTATATE PET/CT imaging, 185 MBq (5 mCi) of 68Ga-DOTATATE was administered intravenously through a peripheral vein. After approximately 60 minutes, the patient was positioned supine in a PET/CT scanner, and images were obtained from mid thighs to the skull. A low-dose, non-contrast–enhanced CT was used for attenuation correction and anatomic localization. SUVmax was measured based on patient total body weight. Patients treated with long-acting octreotide were scanned before the next scheduled monthly dose, while those on short-acting octreotide discontinued treatment for 24 hours before imaging.
Quantification Analysis of 68Ga-DOTATATE PET/CT Studies
Disease burden was assessed by quantifying 68Ga-DOTATATE uptake using the MIM Vista workstation (version 6.5.9). A volume of interest (VOI) encompassing the entire body were drawn, and subsequently an SUVmax threshold–based approach29 customized per patient was applied in order to include all 68Ga-DOTATATE avid lesions (Figure 1). The software enables automatic generation of individual VOIs encircling each separate lesion. Per each scan, a well demarcated 68Ga-DOTATATE-avid lesion was selected. The automatic demarcation by the software was compared with the lesion anatomic cross-sectional image, and the SUVmax threshold was then set, so there will be a maximal overlap between the anatomic and functional measurements, with <5% qualitative difference between the images.
Figure 1.
68Ga-DOTATATE positron-emission tomography (PET)/computerized tomography (CT) of patients with metastatic neuroendocrine tumors (NETs) from each total 68Ga-DOTATATE-avid volume (68Ga-DOTATATE TV) quartile: (A) small-intestine NET (SINET) with metastases to the spine, mesenteric lymph-node and sacrum (red, green and yellow arrows, respectively) and 68Ga-DOTATATE TV of 2·2 mL (first quartile range); (B) pancreatic NET (PNET, blue arrow) with metastases to the left 4th rib (red arrow), liver (green arrows), retroperitoneal and mesenteric lymph nodes (68Ga-DOTATATE TV 6·2 mL, second quartile range); (C) PNET metastases to the liver, mediastinum, spine (green, red and yellow arrows respectively) and pleura (68Ga-DOTATATE TV of 10·8 mL, third quartile range); and (D) PNET with metastases to the spine (green arrows), left scapula (yellow arrow), mediastinum (red arrow) and liver (white arrows) with a 68Ga-DOTATATE TV of 127·8 mL (forth quartile).
Discrete 68Ga-DOTATATE avid lesions with clear delineation of the tumor were used for tumor volume calculations (Supplementary Figures 1 and 2). This involved visual inspection of the automated volume segmentation, to avoid incomplete segmentation of pathologic avidity (Supplementary figures 1C and 2C) and inclusion of background physiologic uptake (Supplementary figures 1D and 2D).
After setting the SUVmax threshold and encircling all lesions, areas of physiologic 68Ga-DOTATATE uptake or uptake not related to disease were manually removed by an experienced nuclear medicine physician who was blinded to the clinical patient data, and then SUVmax and total volume of all 68Ga-DOTATATE-avid lesions (68Ga-DOTATATE TV) were automatically determined. The study cohort was grouped into four quartiles: Q1 [range, 0·1–2·9 ml], Q2 [3·0–9·9 ml], Q3 [9·9–43·1 ml], and Q4 [43·6–1136·6 ml]) by 68Ga-DOTATATE TV and Q1 [8–32], Q2 [33–55], Q3 [56–90], and Q4 [92–307]) by SUVmax.
Disease progression analysis
Only patients with either locally advanced or metastatic disease (n=139) were included in the disease progression analysis. In addition to an annual 68Ga-DOTATATE PET/CT scan, patients underwent annual anatomic imaging (CT/MRI) per protocol, and additional scans and/or 18F-FDG PET/CT as clinically justified. Among patients included in the PFS analysis, all patients had in average more than one scan per year, with a mean rate of 3.1±1.1 (range 1.3–5.5). No difference in the number of scans performed during follow-up was found between patients in the different 68Ga-DOTATATE TV quartiles (3.6±1.8, 3.8±1.5, 3.4±1.5, 3.7±2.3, in quartiles 1, 2, 3 and 4, respectively, p=0.8), or for any specific imaging modality among patients included in the analysis.
Disease progression was defined as a new lesion or a growth of a known lesion, during the interval between baseline 68Ga-DOTATATE PET/CT scan and follow up imaging (14.0±6.0 months, range 1–35 months). Among 51 patients with disease progression during follow-up, 44 were defined as having disease progression based on 68Ga-DOTATATE PET/CT showing new disease, four based on CT, two based on MRI, and one – based on 18F-FDG PET/CT scan.
Statistical Analyses
Statistical calculations were performed using the SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Results are expressed as mean ± standard deviation (SD) unless otherwise indicated. For group comparisons, the independent Student’s t-test or one-way analysis of variance (ANOVA) was used to analyze differences in parametric variables, and the chi-square test was used to analyze differences in categorical variables. Non-parametric tests were used as appropriate. The Kaplan–Meier estimator with a log-rank (Mantel–Cox) test was used to estimate and compare PFS and disease-specific mortality rates by PET/CT indices and biochemical biomarkers levels. Multivariable analysis was used to assess the prognostic utility of 68Ga-DOTATATE TV in terms of disease-specific mortality and PFS after controlling for confounding factors (Supplementary Tables 1 and 2). The joint effects of variables were analyzed by Cox proportional hazards models, using the enter method, to estimate hazard ratios (HR) and 95% confidence intervals (CI) for PFS and disease-specific mortality during follow-up.
After validating the association between 68Ga-DOTATATE TV and PFS and disease-specific mortality in our cohort, we aimed to generalize these findings. Thus, optimal cut-offs for predicting PFS and disease-specific mortality were defined using the receiver operating characteristic (ROC) curve. The discriminative performance of 68Ga-DOTATATE TV was assessed by calculating the Harrell c-statistic, which corresponds to the area under the ROC curve. The P value for statistical significance was set at <0.05.
RESULTS
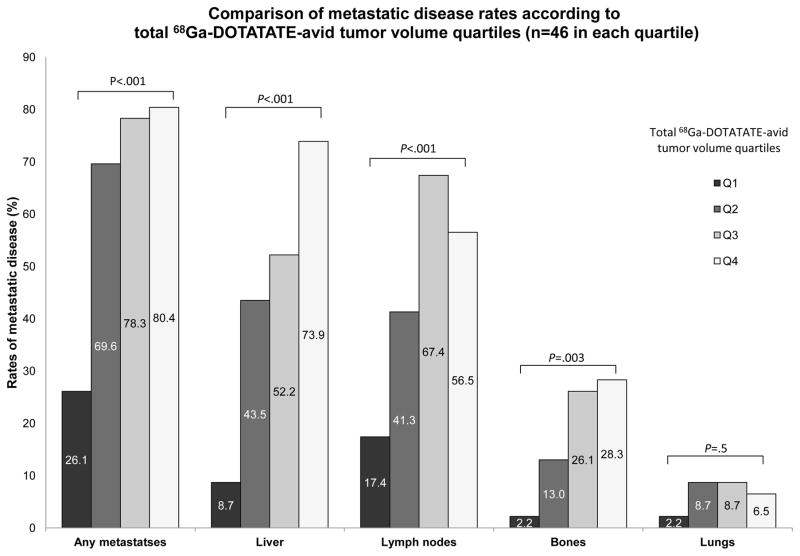
One hundred and eighty-four patients had 68Ga-DOTATATE PET/CT imaging with a median follow-up time of 18 months (range of 4–35 months) and with a mean time from diagnosis of 5.3±6.0 years. The mean age of the study cohort was 55·3±14·2 years, and 95 (51·6%) were women. Ninety-nine subjects (53·8%) had primary PNETs, 57 (31·0%) had primary SINETs, and 13 patients (7·1%) had metastatic NETs with unknown primary tumors. Fifteen subjects had other NET subtypes: gastric (n=3), duodenal (n=7), rectal (n=1), appendiceal (n=1), large intestine (n=2), and bronchial (n=1) NETs. Metastatic disease was present in 128 subjects (69·6%) with 24 patients (13·0%) having only lymph node metastases and 84 subjects (65·6%) having liver metastases. Additional eleven patients (6·0%) had locally advanced disease. Eighty-six patients were treated medically following study enrollment: 47 received octreotide, 24 received lanreotide, 6 received everolimus, 7 received sunitinib and 2 patients were treated with systemic chemotherapy. In addition, 42 patients underwent surgical intervention following their inclusion, ten patients underwent PRRT and 4 patients had liver directed therapy. The clinical characteristics by 68Ga-DOTATATE TV quartiles are summarized in Table 1 and Figure 2.
Table 1.
Study cohort clinical characteristics by total 68Ga-DOTATATE avid tumor volume quartiles
| Q1 n=46 |
Q2 n=46 |
Q3 n=46 |
Q4 n=46 |
P value | |
|---|---|---|---|---|---|
| Age (years) | 52·7±16·1 | 54·7±14·6 | 56·9±13·3 | 56·8±12·4 | NS |
| Female n (%) | 23 (50·0%) | 29 (63·0%) | 24(52·2%) | 19 (41·3%) | NS |
| Disease progression during follow-up n(%) | 3 (8·8%) | 18 (53·9%) | 18 (47·4%) | 18 (54·5%) | 0·001 |
| Died of disease during Follow-up n (%) | 0 | 1 (2·2%) | 2 (4·3%) | 8 (17·4%) | 0·002 |
| Time since diagnosis (months) | 3·2±3·7 | 6.2±6·4 | 7·9±7·7 | 3·8±4·5 | 0.001 |
| F/u duration (months) | 18·4±8·4 | 21·4±8·0 | 19·7±7·8 | 17·0±7·4 | 0·05 |
| NET subtype | NS | ||||
| PNET | 24 (52·2%) | 27 (58·7%) | 23 (50·0%) | 25 (54·3%) | |
| SINET | 11 (23·9%) | 12 (26·1%) | 17 (37·0%) | 17 (37·0%) | |
| Unknown primary | 4 (8·7%) | 5 (10·9%) | 1 (2·2%) | 3 (6·5%) | |
| Other | 7 (15·2%) | 2 (4·3%) | 5 (10·9%) | 1 (2·2%) | |
| SUVmax | 32·7±22·3 | 70·2±52·4 | 83·4±46·9 | 90·3±55·5 | <0·001 |
| 68Ga-DOTATATE TV (mL) | 1·2±0·8 | 6·4±2·0 | 24·1±10·6 | 245·1±227·1 | <0·001 |
| Treatment after inclusion | |||||
| Medical n (%) | 14 (31·1%) | 23 (50·0%) | 18 (39·1%) | 31 (68·9%) | 0·002 |
| Surgical n (%) | 11 (24·4%) | 10 (21·7%) | 12 (26·1%) | 9 (20·0) | NS |
| PRRT n(%) | 0 | 3 (6·5%) | 2 (4·3%) | 5 (11·1%) | NS |
| LDT n(%) | 0 | 2 (4·3%) | 0 | 2 (4·3%) | NS |
| WHO 2010 grading | NS | ||||
| G1 | 11 (64·7%) | 6 (40·0%) | 11 (45·8%) | 11 (44·0%) | |
| G2 | 5 (29·4%) | 9 (60·0%) | 13 (54·2%) | 11 (44·0%) | |
| G3 | 1 (5·9%) | 0 | 0 | 3 (12·0%) | |
| Disease stage n (%)* | <0·001 | ||||
| I | 21 (45·7%) | 10 (21·7%) | 3 (6·5%) | 0 | |
| II | 7 (15·2%) | 1 (2·2%) | 3 (6·5%) | 1 (2·2%) | |
| III | 10 (21·7%) | 10 (21·7%) | 9 (19·6%) | 5 (10·9%) | |
| IV | 8 (17·4%) | 25 (54·3%) | 31 (67·4%) | 40 (87·0%) | |
| Plasma CGA (ng/mL) | 213±374 | 627±1242 | 693±1353 | 7220±37446 | NS |
| Urinary 5HIAA (mg/24h) | 4·8±1·8 | 5·7±3·7 | 7·6±5·3 | 30·6±47·0 | <0·001 |
| Plasma NSE (ng/mL) | 9·4±3·8 | 28·6±59·4 | 10·8±7·4 | 14·3±10·9 | NS |
| Plasma gastrin (pg/mL) | 81±126 | 125±241 | 461±1398 | 159±454 | NS |
| Plasma glucagon (pg/mL) | 37·9±20·4 | 42·2±36·9 | 40·4±33·3 | 55·8±73·1 | NS |
| Plasma PP (pg/mL) | 147±84 | 240±293 | 198±248 | 427±995 | NS |
| Plasma VIP (pg/mL) | 33·3±13·3 | 37·9±15·9 | 36·8±12·8 | 47·7±53·0 | NS |
NS, non-significant; f/u, follow-up; NET, neuroendocrine tumor; PNET, pancreatic neuroendocrine tumor; SINET, small intestine neuroendocrine tumor; SUVmax, maximal standardized uptake values in 68Ga-DOTATATE PET/CT; 68Ga-DOTATATE TV, total 68Ga-DOTATATE-avid tumor volume; SSA, somatostatin analogs; LDT, liver-directed therapy; WHO, World Health Organization; CGA, chromogranin A; 5HIAA, 5-hydroxyindoleacetic acid; NSE, neuron-specific enolase; PP, pancreatic polypeptide; VIP, vasoactive intestinal peptide
Continuous variables are presented as mean±standard deviation
Tumor staging was determined according to the American Joint Committee on Cancer, 8th edition.
Figure 2.
Comparison of metastatic disease rates according to total 68Ga-DOTATATE-avid tumor volume quartiles (n=46 in each quartile)
Eleven patients died of their disease during follow-up, with no significant difference in age (57·4±17·5 vs. 55·2±14·0 years, P=0·6) or gender (63·6% vs. 50·9% women, P=0·4). Among patients who died of their disease, 6 had PNETs, 4 had SINETs, and 1 had an unknown primary. As expected, patients who died of their disease had higher rates of metastatic disease (100% vs. 61·3%, P=0·01): liver (81·8% vs. 42·2%, P=0·01), lymph nodes (81·8% vs. 43·4%, P=0·01) and bone (45·5% vs. 15·6%, P=0·01). They also had higher WHO tumor grade (G2 or G3, 100% vs. 48%, P=0·001, Supplementary Figure 3), and higher plasma CGA (3,366±4,838 vs. 2,121±19,675, P=0·001) and NSE levels (20·6±11·5 vs. 9·7±3·5, P=0·001).
The optimal 68Ga-DOTATATE TV cutoff points for PFS and disease-specific mortality were ≥ 7·0 mL (c-statistic 0·683, p<0.001) and ≥35·8 mL (c-statistic 0·844, p<0·001), respectively. These cut-offs points identified 78·9% of patients who developed disease progression, and 81·8% of patients died of their disease during the follow-up.
68Ga-DOTATATE TV and progression-free survival
Patients with disease progression during follow-up (n=51) had higher rate of metastases to the liver (72·5% vs. 50·8%, P=0·02), and/or bones (35·3% vs. 11·1%, P=0·002), and received more medical treatment (72·0% vs. 44·4%, P=0·003) and PRRT (18·0% vs. 1·6%, P=0·002) compared to patients with stable disease (n=88). 68Ga-DOTATATE TV ≥7·0 ml was associated with higher risk for disease progression both on univariate (P=0·02, Table 2 and Figure 3A) and multivariable analyses (HR 3·0, 95% CI 1·1–8·7, P=0·04). In order to control for the effect of treatment on disease progression, we performed a subgroup analysis according to medical therapy during follow-up. A trend for lower PFS among patients with 68Ga-DOTATATE TV ≥7 mL was found among patients with no treatment during follow-up (Log-rank test, p=0.05), with a similar trend among patients receiving medical treatment (p=0.086).
Table 2.
Results of univariate analysis for progression-free survival and disease-specific mortality in patients with neuroendocrine tumors
| Progression-free survival | Disease-specific mortality | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Female | 1·1 | 0·6 – 2·0 | 0·7 | 1·7 | 0·5 – 5·8 | 0·4 |
| PNET vs. other subtypes | 0·7 | 0·4 – 1·2 | 0·2 | 0·9 | 0·3 – 3·1 | 0·9 |
| WHO 2010 grade G3 vs. G1/G2 | 1·2 | 0·2 – 8·9 | 0·8 | 66·5 | 6·0 – 743·0 | 0·001 |
| Presence vs. absence of metastases | ||||||
| Liver | 2·0 | 1·1 – 3·7 | 0·03 | 6·3 | 1·4 – 29·4 | 0·02 |
| Lymph nodes | 1·5 | 0·8 – 2·8 | 0·2 | 5·4 | 1·2 – 25·0 | 0·03 |
| Bone | 2·7 | 1·5 – 4·8 | 0·001 | 4·9 | 1·5 – 16·1 | 0·009 |
| Lung | 0·6 | 0·2 – 1·9 | 0·4 | 1·3 | 0·2 – 10·5 | 0·8 |
| Hereditary vs. sporadic | 0·4 | 0·2 – 0·9 | 0·02 | 0·3 | 0·1 – 1·4 | 0·1 |
| Functional vs. non-functional tumor | 0·6 | 0·4 – 1·1 | 0·1 | 0·9 | 0·3 – 2·9 | 0·8 |
| Treatment after inclusion | ||||||
| Surgery | 1·5 | 0·8 – 2·8 | 0·3 | 0·4 | 0·04 – 2·8 | 0·3 |
| Medical | 2·4 | 1·3 – 4·5 | 0·006 | 0·9 | 0·2 – 3·1 | 0·8 |
| PRRT/LDT | 2·2 | 1·1 – 4·4 | 0·02 | 2·3 | 0·5 – 10·8 | 0·3 |
| SUVmax ≥ 55.9+ | 1·0 | 0·6 – 1·8 | 0·9 | 0·6 | 0·2 – 1·9 | 0·4 |
| Total 68Ga-DOTATATE volume ≥7.0 ml | 2·4 | 1·2 – 4.9 | 0.02 | NA* | NA | NA |
| Total 68Ga-DOTATATE volume ≥35.8 ml | 1·4 | 0·8 – 2·5 | 0·2 | 12·5 | 2·7 – 57·7 | 0·001 |
| Biochemical biomarkers | ||||||
| Elevated plasma NSE (>15 ng/mL) | 2·2 | 1·02 – 4·7 | 0·04 | 3·6 | 0·6 – 21·0 | 0·1 |
| Elevated plasma CGA (>73 ng/mL) | 1·4 | 0·8 – 2·8 | 0·3 | NA* | NA | NA |
| Elevated urinary 5HIAA (>8 mg/24h) | 1·7 | 1·0 – 3·0 | 0·07 | 5·2 | 1·5 – 18·8 | 0·01 |
HR, hazard ratio; CI, confidence interval; PNET, pancreatic neuroendocrine tumors; WHO, World Health Organization; SSA, somatostatin analogues; PRRT, peptide receptor radionuclide therapy; LDT, liver-directed therapy; SUVmax, maximum standardized uptake value; NA, not applicable.
Median values,
Cox regression analysis was not performed as one of the groups had no events.
Figure 3.
Survival analysis in patients with neuroendocrine tumors. Progression-free survival by 68Ga-DOTATATE-avid tumor volume (n=139, A). Kaplan-Meier survival analysis by 68Ga-DOTATATE-avid tumor volume in the entire cohort (n=184, B), and among patients with pancreatic (n=99, C) and small-intestine neuroendocrine tumors (n=57, D).
HR, hazard ratio; CI, confidence interval
68Ga-DOTATATE TV and disease-specific mortality
There was no significant difference in disease-specific mortality by mean SUVmax values (63·3±46·4 vs. 69·3±51·3, P=0·7). However, disease-specific mortality was significantly different by 68Ga-DOTATATE TV (301·7±349·3 vs. 54·4±117·7, dead vs. alive, respectively, P<0·001). Moreover, survival analysis revealed a significant difference when compared by 68Ga-DOTATATE TV quartiles, but not by SUVmax quartiles (Supplementary figures 5A and 5B).
On univariate analysis (Table 2), tumor WHO G3 grade, presence of liver, lymph node and/or bone metastases, elevated urinary 5HIAA (>8 mg/24h), and high 68Ga-DOTATATE TV (≥35·8 mL) were associated with higher disease-specific mortality in patients with NETs (Figure 3B). On multivariable analysis, only high 68Ga-DOTATATE TV ≥35·8 mL was associated with a higher disease-specific mortality (HR 10·6, 95% CI 1·6–68·9, P=0·014).
Subgroup Analysis
68Ga-DOTATATE TV ≥ 35·8 mL was associated with a high disease-specific mortality rate in patients with PNETs (Log-Rank test, P=0·001, HR 16·4, 95% CI 1·9–140·2, P=0·01; Figure 3C), with a trend in patients with SINETs (HR 5·4, 95% CI 0·6–51·8, P=0·1, Figure 3D). High 68Ga-DOTATATE TV was associated with disease-specific mortality even when excluding patients with no metastases both on univariate (HR = 8·5, 95% CI 1·8–39·4, P=0·006) and multivariable analyses (HR = 11·2, 95% CI 1·2–107·7, P=0·04).
We performed subgroup analysis in patients with PNETs, comparing the utility of tumor markers used mostly in PNETs. We did not find increased risk for DSM among patients with high plasma levels of gastrin (Log-rank test, p=0.99), glucagon (p=0.68), VIP (p=0.62) or pancreatic polypeptide (p=0.68).
DISCUSSION
In this prospective study, we assessed the prognostic utility of 68Ga-DOTATATE PET/CT imaging indices in a large cohort of patients with NETs. 68Ga-DOTATATE TV was significantly higher among subjects who had disease progression and among those who died of their disease. Survival analysis demonstrated a stepwise increase in cumulative risk for disease progression and for disease-specific mortality by increasing 68Ga-DOTATATE TV quartiles. Cut-offs for 68Ga-DOTATATE TV and PFS (≥7·0 mL) and disease-specific mortality (≥35·8 mL) were calculated, with high c-statistic (0·844) for disease specific mortality and moderate (0·683) for PFS. In multivariable analysis, 68Ga-DOTATATE TV was associated with lower PFS and higher disease-specific mortality. Furthermore, there was an inverse association between 68Ga-DOTATATE TV levels and survival rates in patients with PNETs with a similar trend in patients with SINETs.
A number of retrospective studies have assessed radiolabeled SSR–binding ligands as prognostic markers for NETs. In these studies, a low SUVmax was reported to be associated with poor prognosis in patients with NETs.31–33 In our cohort we did not find any association between SUVmax and the prognosis of patients with gastrointestinal and pancreatic NETs, but when utilizing SUV for setting the threshold for volumetric measurements, 68Ga-DOTATATE TV was associated with prognosis. The fact that SUVmax only reflects the expression of SSR in the tumor tissue, whereas 68Ga-DOTATATE TV utilizes both volumetric tumor measurements and SSR expression,29 may explain this difference. In addition, although 68Ga-DOTATATE detection of NET is high, ranging between 79–96% in different studies28,34,35, we included only patients with 68Ga-DOTATATE-avid NETs, possibly excluding patients harboring poorly-differentiated NET, with higher risk for disease-specific mortality.
The presence of NET liver metastases was associated with higher disease-specific mortality by univariate analysis as expected.36–39 However, by multivariable analysis, presence of metastases was not associated with disease-specific mortality, whereas 68Ga-DOTATATE TV was significantly associated, suggesting a more accurate assessment of tumor burden. Interestingly, although 68Ga-DOTATATE SUVmax was reported as a potential prognostic marker in NET,33 it was not associated with disease-specific mortality in the current study. This may be due to SUVmax measurement being representative of a single region of the tumor and thus not representative of the total tumor burden. Moreover, the known heterogeneity in SSR-based ligand uptake, even within the same individual’s scan, might further limit its utility.40 Finally, WHO tumor grade is an important prognostic factor in NETs,38 as was also found in the current study. However, in practice data on tumor grade is often incomplete as not all patients have resection of their tumor or biopsy of all tumor sites and analysis of the tumor grade, and patients may have different tumor grade depending on the tumors site41.
The incidence of PNETs in the general population accounts for 7–10% of all gastrointestinal and pancreatic NETs,2,42 whereas in our cohort PNETs accounted for the majority of the NETs (53.4%). The high proportion of PNETs in our study may be due to the focus of the clinical protocol on gastrointestinal and pancreatic NETs and the referral of many patients with PNETs to our center.
In our analysis, 68Ga-DOTATATE TV was an independent prognostic factor for disease-specific mortality in patients with PNETs with a trend in patients with SINETs. This finding is expected, as patients with PNETs have a worse survival as compared to patients with other NET subtypes.38,43 Furthermore, several studies have observed lower survival rates among patients with foregut tumors39 and among patients with liver metastases from PNETs vs. other gastrointestinal NETs.38
The 68Ga-DOTATATE TV optimal cut-off value for disease-specific mortality had high accuracy, thus suggesting it could be useful for selecting patients for closer follow-up and early intervention. Also, the c-statistic calculated for PFS identified most patients at risk for progression and thus could help guide the need for treatment before disease progression occurs.
Our study findings have several important clinical implications in patients with NETs. First, analysis of 68Ga-DOTATATE TV could help identify which patients are likely to progress or die of their disease. Second, measurement of 68Ga-DOTATATE TV can be used to identify patients who should have treatment intervention because of a higher-risk of disease progression or disease-specific mortality. This is important because most newly developed therapies, when evaluated in clinical trials in patients with unresectable NETs, have used disease-progression on anatomic imaging (within 6–18 months) as a criterion for treatment intervention and as the primary endpoint to evaluate treatment efficacy. Therefore, the application of 68Ga-DOTATATE TV measurement could more precisely identify those patients likely to have progressive disease early or die of their disease. Third, given the ever-expanding treatment options available to patients with unresectable NETs—including active surveillance, as some patients could have indolent metastatic disease for decades that does not require intervention—the use of 68Ga-DOTATATE TV measurement can stratify patients into low- and high-risk groups for which treatment intervention should be considered. Such an approach could represent the potential of 68Ga-DOTATATE TV as a method to implement precision medicine in patients with NETs. Our findings are likely to be generalizable and can be applied by other medical centers as 68Ga-DOTATATE was recently approved by the United States Food and Drug Administration for NET imaging.
Our study limitations include the heterogenous study cohort, which consisted of patients with various primary NET locations, and of different disease stages and grades. In addition, our results need to be validated in future studies.
In conclusion, 68Ga-DOTATATE TV is independently associated with PFS and disease-specific mortality in patients with NETs, with higher TV values associated with a lower PFS and higher disease-specific mortality. This new data could be used to determine the need for treatment intervention, frequency of follow up and ultimately lead to precision medicine in patients with NET.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families, as well as the health care providers who participated in this study.
Footnotes
Authors contribution
Amit Tirosh – Data analysis and manuscript writing
Georgios Z. Papadakis – Analysis of imaging studies
Corina Millo – Analysis of imaging studies
Dima Hammoud – Analysis of imaging studies
Samira M. Sadowski - Study concept and design
Peter Herscovitch – Analysis of imaging studies
Karel Pacak - Interpretation of data
Stephen J. Marx - Interpretation of data
Lily Yang – Data analysis
Pavel Nockel - Interpretation of data
Jasmine Shell - Interpretation of data
Patience Green - Interpretation of data
Xavier M. Keutgen - Study concept and design
Dhaval Patel - Interpretation of data
Naris Nilubol - Interpretation of data
Electron Kebebew - Study concept and design
Critical revision of the manuscript for important intellectual content: all authors.
Disclosure summary - I certify that neither I nor my co-authors have a conflict of interest as described above that is relevant to the subject matter or materials included in this Work.
The study was funded by the intramural research program of the National Cancer Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. [Accessed September 12, 2016];Cancer. 2003 97:934–59. doi: 10.1002/cncr.11105. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12569593. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Pavel M, O’Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. [Accessed August 22, 2016];Neuroendocrinology. 2016 103:172–85. doi: 10.1159/000443167. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26731013. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. [Accessed August 22, 2016];Endocrinol Metab Clin North Am. 2011 40:1–18. vii. doi: 10.1016/j.ecl.2010.12.005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21349409. [DOI] [PubMed] [Google Scholar]
- 5.Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. [Accessed August 22, 2016];Lancet Oncol. 2014 15:e8–21. doi: 10.1016/S1470-2045(13)70362-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24384494. [DOI] [PubMed] [Google Scholar]
- 6.Rinke A, Müller H-H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. [Accessed September 15, 2016];J Clin Oncol. 2009 27:4656–63. doi: 10.1200/JCO.2009.22.8510. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19704057. [DOI] [PubMed] [Google Scholar]
- 7.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. [Accessed September 15, 2016];N Engl J Med. 2014 371:224–33. doi: 10.1056/NEJMoa1316158. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25014687. [DOI] [PubMed] [Google Scholar]
- 8.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, nonfunctional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. [Accessed September 15, 2016];Lancet (London, England) 2016 387:968–77. doi: 10.1016/S0140-6736(15)00817-X. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26703889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulke MH, Lenz H-J, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. [Accessed September 15, 2016];J Clin Oncol. 2008 26:3403–10. doi: 10.1200/JCO.2007.15.9020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18612155. [DOI] [PubMed] [Google Scholar]
- 11.Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. [Accessed October 16, 2016];N Engl J Med. 2011 364:501–13. doi: 10.1056/NEJMoa1003825. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21306237. [DOI] [PubMed] [Google Scholar]
- 12.de Baere T, Deschamps F, Tselikas L, et al. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. [Accessed October 17, 2016];Eur J Endocrinol. 2015 172:R151–66. doi: 10.1530/EJE-14-0630. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25385817. [DOI] [PubMed] [Google Scholar]
- 13.van der Zwan WA, Bodei L, Mueller-Brand J, et al. GEPNETs update: Radionuclide therapy in neuroendocrine tumors. [Accessed September 15, 2016];Eur J Endocrinol. 2015 172:R1–8. doi: 10.1530/EJE-14-0488. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25117465. [DOI] [PubMed] [Google Scholar]
- 14.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. [Accessed October 16, 2016];N Engl J Med. 2009 361:32–9. doi: 10.1056/NEJMoa0900043. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19571281. [DOI] [PubMed] [Google Scholar]
- 15.Mehanna H, Wong W-L, McConkey CC, et al. PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer. [Accessed October 16, 2016];N Engl J Med. 2016 374:1444–54. doi: 10.1056/NEJMoa1514493. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27007578. [DOI] [PubMed] [Google Scholar]
- 16.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. [Accessed October 16, 2016];N Engl J Med. 2015 372:1598–607. doi: 10.1056/NEJMoa1408648. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25901426. [DOI] [PubMed] [Google Scholar]
- 17.Johnson P, Federico M, Kirkwood A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. [Accessed October 16, 2016];N Engl J Med. 2016 374:2419–29. doi: 10.1056/NEJMoa1510093. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27332902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meignan M, Cottereau AS, Versari A, et al. Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. [Accessed October 17, 2016];J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.9440. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27551111. [DOI] [PubMed]
- 19.Kim TH, Yoon J-K, Kang DK, et al. Value of volume-based metabolic parameters for predicting survival in breast cancer patients treated with neoadjuvant chemotherapy. [Accessed October 17, 2016];Medicine (Baltimore) 2016 95:e4605. doi: 10.1097/MD.0000000000004605. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27741099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo MY, Paeng JC, Cheon GJ, et al. Prognostic Value of Metabolic Tumor Volume on (11)C-Methionine PET in Predicting Progression-Free Survival in High-Grade Glioma. [Accessed October 17, 2016];Nucl Med Mol Imaging (2010) 2015 49:291–7. doi: 10.1007/s13139-015-0362-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26550048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald JE, Kessler MM, Gardner MW, et al. Assessment of Total Lesion Glycolysis by 18F FDG PET/CT Significantly Improves Prognostic Value of GEP and ISS in Myeloma. [Accessed October 17, 2016];Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0235. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27698001. [DOI] [PMC free article] [PubMed]
- 22.Satoh K, Patel D, Dieckmann W, et al. Whole Body Metabolic Tumor Volume and Total Lesion Glycolysis Predict Survival in Patients with Adrenocortical Carcinoma. [Accessed October 17, 2016];Ann Surg Oncol. 2015 22:714–720. doi: 10.1245/s10434-015-4813-8. Available at: http://link.springer.com/10.1245/s10434-015-4813-8. [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Fan M, Liu B, et al. Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. [Accessed October 17, 2016];J Nucl Med. 2014 55:1584–90. doi: 10.2967/jnumed.114.142919. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25214640. [DOI] [PubMed] [Google Scholar]
- 24.Moller S, Law I, Munck Af, Rosenschold P, et al. Prognostic value of (18)F-FET PET imaging in re-irradiation of high-grade glioma: Results of a phase I clinical trial. [Accessed October 17, 2016];Radiother Oncol. 2016 doi: 10.1016/j.radonc.2016.08.014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27622554. [DOI] [PubMed]
- 25.Binderup T, Knigge U, Loft A, et al. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Survival of Patients with Neuroendocrine Tumors. [Accessed June 8, 2017];Clin Cancer Res. 2010 16:978–985. doi: 10.1158/1078-0432.CCR-09-1759. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20103666. [DOI] [PubMed] [Google Scholar]
- 26.Panagiotidis E, Alshammari A, Michopoulou S, et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. [Accessed June 8, 2017];J Nucl Med. 2017 58:91–96. doi: 10.2967/jnumed.116.178095. Available at: http://jnm.snmjournals.org/lookup/doi/10.2967/jnumed.116.178095. [DOI] [PubMed] [Google Scholar]
- 27.Baumann T, Rottenburger C, Nicolas G, et al. Gastroenteropancreatic neuroendocrine tumours (GEP-NET) - Imaging and staging. [Accessed August 31, 2016];Best Pract Res Clin Endocrinol Metab. 2016 30:45–57. doi: 10.1016/j.beem.2016.01.003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26971843. [DOI] [PubMed] [Google Scholar]
- 28.Sadowski SM, Neychev V, Millo C, et al. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. [Accessed August 31, 2016];J Clin Oncol. 2016 34:588–96. doi: 10.1200/JCO.2015.64.0987. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26712231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etchebehere EC, Araujo JC, Fox PS, et al. Prognostic Factors in Patients Treated with 223Ra: The Role of Skeletal Tumor Burden on Baseline 18F-Fluoride PET/CT in Predicting Overall Survival. [Accessed September 1, 2016];J Nucl Med. 2015 56:1177–84. doi: 10.2967/jnumed.115.158626. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26069307. [DOI] [PubMed] [Google Scholar]
- 30.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. [Accessed September 9, 2016];Pancreas. 2010 39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20664470. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Naswa N, Kc SS, et al. Comparison of the prognostic values of 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. [Accessed September 9, 2016];Eur J Nucl Med Mol Imaging. 2014 41:2194–202. doi: 10.1007/s00259-014-2850-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25030618. [DOI] [PubMed] [Google Scholar]
- 32.Campana D, Ambrosini V, Pezzilli R, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. [Accessed September 9, 2016];J Nucl Med. 2010 51:353–9. doi: 10.2967/jnumed.109.066662. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20150249. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosini V, Campana D, Polverari G, et al. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients with Neuroendocrine Tumors of the Pancreas. [Accessed August 31, 2016];J Nucl Med. 2015 56:1843–8. doi: 10.2967/jnumed.115.162719. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26405169. [DOI] [PubMed] [Google Scholar]
- 34.Alonso O, Rodríguez-Taroco M, Savio E, et al. (68)Ga-DOTATATE PET/CT in the evaluation of patients with neuroendocrine metastatic carcinoma of unknown origin. [Accessed June 8, 2017];Ann Nucl Med. 2014 28:638–45. doi: 10.1007/s12149-014-0856-3. Available at: http://link.springer.com/10.1007/s12149-014-0856-3. [DOI] [PubMed] [Google Scholar]
- 35.Haug A, Auernhammer CJ, Wängler B, et al. Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. [Accessed November 30, 2016];Eur J Nucl Med Mol Imaging. 2009 36:765–770. doi: 10.1007/s00259-008-1030-8. Available at: http://link.springer.com/10.1007/s00259-008-1030-8. [DOI] [PubMed] [Google Scholar]
- 36.Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. [Accessed September 12, 2016];Ann Oncol. 2005 16:1806–10. doi: 10.1093/annonc/mdi358. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16085691. [DOI] [PubMed] [Google Scholar]
- 37.Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. [Accessed September 12, 2016];World J Surg. 2002 26:991–7. doi: 10.1007/s00268-002-6630-z. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12016480. [DOI] [PubMed] [Google Scholar]
- 38.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. [Accessed September 9, 2016];Endocr Relat Cancer. 2005 12:1083–92. doi: 10.1677/erc.1.01017. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16322345. [DOI] [PubMed] [Google Scholar]
- 39.Söreide JA, van Heerden JA, Thompson GB, et al. Gastrointestinal carcinoid tumors: long-term prognosis for surgically treated patients. [Accessed September 12, 2016];World J Surg. 2000 24:1431–6. doi: 10.1007/s002680010236. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11038218. [DOI] [PubMed] [Google Scholar]
- 40.Velikyan I, Sundin A, Sörensen J, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. [Accessed September 12, 2016];J Nucl Med. 2014 55:204–10. doi: 10.2967/jnumed.113.126177. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24379222. [DOI] [PubMed] [Google Scholar]
- 41.Adesoye T, Daleo MA, Loeffler AG, et al. Discordance of Histologic Grade Between Primary and Metastatic Neuroendocrine Carcinomas. [Accessed October 24, 2016];Ann Surg Oncol. 2015 22(Suppl 3):S817–21. doi: 10.1245/s10434-015-4733-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26193965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. [Accessed June 13, 2017];JAMA Oncol. 2017 26:2124–2130. doi: 10.1001/jamaoncol.2017.0589. Available at: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. [Accessed September 9, 2016];J Clin Oncol. 2011 29:2372–7. doi: 10.1200/JCO.2010.33.0688. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21555696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.