Abstract
Background & Aims
It is not clear how the complex interactions between diet and the intestinal microbiota affect development of mucosal inflammation or inflammatory bowel disease. We investigated interactions between dietary ingredients, nutrients, and the microbiota in specific pathogen-free (SPF) and germ-free (GF) mice given more than 40 unique diets; we quantified individual and synergistic effects of dietary macronutrients and the microbiota on intestinal health and development of colitis.
Methods
C56BL/6J SPF and GF mice were placed on custom diets containing different concentrations and sources of protein, fat, digestible carbohydrates, and indigestible carbohydrates (fiber). After 1 week, SPF and germ-free mice were given dextran sulfate sodium (DSS) to induce colitis. Disease severity was determined based on the percent weight change from baseline, and modeled as a function of the concentration of each macronutrient in the diet. In unchallenged mice, we measured intestinal permeability by feeding mice labeled dextran and measuring levels in blood. Feces were collected and microbiota were analyzed by 16S rDNA sequencing. We collected colons from mice and performed transcriptome analyses.
Results
Fecal microbiota varied with diet; the concentration of protein and fiber had the strongest effect on colitis development. Among 9 fiber sources tested, psyllium, pectin, and cellulose fiber reduced the severity of colitis in SPF mice, whereas methylcellulose increased severity. Increasing dietary protein increased the density of the fecal microbiota and the severity of colitis in SPF mice, but not in GF mice or mice given antibiotics. Psyllium fiber reduced the severity of colitis through microbiota-dependent and microbiota-independent mechanisms. Combinatorial perturbations to dietary casein protein and psyllium fiber in parallel accounted for most variation in gut microbial density and intestinal permeability in unchallenged mice, as well as the severity of DSS-induced colitis; changes in 1 ingredient could be offset by changes in another.
Conclusions
In an analysis of the effects of different dietary components and the gut microbiota on mice with and without DSS-induced colitis, we found complex mixtures of nutrients affect intestinal permeability, gut microbial density, and development of intestinal inflammation.
Keywords: IBD, Microbiota, Mouse Models, Systems Biology
Despite strong patient interest and numerous studies implicating diet in the exacerbation or prevention of inflammatory bowel disease (IBD), exclusive enteral nutrition (EEN), whereby patients consume a nutritionally complete simple diet, provides the only clinically validated diet that can treat IBD, specifically pediatric Crohn’s disease (CD).1–3 However, the specific mechanisms driving therapeutic response to EEN are poorly understood, and the return to a normal diet is typically accompanied by the return of the colitis. Furthermore, consumption of EEN is too divergent from normal dietary customs to represent a practical maintenance therapy. The ideal IBD diet would have higher efficacy than EEN and allow the consumption of a diet with few restrictions to maximize patient compliance. Developing such a diet for IBD is a major challenge, as diet’s influence on mucosal inflammation likely involves complex interactions between multiple dietary ingredients, nutrients, host physiology, disease heterogeneity, and the gut microbiota.
Many of the microbes that colonize our gut are stably colonized for decades,4 but the relative abundances of these microbes change as we vary our diet consumption.5–10 In both mice and humans, the abundance of bacterial community members can change several orders of magnitude within 24 hours of changing diet, remain stable while diet is held constant, and reverse when diet is reverted.5–8,11 There are numerous lines of evidence suggesting the gut microbiota plays a role in IBD, including differences in the microbiota composition of individuals with IBD compared to healthy controls,12,13 the requirement of the gut microbiota for disease initiation in multiple animal colitis models,14 and the impact of intensive fecal microbiota transplantation in treating ulcerative colitis (UC).15,16 Furthermore, diet and the microbiota can work in concert to influence host physiology through both metabolites and immune interactions.17–20 To understand the complex interactions between multiple dietary ingredients, nutrients, and the microbiota in a controlled manner, we used specific pathogen free (SPF) and germ free (GF) animals combined with over 40 unique diets to quantify the individual and synergistic influence of dietary macronutrients and the microbiota on host physiology and experimental colitis.
Materials and Methods
Mice
SPF mice were purchased from Jackson Labs (C57BL/6J mice) or bred in-house (Rag1−/− C57BL/6J mice). GF mice were housed in standard flexible film isolators. Male mice were used for dextran sulfate sodium (DSS) experiments in SPF mice. All other experiments used both male and female mice. To colonize gnotobiotic mice, fecal slurries were gavaged into GF mice from either an SPF mouse donor or a human donor.21
Diets
Custom refined mouse chows (Table S1) were designed in collaboration with a nutritionist at Envigo. Carbohydrates were subdivided into host digestible carbohydates (which we will simply refer to as carbohydrates) and host indigestible carbohydrates (fiber). For each macronutrient source we preferentially choose refined sources that contain only a single macronutrient although some tested ingredients (e.g., fiber sources), contain a mixture of macronutrients. The macronutrient sources included animal and plant proteins, animal and plant fats (with diverse fatty acid profiles), simple and complex sugars, and dietary fibers. All custom diets were pelleted, dried (leaving only ~5–7% moisture), irradiated and vacuum-sealed.
Dextran Sulfate Sodium Colitis Model
One week prior to the administration of DSS, mice were provided a custom diet (Table S1) ad libitum and were weighed daily for the duration of the experiment. After a week of diet ad libitum, the mice were continued on the same diet and given 3% DSS in drinking water to induce mucosal injury (Fig. 1). Fluid intake did not significantly differ between the diets in mice consuming water with 3% DSS or water alone. Mice were sacrificed at day 7 or upon reaching a humane endpoint (i.e., >18% weight loss, a body condition score of 2, or based on humane indicators). GF mice were given 2% DSS instead of 3% DSS to induce roughly equivalent survival times in SPF and GF mice. For animals sacrificed for ethical reasons prior to day 7, we extrapolated the weight loss curve of each animal by linear regression using their last three measured time points. These extrapolated values are highlighted in yellow in Table S2A and as a dotted line in Fig. 2A. Survival was defined as the time at which animals reach 18% weight loss or other humane endpoint.
Figure 1. Dietary screening demonstrated that protein and fiber have the greatest effect upon DSS colitis.
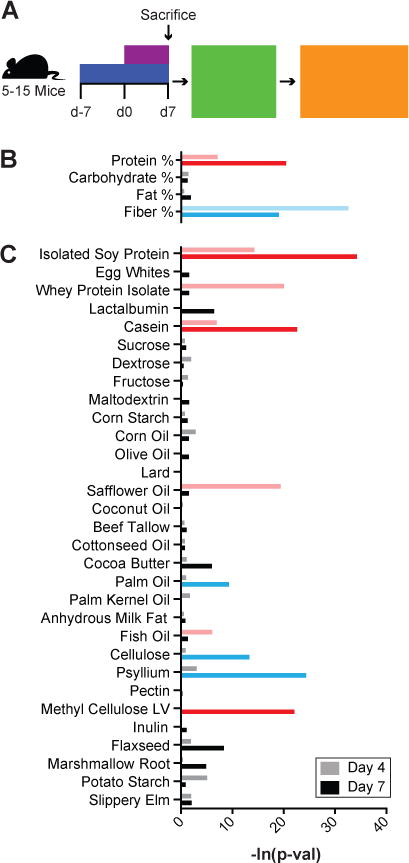
(A) Mice were fed one of thirty-two unique diets for a week before giving 3% DSS in drinking water. Disease severity was measured as weight loss. Stepwise regression and lasso were used to identify (B) macronutrients and (C) dietary ingredients that contributed to differences in weight change at 4 and 7 days after beginning 3% DSS. Factors significantly associated with increased and decreased colitis severity are shown in red and blue respectively.
Figure 2. Casein promotes colitis and psyllium protects from colitis.
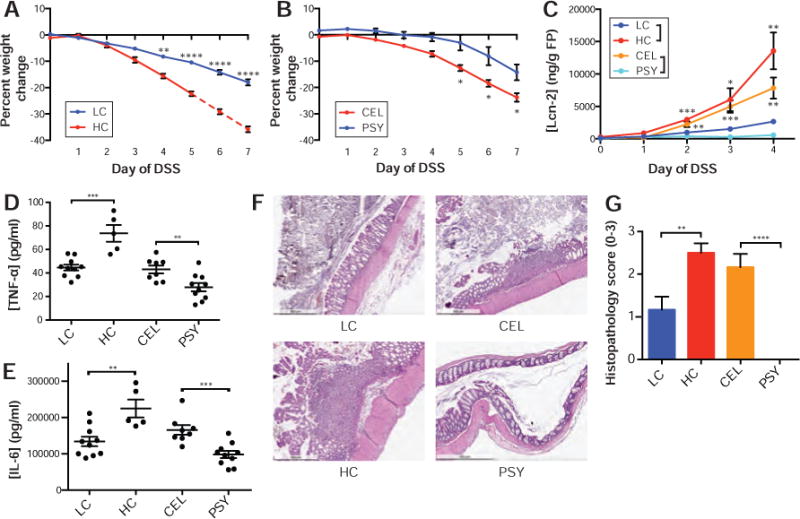
Mice fed (A) LC diet had less severe weight loss after one week on DSS than those fed a HC diet, while mice consuming the (B) PSY diet had less severe disease than those consuming the CEL diet. (C) After 4 days of DSS, fecal lipocalin-2 was almost undetectable for the PSY diet and highly increased in the HC diet. After 7 days of DSS or at the humane endpoint, (D) TNF-α and (E) IL-6 from whole colonic explants demonstrated increases in HC and decreases in PSY. (F) Representative H&E staining and (G) histopathological scoring of colons indicate that HC and CEL drive more severe disease. The mean ± SEM are plotted for each time point and group (AC, G). Points represent individual animals (D–E). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Results
Identifying dietary manipulations that influence colitis severity
To identify macronutrients and individual ingredients that modulate experimental colitis, we used 32 unique diets (Table S1 and Table S2A) with varied concentrations and sources of macronutrients comprising 31 ingredients whose nutritional content was dominated by a single macronutrient. For the macronutrients protein and fat, we tested a concentration range (wt/wt) of 5–41% and 1–21% respectively with soy protein, whey, casein, and egg whites as protein sources and corn oil, olive oil, lard, safflower oil, coconut oil, beef tallow, cotton seed oil, cocoa butter, palm oil, palm kernel oil, milk fat, and fish oil as fat sources. We separated carbohydrates into those that are largely host digestible, consisting of sucrose, fructose, glucose, maltodextrin, and corn starch tested over a range of 24–83%, and those that are largely host indigestible (fiber), consisting of cellulose, methylcellulose, psyllium, pectin, inulin, flaxseed, marshmallow root, potato starch, and slippery elm tested over a range of 5–15%. All diets were not limited for vitamins or minerals, and ranges for each macronutrient were selected to perturb each macronutrient concentration several fold.
Each dietary combination was fed ad libitum to 5–15 C56BL/6J SPF mice for one week, whereupon the animals were continued on the same diet while consuming 3% DSS in their drinking water to induce intestinal injury and inflammation (Fig. 1A). Importantly, unlike many colitis models, the DSS injury model is effective in both conventional mice and GF animals,22,23 allowing us to determine both the microbiota-dependent and microbiota-independent influences of diet on intestinal inflammation. During the initial dietary screen, disease severity was measured each day for seven days as the percent weight change of each animal relative to their mean baseline weight in the four days prior to DSS administration (Table S2A). Weight change was chosen as a metric of disease severity due to its ease of longitudinal measurement and its consistency with other inflammatory markers.24 We observed substantial variation in disease severity as a function of diet (p<0.0001, ANOVA).
To understand the influence of macronutrients on disease severity, we modeled each animal’s weight loss during DSS administration as a function of the concentration of each macronutrient in the diet. We used stepwise regression and lasso25 to identify dietary macronutrients across the 32 dietary combinations that best explain the variation in disease severity estimated by weight loss at day 4 and at day 7 (Fig. 1B). Both stepwise regression and lasso identified protein as explaining increased weight loss at days 4 and 7 (p<0.001 for stepwise regression and lasso), while they identified fiber as explaining decreased weight loss at days 4 and 7 (stepwise regression; p=1.9×10−14 and p=9.5×10−9 respectively) or day 7 alone (p<0.0001, lasso; Table S3A).
To understand the role of individual ingredients on the observed weight changes, we performed a regression analysis using the concentrations of the 31 different macronutrient sources used across the diets (Fig. 1C, Table S3B). Most protein sources (casein, isolated soy protein and whey) were identified as increasing weight loss. Lactalbumin and egg whites, the two protein sources not significantly associated with colitis severity, had ingredient concentrations highly correlated with other ingredients, perhaps limiting our ability to detect their effects with the modeling approach. To test more generally if protein sources increased weight loss, we tested six additional diets with high (41%) and low (6%) protein from different sources including chicken and beef, as well as a repeat of egg whites without the confounding changes to other ingredients. As in the screen, we found mice consuming high protein diets had increased colitis severity relative to those consuming low protein diets (p=0.0031, 0.0072, 0.0018; weight loss day 4 for egg whites, chicken, and beef respectively). Amongst the nine fiber sources, only psyllium, pectin, and cellulose fiber were associated with decreased weight loss, while methylcellulose was associated with more severe disease. As in previous studies,26,27 we identified a small but significant influence of select dietary fats on experimental colitis severity (Fig. S1 and Supplemental Materials).
Across all diets and macronutrients, protein, in the form of casein, was identified as most significantly associated with increased weight loss while fiber, in the form of psyllium, was associated with the largest protection from disease. To explore these macronutrients with the greatest influence on disease severity, we focused on high casein (41%, TD.09054; HC) and low casein (6%, TD.09052; LC) diets, as well as diets containing psyllium (5%, TD.150229; PSY) or cellulose (5%, TD.09053; CEL). Mice fed a HC diet, while consuming 3% DSS, lost approximately twice as much weight by day 4 and 7 respectively as mice consuming LC diet (15.6% vs 8.2% on d4 and 35.8% vs 18.0% on d7; p<0.0001 both days, t-test; Fig. 2A; Table S2A). The HC-associated weight loss was associated with increased concentrations of fecal lipocalin-2 (p=0.0035, t-test; Fig. 2C Table S4B), increased TNF-α and IL-6 from colon explants (p=0.0003 and p=0.0033 respectively, t-test; Fig. 2D–E; Table S4A), more severe colon histopathology (p=0.0056, t-test; Fig. 2F–G; Table S4C), and decreased colon lengths (p=0.0003, t-test; Fig. S2; Table S4D). In contrast, mice fed PSY diet experience approximately 9-fold and 2-fold less weight loss on day 4 and 7 respectively when compared to mice fed CEL diet (0.8% vs 7.2% on d4 and 14.2% vs 23.8% on d7; p=0.010, p=0.011, t-test; Fig 2B; Table S2A). We also observed concomitant differences in fecal lipocalin-2 (p=0.0013, t-test; Fig. 2C; Table S4B), inflammatory cytokines from colon explants (TNF-α and IL-6; p=0.0077, p=0.0007, t-test; Fig. 2D–E; Table S4A), colon histopathology (p<0.0001, t-test; Fig. 2F–G; Table S4C), and colon length (p=0.0003, t-test; Fig. S2; Table S4D). Together, these immune monitoring and pathology findings support the observations from our diet screen that changes in dietary protein and fiber are major determinants of disease outcome in the mouse DSS colitis model.
Casein modulates colitis severity through microbiota-dependent mechanisms
To determine if the deleterious influence of protein on disease severity was dependent upon the microbiota, we provided HC or LC diet to both GF and SPF animals. Since GF mice are more susceptible to disease than SPF in the DSS colitis model,22 we used 2% DSS in the GF group to induce equivalent disease to 3% DSS in the SPF mice (Fig. S3). We found a 1.8-fold increase (4.4 days to 7.8 days; Fig. 3A) in survival time following the start of DSS treatment for SPF mice on the LC diet compared with the HC diet (p<0.0001, Log-Rank test), while there was no difference in survival between mice consuming the LC and HC diets in GF animals (5.6/5.2 days, respectively; p=0.22, Log-Rank test), demonstrating that the influence of dietary casein concentration on disease severity is microbiota-dependent.
Figure 3. The effects of casein upon health and colitis are microbiota-dependent.
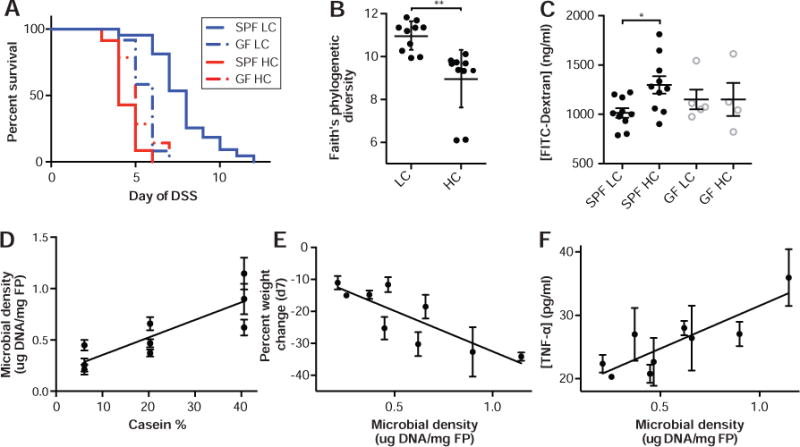
(A) In the DSS colitis model, HC diet reduces survival relative to LC diet in SPF mice but not in GF animals. Alpha diversity measured by (B) Faith’s phylogenetic diversity rarified to 10k reads per sample demonstrated decreased microbial diversity in the HC diet. Furthermore, mice on HC diet had (C) decreased barrier function in healthy SPF mice, while barrier was not influence by casein in GF mice. In panes D–F, animals were given one of 9 controlled diets with different casein concentrations (TD.09049-TD.09057) with varying amounts of nutrients for a week and then given DSS. (D) Increased gut microbial density, driven by casein concentration, was associated with more severe disease in terms of both (E) weight change on DSS at d7 and (F) TNF-α. Lines represent surviving number of animals (A). SPF animals were given 3% DSS and GF animals were given 2% DSS. Black (SPF) and gray (GF) points represent individual animals (B–C). The mean ± SEM are plotted for each point (D–F). *p<0.05, **p<0.01
To understand if casein might configure the microbiota to increase host susceptibility to DSS colitis, we analyzed the microbiota in animals fed HC and LC diets that were unchallenged with DSS. Culture independent 16S rDNA sequencing demonstrated a reduced diversity in the fecal microbiota of mice fed HC compared to LC diet (Faith’s phylogenetic diversity; p=0.0022, t-test; Fig. 3B). We also observed significantly different shifts in fecal microbiota composition away from conserved microbial baselines across experiments between mice fed the LC and HC diets (weighted unifrac; p=0.001, permanova; Fig. S4 and Fig. S5A), consisting of phylum-level increases in the relative abundance of Bacteriodetes and decreases the Firmicutes in the mice fed HC diet (Fig. S4). These changes in gut microbiota composition occur over 48hr and remain stable while consuming each diet (Fig. S5B) and might modulate the microbiota-dependent influence of protein on colitis severity. To determine if the casein-altered microbiota is sufficient to cause increased severity in the DSS colitis model, we transplanted feces from mice consuming either HC or LC diet into GF recipients consuming a standard polysaccharide-rich mouse chow (Fig. S6; Harlan TD.2018S). After transfer, the gut microbiota again clustered with the baseline standard mouse chow samples (Fig. S6E). We observed no significant difference in disease severity between the microbiota transplant recipients (Fig. S6), demonstrating that both the casein and the microbiota are required.
We investigated features of unchallenged mice fed HC or LC diets that might explain the microbiota-dependent influence of protein on DSS colitis. In transcriptional profiles of colonic tissue from mice fed HC or LC diet, we found decreased casein associated with increased expression of genes involved in cell adhesion, such as AOCS3, LAMC1, KRT17, and DES, as well as increases in genes associated actin cytoskeleton and intermediate filament organization (Fig. S7). As we observed decreased expression of cell adhesion genes in mice fed a HC diet, we hypothesized that dietary protein composition may affect the integrity of the intestinal barrier. By measuring translocation of orally administered FITC-dextran in the bloodstream of mice, we found mice fed HC diet had a microbiota-dependent increase in intestinal permeability compared to those consuming LC diet (Fig. 3C; Table S6A) with no difference in gut motility between diets.
Previous studies have shown that antibiotics can reduce disease severity in DSS mouse colitis models28 and provide some benefit in patients with IBD.29 Importantly, work in both rat and mouse DSS colitis suggests the reduction in disease severity upon antibiotic treatment is proportional to the ability of the antibiotic to deplete the microbiota.30 We previously identified casein as a potential driver of gut microbial density.11 We therefore hypothesized that increased microbial density, associated with high casein diets relative to low casein diets, could drive the diet and microbiota dependent changes in pathology. We analyzed the microbiota density of the 9 different diets, from our initial screen (TD.09049-TD.09057; 5–10 mice per diet, Table S2A see green highlights), that contained various amounts of casein (low, medium, and high), corn oil (low, medium, and high) and sucrose (low, medium, and high). As expected in mice unchallenged with DSS, increasing casein was significantly correlated with an increase in gut microbial density (R2=0.71, p=0.0045, F-test; Fig. 3D; Table S2A) with a 3-fold average difference in microbial density between the HC and LC diets.
To determine if increases in gut microbial density might create an environment for elevated colitis susceptibility, we measured the gut microbiota density in the same mice after administration of 3% DSS and found increased dietary protein also increased gut microbial density under inflammatory conditions (R2=0.88, p=0.0002, F-test; Fig. S8; Table S2A). Protein-driven changes in gut microbial density were significantly associated with disease severity estimated as weight change on day 7 of DSS administration as well as with colonic TNF-α and IL-10 from colon explants (R2=0.59, 0.73, 0.45; p=0.016, 0.0035, 0.049 respectively, F-test; Fig. 3E–F, Fig. S8, and Table S2A). These results suggest the level of inflammation in DSS injury is driven in part by the density of bacteria within the intestinal lumen, which would also explain the microbiota-dependent nature of the influence of casein on disease severity. To validate that casein-driven increases in microbial density are causally linked to increased pathology in the DSS model, we fed groups of mice the LC diet, the HC diet, or the HC diet + 3mg/ml metronidazole in drinking water, which induced a similar gut microbial density to the LC diet (Fig. S9). We found no significant difference in survival between mice receiving the HC + metronidazole or the LC diet when administered 3% DSS (Log-Rank test; Fig. S9). Both of these microbial-density lowering interventions enabled significantly longer survival than seen in mice fed HC diet (p=0.0039, p=0.0033, respectively, Log-Rank test; Fig. S9). Together these results show that the microbiota-dependent increase in DSS pathology in animals fed HC diet compared to LC diet is linked to increased microbial density. This increase in microbial density in HC diet, which also correlates with a reduced microbial diversity and decreased intestinal barrier function, might exacerbate DSS induced intestinal injury either through increases in antigenic load that elevate the likelihood of microbial invasion into the nearby tissue or increased capacity to generate pathogenic metabolites.
Psyllium modulates intestinal permeability and colitis severity
To determine if psyllium, the most protective ingredient in our dietary screen, mitigated disease in a microbiota-dependent manner, we measured survival of SPF and GF mice fed either a high psyllium diet (15% psyllium, TD.130633; HPSY) or high cellulose diet (15% cellulose, TD.130630; HCEL) for a week prior to and during induction of colitis. SPF mice fed the HPSY diet survived on average for 27.6 days, 4.6 times longer than mice fed the HCEL diet (6 days, p<0.0001, Log-Rank test; Fig. 4A). In contrast to the microbiota-dependent effects of casein, GF animals experienced some of the protective benefit from consuming HPSY diet and survived for an average of 16.7 days, 3.6 times longer than GF mice fed HCEL diet (4.7 days, p<0.0001, Log-Rank test; Fig. 4A). Although psyllium provided some microbiota-independent benefit in the context of DSS-colitis, we observe significantly greater benefit in SPF animals on the HPSY diet relative to GF animals (p<0.0001; Log-Rank test; Fig. 4A) suggesting that some of the benefit is also microbiota-dependent.
Figure 4. Dietary psyllium has both microbiota-dependent and microbiota-independent effects upon host health and colitis.
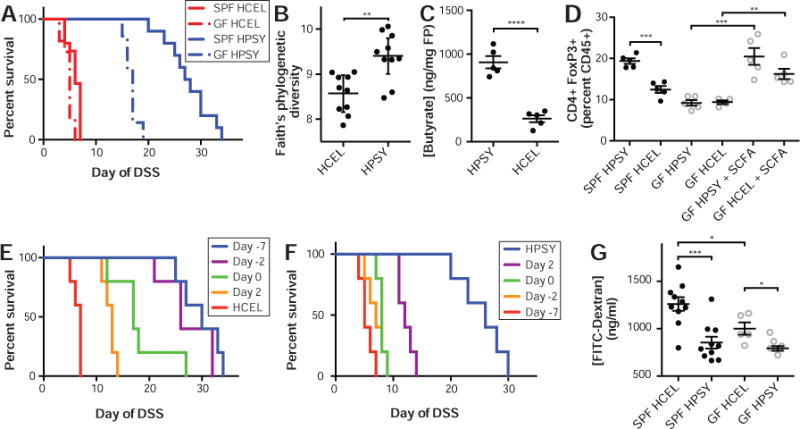
The HPSY diet led to (A) increased survival in both SPF and GF mice with the greatest difference observed in SPF animals. Alpha diversity measured by (B) Faith’s phylogenetic diversity rarified to 7k reads per sample demonstrated increased microbial diversity in the HPSY diet. Mice fed HPSY had increased colonic (C) butyrate, isobutyrate (p=0.028, t-test; Table S5), and valerate (p<0.0001, t-test; Table S5), which was coupled with (D) an increase in colonic Tregs in SPF animals. In GF animals, Tregs were increased via oral supplementation of SCFA in the drinking water. (E) To determine the dietary pretreatment time necessary to obtain the protective benefit of psyllium in DSS colitis, mice were initially given the HCEL diet and were transferred to the HPSY diet at various time points. Administering the HPSY diet at least two days prior to the administration of DSS was sufficient to provide the same protection as a week of diet. (F) To determine the duration of the benefit of psyllium in DSS colitis, mice were initially given the HPSY diet and were switched to the HCEL diet at different time points. The replacement of psyllium with cellulose almost immediately halted the protective benefits of psyllium in mouse survival. (G) The HCEL diet was associated with increased intestinal permeability with a larger barrier defect in SPF relative to GF mice. SPF animals were given 3% DSS and GF animals were given 2% DSS. Lines represent surviving number of animals (A, E–F). Black (SPF) and grey (GF) points represent individual animals (B–D, G). **p<0.01, ***p<0.001, ****p<0.0001.
To better understand the potential role of the microbiota in the beneficial properties of psyllium dietary fiber, we measured gut microbial density and performed 16S rDNA sequencing of fecal pellets taken after a week of HPSY or HCEL diet in mice unchallenged with DSS. HPSY diet significantly reduced microbial density relative to HCEL diet (p=0.047, t-test). The fecal microbiota in mice fed HPSY was more diverse than those fed HCEL (Faith’s phylogenetic diversity, rarefied to 7K reads; p=0.0012, t-test; Fig. 4B) and formed a distinct cluster from the gut microbiota of animals consuming the HCEL diet (weighted unifrac, rarefied to 7K reads; p=0.006, permanova; Fig. S10). Independent batches of mice with comparable baseline microbiotas displayed similar, stable responses to the same dietary intervention (Fig. S5) with increased psyllium driving increased microbiota diversity in unchallenged animals and less severe disease in those animals upon administration of DSS. Microbiota transplant experiments demonstrate that the psyllium-altered microbiota is not sufficient to transfer the colitis protection (Fig. S6).
Some dietary fibers provide a substrate for bacterial fermentation that generates SCFA, which increase the number and function of CD4+FoxP3+ regulatory T cells (Tregs) and have a protective effect in mouse colitis models.31–33 We therefore hypothesized that psyllium minimized disease in DSS colitis in a microbiota-dependent manner through increased production of SCFA and Tregs. Replacing dietary cellulose with psyllium led to increased concentrations of butyrate in the intestinal lumen (p<0.0001, t-test; Fig. 4C, Table S5), increased colon Treg cells (p=0.0002, t-test; Fig. 4D), and less severe colitis in the T cell transfer model of colitis (Fig. S11). To determine if increased Tregs were responsible for the microbiota-dependent protective effects of psyllium in the DSS model, we directly increased Tregs in GF mice through oral administration of SCFA.31 However, exogenous SCFA were insufficient to protect GF mice in the DSS model (p=0.31, Log-Rank test; Fig. S12), suggesting that psyllium-driven, microbiota-dependent increases in SCFA and increased Tregs could not explain the microbiota-dependent protective effects of psyllium in the context of DSS colitis.
To test the kinetics of dietary psyllium protection, we fed mice the HCEL diet and then replaced it with the HPSY diet at various time points before and after beginning DSS treatment. We observed similar protective effects when we provided HPSY diet at either 2 or 7 days prior to DSS treatment (4.3/4.7 fold increased survival; p=0.0034, p=0.0030, respectively, Log-Rank test; Fig. 4E) with significant but less pronounced effects when psyllium was provided at the start of DSS administration or thereafter. To determine the duration of protection after psyllium consumption was halted, we replaced HPSY diet with HCEL diet at different time points before or after beginning DSS treatment. Animals given psyllium for the duration of the experiment had the longest survival (4.7 fold increased survival; p=0.0018, Log-Rank test; Fig. 4F) with a rapid decrease in benefit when the HPSY diet is removed (2.3 fold increased survival for d2; p=0.0045, Log-Rank test). Together these results demonstrate that the beneficial effects of psyllium are rapid and short-lived, which argues against an important role for the adaptive immune system and in favor of more rapid responses, like innate immune mechanisms, altered barrier function, or transcriptional changes.
To test the impact of psyllium on barrier function, we measured intestinal permeability with FITC-dextran in unchallenged SPF and GF mice fed the HPSY or HCEL diet. In both SPF and GF animals, mice fed HCEL had higher intestinal permeability than HPSY (p=0.0005, p=0.017 respectively, t-test; Fig. 4G; Table S6A). However, as in the protein dietary interventions, there was a microbiota-dependent difference in the influence of dietary fiber on intestinal permeability with decreased barrier function in SPF consuming HCEL relative to GF mice (p=0.040, t-test; Fig. 4G; Table S6A) and no difference in gut motility between HPSY and HCEL diets. To better understand the mechanism of the increased barrier function, transcriptional profiling was performed on colonic tissue (Fig. S7). We found HPSY diet drove changes in expression of genes related to the innate immune response (i.e., neutrophils and macrophages) and related to host responses to bacteria/LPS (e.g., NCF2, PLSCR4, and TIMP4).
Overall these results indicate the benefits of psyllium in reducing DSS pathology are rapid acting and short-lived and unlikely to result from changes to the adaptive immune system. In unchallenged mice, microbiota-independent and microbiota-dependent benefits of psyllium are associated with improved intestinal barrier and increased gene expression related to innate immunity and responses to bacteria. These results suggest psyllium is strengthening antimicrobial immunity, limiting access of microbes into the host tissue, and limiting microbial density with perhaps some benefit resulting from the increased diversity of the microbial community associated with psyllium.
The combinatorial influence of casein and psyllium on host physiology and inflammation
A critical feature of human diets is the continuous variation and combinations of multiple ingredients that, in concert, might alter disease risk or pathology. To quantify the combined influence of the two ingredients with the most significant impact on disease variation, we generated diets with all nine possible combinations of low, medium, and high casein (6, 20, 41%) and psyllium (0, 0.5, 5%; Table S1 and S2B). We modeled weight change following DSS administration as the combined influence of both ingredients. We observed a significant influence of both psyllium and casein on weight change, as the beneficial influence of increased psyllium could offset the damaging effects of increased casein. The two extremes of high casein with low psyllium and low casein with high psyllium had the most and least severe disease respectively (Fig. S13A). To quantify the individual and synergistic influence of each ingredient on weight change, we performed a linear regression with each ingredient separately and combined. A linear regression with casein alone explains 33% of the variation in disease (estimated as weight change at DSS day 7; p=3.47×10−5; F-test) compared to 31% for psyllium alone (p=7.41×10−5, F-test) and 62% for casein and psyllium together (p=4.64×10−10, F-test; best model by BIC). Similar to our analyses of the effects of casein and psyllium individually, we also found a combinatorial influence of these two dietary ingredients on host physiology and the microbiota in unchallenged mice for both gut microbial density (R2=0.46, p=2.80×10−6, F-test) and intestinal permeability estimated with FITC-dextran translocation (R2=0.63, p=2.41×10−4, F-test; Fig. S13B; Table S6B). The combination of gut microbial density and intestinal permeability in unchallenged mice also explain most variation in DSS disease severity (71%; p=2.61×10−5, F-test). As in our previous study,11 we found dietary combinations also predictably alter the abundance of different phylogenetic groups, as casein and psyllium fiber concentrations explain changes in both the Firmicutes and Bacteroidetes phyla (R2 = 0.85 and 0.72; p=0.003 and 0.023, respectively; F-test) with both ingredients positively associated with Bacteroidetes and negatively associated with Firmicutes.
To determine if casein and psyllium have the same combinatorial effects upon DSS colitis in the context of human gut microbes, we gavaged a human fecal microbiota into GF mice one week prior to starting one of five casein and psyllium diet variations (i.e., all four combinations of low and high psyllium and casein as well as one diet with medium casein and psyllium). One week later, 3% DSS was administered with disease severity measured as weight loss at day 7 and fecal lipocalin-2 measured at day 4. In these humanized microbiota mice, we again find the combined influence of casein and psyllium best explains disease severity (R2=0.72 and 0.69, p=3.27×10−5 and 8.37×10−5, F-test; for weight loss d7 and fecal lipocalin-2, respectively). Like SPF mice microbiota, the human microbiota cluster together with control diet and diverge with dietary change (Fig. S5). Altogether, these results further suggest colitis severity is the result of the combined influence of multiple ingredients and indicate the influence of diet on colitis is conserved across very different microbiotas (i.e., mouse and human).
Discussion
Deciphering the complex interactions between the gut microbiota, diet, and IBD remains a major challenge in the development of diet-based therapies for the prevention and treatment of IBD. Towards this, we used mouse models of colitis to control host genotype and the gut microbiota, while systematically manipulating dietary composition. In our study of over 40 dietary combinations tested in the DSS model of colitis, we observed significant microbiota-dependent and microbiota-independent influences of diet on experimental colitis, as well as on host physiology in the context of healthy, unchallenged animals. Overall, we observed a remarkable 6.3-fold difference in survival from the most deleterious (4.4 days survival) to the most beneficial diet (27.6 days survival) in the DSS model, highlighting the importance of diet in experimental colitis. By including levels of protein and fiber that are typically found in more extreme human diets such as low-carb, high protein diets or high fiber diets, we explored a wide-range of dietary possibilities. Importantly, even across a more limited range we observed a significant impact of diet on disease severity, as low casein (LC) was more beneficial than medium casein (MC) (p=0.015, t-test), while psyllium fiber provided a significant benefit down to 0.5% (p=0.022, t-test).
Our data demonstrate that the influence of diet on gut inflammation involves numerous potentially additive or offsetting effects between dietary components and the gut microbiota that should be considered when developing specific IBD diets in the clinic. Dietary trials that exclude specific proinflammatory ingredients will only be successful in extreme cases, as the majority of diet’s influence on gut inflammation is likely the sum of multiple dietary components—balancing these components represents the key to effective diet therapy.
The observed combinatorial influence of dietary protein and psyllium fiber might also help interpret existing clinical data and inform future clinical trials. The deleterious role observed for protein is supported by a prospective human cohort study that found meat and protein consumption associated with increased likelihood of UC relapse.34 The ability of protein to drive an expansion of the gut microbiota also provides a novel biomarker for treatment efficacy. Contrary to previous clinical recommendations to reduce fiber consumption in IBD patients, recent results have highlighted the important role of fiber in maintaining intestinal homeostasis.19,20 In the Nurses’ Health study, higher intake of fruit and vegetables in high school students was associated with a 53% lower risk of CD,35 and a recent dietary survey of over 1500 individuals with IBD found the avoidance of fiber is associated with a greater risk of a flare in CD.36 In the specific context of psyllium fiber, a double-blind, placebo-controlled crossover trial in 29 patients found psyllium was associated with a significantly higher rate of improvement in gastrointestinal symptoms in UC in remission compared to a non-gel-forming placebo fiber.37 Our results suggest these protein and fiber interventions in IBD could prove even more beneficial in combination. In the context of mild to moderate IBD, a combination of limiting microbial load with reduced protein intake and maximizing intestinal barrier function through consumption of selectively beneficial dietary fiber could provide a unique non-invasive path to minimize intestinal damage. Dietary changes might be effective alone or in association with current immunosuppressive therapies in the treatment of CD and UC and eventually to prevent disease onset in individuals at risk of developing IBD.38,39
Supplementary Material
Acknowledgments
We are grateful to C. Fermin, E. Vazquez, R. Ng, and G. Escano in the Mount Sinai Immunology Institute Gnotobiotic facility for their help with gnotobiotic animal husbandry. C. Berin, C. Yang, O. and B. Mickelson provided helpful suggestions during the course of this work. Metabolite measurements were performed by the Stable Isotope and Metabolomics Core Facility of the Diabetes Research and Training Center (DRTC) of the Albert Einstein College of Medicine supported by NIH/NCI grant P60DK020541. Next generation sequencing was performed at NYU School of Medicine by the Genome Technology Center partially supported by the Cancer Center Support Grant, P30CA016087. The RNA-Seq and 16S rDNA datasets analyzed in the manuscript are available through NCBI under accession numbers GSE104461 and PRJNA403997 respectively. This work was supported in part by the staff and resources of Scientific Computing and of the Flow Cytometry Core at the Icahn School of Medicine at Mount Sinai.
Funding: This work was supported by grants from the NIH (NIGMS GM108505, NCCIH AT008661, and NIDDK DK108487) and SUCCESS.
Abbreviations
- CD
Crohn’s Disease
- CEL
cellulose diet
- DSS
dextran sodium sulfate
- EEN
exclusive enteral nutrition
- GF
germ free
- HC
high casein diet
- HCEL
high cellulose diet
- HPSY
high psyllium diet
- IBD
Inflammatory Bowel Disease
- LC
low casein diet
- MC
medium casein diet
- PSY
psyllium diet
- SCFA
short chain fatty acids
- SPF
specific pathogen free
- Tregs
T regulatory cells
- UC
ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: We have nothing to disclose
Writing Assistance: S.R.L. and J.J.F. wrote the paper.
Author contributions: S.R.L. and J.J.F. designed the experiments; S.R.L., O.V., A.M., and G.J.B. generated the data involving immune function and inflammation; E.J.C. developed high throughput methods for measuring gut microbial density; S.R.L., G.B., A.G., E.J.C, J.F.C., J.C.C., M.M., and J.J.F. analyzed the data; S.R.L. and J.J.F. wrote the paper.
References
- 1.Lee D, Baldassano RN, Otley AR, et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm Bowel Dis. 2015;21:1786–93. doi: 10.1097/MIB.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152:398–414. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000542.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Faith JJ, Guruge JL, Charbonneau M, et al. The Long-Term Stability of the Human Gut Microbiota. Science (80-) 2013;341:1237439–1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNulty NP, Wu M, Erickson AR, et al. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol. 2013;11:e1001637. doi: 10.1371/journal.pbio.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes AJ, Chew YV, Colakoglu F, et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2016:1–12. doi: 10.1016/j.cmet.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenburg ED, Zheng H, Joglekar P, et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faith JJ, McNulty NP, Rey FE, et al. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–4. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs JP, Goudarzi M, Singh N, et al. A Disease-Associated Microbial and Metabolomics State in Relatives of Pediatric Inflammatory Bowel Disease Patients. C Cell Mol Gastroenterol Hepatol. 2016;2:750–766. doi: 10.1016/j.jcmgh.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen JJ, Sartor RB. Insights from animal models. 2007 [Google Scholar]
- 15.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;6736:1–11. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 17.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chassaing B, Miles-Brown J, Pellizzon M, et al. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol - Gastrointest Liver Physiol. 2015;309:G528–G541. doi: 10.1152/ajpgi.00172.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earle KA, Billings G, Sigal M, et al. Quantitative Imaging of Gut Microbiota Spatial Organization. Cell Host Microbe. 2015;18:478–488. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bylund-Fellenius A, Landstrom E, Axelsson L, et al. Experimental Colitis Induced by Dextran Sulphate in Normal and Germfree Mice. Microbioal Ecol Heal Dis. 1994;7:207–215. [Google Scholar]
- 23.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chassaing B, Srinivasan G, Delgado Ma, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efron B, Hastie T, Johnstone I, et al. Least Angle Regression. Ann Stat. 2004;32:407–499. [Google Scholar]
- 26.van der Logt EMJ, Blokzijl T, van der Meer R, et al. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J Nutr Biochem. 2013;24:1159–65. doi: 10.1016/j.jnutbio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Laroui H, Ingersoll SA, Liu HC, et al. Dextran sodium sulfate (dss) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hans W, Schölmerich J, Gross V, et al. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267–273. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 29.Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 30.Rath HC, Schultz M, Freitag R, et al. Different Subsets of Enteric Bacteria Induce and Perpetuate Experimental Colitis in Rats and Mice. Infect Immun. 2001;69:2277–2285. doi: 10.1128/IAI.69.4.2277-2285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 33.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananthakrishnan AN, Khalili H, Song MY, et al. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2015;21:2311–2319. doi: 10.1097/MIB.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brotherton CS, Martin CA, Long MD, et al. Avoidance of Fiber Is Associated With Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clin Gastroenterol Hepatol. 2016;14:1130–1136. doi: 10.1016/j.cgh.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallert C, Kaldma M, Petersson BG. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol. 1991;26:747–50. doi: 10.3109/00365529108998594. [DOI] [PubMed] [Google Scholar]
- 38.Torres J, Burisch J, Riddle M, et al. Pre-clinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 2016;65:1061–1069. doi: 10.1136/gutjnl-2016-311785. [DOI] [PubMed] [Google Scholar]
- 39.Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152:327–339. doi: 10.1053/j.gastro.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


