Abstract
Background and Aims
CD4+CD25+Foxp3+ T-regulatory (Treg) cells control immune responses and maintain immune homeostasis. However, under inflammatory conditions, Treg cells produce cytokines that promote inflammation. We investigated production of tumor necrosis factor (TNF) by Treg cells in patients with acute hepatitis A (AHA), and examined the characteristics of these cells and association with clinical factors.
Methods
We analyzed blood samples collected from 63 patients with AHA at the time of hospitalization (and some at later time points) and 19 healthy donors in South Korea. Liver tissues were collected from patients with fulminant AHA during liver transplantation. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood and lymphocytes were isolated from liver tissues and analyzed by flow cytometry. Cytokine production from Treg cells (CD4+CD25+Foxp3+) was measured by immunofluorescence levels following stimulation with anti-CD3 and anti-CD28. Epigenetic stability of Treg cells was determined based on DNA methylation patters. Phenotypes of Treg cells were analyzed by flow cytometry, and an RORγt inhibitor, ML-209 was used to inhibit TNF production. Treg cell suppression assay was performed by co-culture of Treg-depleted PBMCs and isolated Treg cells.
Results
A higher proportion of CD4+CD25+Foxp3+ Treg cells from patients with AHA, compared with controls, produced TNF upon stimulation with anti-CD3 and anti-CD28 (11.2% vs 2.8%). DNA methylation analysis confirmed the identity of the Treg cells. TNF-producing Treg cells had features of T-helper 17 cells, including upregulation of RORγt, which was required for TNF production. The Treg cells had reduced suppressive functions compared to Treg cells from controls. The frequency of TNF-producing Treg cells in AHA patients’ blood correlated with their serum level of alanine aminotransferase.
Conclusions
Treg cells from patients with AHA have altered functions, compared with Treg cells from healthy individuals. Treg cells from patients with AHA produce higher levels of TNF, gain features of T-helper 17 cells, and have reduced suppressive activity. The presence of these cells is associated with severe liver injury in patients with AHA.
Keywords: hepatitis A virus infection, ALT, liver injury, inflammation
Graphical Abstract

Introduction
Primary infection with the hepatitis A virus (HAV), a positive-strand RNA virus in the family of Picornaviridae, is a common cause of acute hepatitis worldwide.1, 2 Even though patients with acute hepatitis A (AHA) may experience varying degrees of hepatitis, most experience complete clinical and virological recovery and acquire lifelong immunity to the virus; however, some cases are complicated by fulminant hepatic failure.3 The severity of AHA is related to the age of the patient at the time of infection, as adults show more serious clinical manifestation than do children.3, 4 In patients with AHA, liver injury is known to be mediated by T cells rather than the direct cytopathic effects of the virus itself.5, 6 From an immunologic perspective, AHA in HAV-infected adults provides a unique opportunity to investigate mechanisms of immune-mediated tissue injury during acute human viral infections.
CD4+CD25+Foxp3+ regulatory T (Treg) cells are a distinct subset of CD4+ T cells capable of inhibiting the proliferation and effector functions of various immune cells both in vitro and in vivo.7–9 Treg cells maintain immune homeostasis by preventing the activation of self-reactive T cells and control immune responses in pathologic conditions.10 In fact, many human studies have indicated the involvement of quantitative and/or qualitative impairment of the Treg cell population in the inflammatory tissue damage observed in autoimmune diseases.11–14 Treg cells also play a role in host injury that occurs during many viral infections, including HAV infection. In a previous study, we reported the role of Treg cells in the prevention of immune-mediated liver injury during AHA.15 We found that the frequency of Treg cells is decreased by Fas-mediated apoptosis during AHA and that the reduced Treg cell frequency is associated with a high degree of immune-mediate liver injury in AHA patients.15 Moreover, it was reported that HAV itself is able to bind its receptor on Treg cells and directly inhibit their suppressive activity.16
Intriguingly, transcription factor expression and the ability to produce inflammatory cytokines varies in Treg cells under different inflammatory conditions.17, 18, 19 For example, it has been shown that Treg cells produce IFN-γ in multiple sclerosis and type I diabetes20, 21 and IL-17A in Crohn’s disease and rheumatoid arthritis.22, 23 Moreover, such Treg cells present in the inflammatory milieu, known as ‘inflammatory Treg cells’, express helper T (Th) cell type-specific transcription factors, either T-bet or RORγt together with Foxp3, and chemokine receptors responsible for homing to inflammatory sites24–26 and show varying degrees of suppression.20–26 These reports support the potential role of the pathologic conversion of Foxp3+ Treg cells in inflammatory diseases. Likewise, the acquisition of effector cell phenotypes by Treg cells has also been studied in an experimental infection model and it was found that environmental factors provided by both local dendritic cells and effector T cells can induce T-bet expression in Treg cells, which leads to IFN-γ production, in the context of a lethal infection with Toxoplasma gondii.27 These findings provide new insights into immunopathogenesis by showing the development of a disorganized regulatory pathway under overwhelming infectious conditions. However, the inflammatory conversion of Foxp3+ Treg cells has not been reported in human viral diseases and the clinical implications of these cells have not been explored.
In the present study, we investigated alterations of Treg cells in patients with AHA, focusing on the functional conversion of Treg cells and in particular, their production of inflammatory cytokines. We also analyzed the association between the functional change of Treg cells and immunopathological liver injury during AHA. To this end, we found that a considerable proportion of CD4+CD25+Foxp3+ Treg cells from patients with AHA produced TNF-α following T cell receptor (TCR) stimulation. Interestingly, TNF-α-producing Treg cells have reduced suppressive activity and share various immunophenotypes with helper 17 T (Th17) cells including the expression of RORγt and CCR6. Furthermore, we found a strong correlation between these cells and the degree of liver injury, suggesting possible roles of TNF-α-producing Treg cells having reduced suppressive activity in the immunopathological liver injury during AHA.
Materials and Methods
Study subjects and isolation of lymphocytes
Sixty-three patients, who were diagnosed with AHA and hospitalized in Yonsei University Severance Hospital (Seoul, Korea) and Chung-Ang University Hospital (Seoul, Korea), were included in the current study. The criteria for inclusion were age 20 or older; seropositivity for anti-HAV IgM antibody; serum ALT level higher than three times the upper limit of normal; and clinical symptoms compatible with acute hepatitis. Whole blood samples were drawn at the time of hospitalization. In some cases, serial samples in the convalescent phase were also obtained. In the case of fulminant AHA, liver tissues were obtained during liver transplantation. In addition, patients with other liver diseases (acute hepatitis B, n=6; chronic hepatitis B, n=14; acute hepatitis C, n=3; chronic hepatitis C, n=12; and toxic/drug-induced hepatitis, n=4) were included. This study protocol was reviewed and approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all study subjects.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by standard Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation and cryopreserved until use. Liver-infiltrating lymphocytes (LILs) were isolated from liver tissues by Ficoll-Paque density gradient centrifugation following meticulous mechanical homogenization.
Antibodies
The following fluorochrome-conjugated monoclonal antibodies (mAbs) were used for multicolor flow cytometry: anti-CD3-V500 or -Alexa Fluor 700 (UCHT-1), anti-CD4-V450, -APC-H7 or -Alexa Fluor 700 (RPA-T4), anti-CD14-PerCP-Cy5.5 (M5E2), anti-CD19-PerCP-Cy5.5 (HIB19), anti-CD25-PerCP-Cy5.5, -PE-Cy7 or -APC (M-A251), anti-CD45RA-PE-Cy7 or -APC-H7 (HI100), anti-CD195-FITC or -APC (2D7/CCR5), anti-CD196-PE-Cy7 (11A9), anti-Ki-67-FITC (B56) (all from BD Biosciences, San Jose, CA); anti-CD39-PE-Cy7 (eBioA1) (eBioscience, San Diego, CA); anti-CD127-Alexa Fluor 488 (40131), anti-CD183-FITC or -APC (49810) and anti-CD194-FITC (205410) (all from R&D Systems, Minneapolis, MN). For intracellular staining, PE-conjugated anti-Foxp3 mAb (PCH101) and anti-RORγt-APC (AFKJS-9) (all from eBioscience); anti-T-bet-Alexa Fluor 647 (O4-46) and anti-GATA3-PE-Cy7 (L50-823) (all from BD Biosciences) were used.
Flow cytometry and immunophenotyping
PBMCs were stained with fluorochrome-conjugated antibodies against surface markers for 30 minutes on ice and then washed. Dead cells were excluded by staining with either a Live/Dead fixable cell stain kit (Invitrogen, Carlsbad, CA) or ethidium monoazide (Invitrogen). For intracellular staining, surface-stained cells were permeabilized using a Foxp3 staining buffer kit (eBioscience) according to the manufacturer’s instructions and were further stained for intracellular proteins. Flow cytometry was performed on an LSR II instrument using FACSDiva software (BD Biosciences), and the data were analyzed using FlowJo software (Treestar, San Carlos, CA).
In vitro stimulation of T cells and intracellular cytokine staining (ICS)
Cryopreserved PBMCs were thawed, resuspended in RPMI 1640 containing 5% FBS and 2 mM L-glutamine, and rested overnight at 37°C. The next morning, PBMCs were stimulated with anti-CD3 antibody (0.1 μg/mL; Beckman Coulter Immunotech, Marseille, France) and anti-CD28 antibody (1 μg/ml; BD Biosciences), HAV VP2 protein or a mix of HAV VP2 overlapping peptides (OLPs). Brefeldin A (GolgiPlug, BD Biosciences) and monensin (GolgiStop, BD Biosciences) were added 1 hour later. After another 5 hours of incubation, cells were first stained using a Live/Dead fixable cell stain kit to exclude dead cells and then stained with fluorochrome-conjugated antibodies against surface markers including anti-CD3 (V500 or Alexa Fluor 700), anti-CD4 (V450 or Alexa Fluor 700), anti-CD25 (PE-Cy7), and anti-CD127 (eFluor450). Stained cells were permeabilized using the Foxp3 staining buffer kit and further stained with anti-Foxp3 (PE), anti-TNF-α (APC or Alexa Fluor 700; BD Biosciences), and either anti-IFN-γ (FITC; BD Biosciences) or anti-IL-17A (FITC; eBioscience). FACS analysis was performed on an LSRII flow cytometer, and the data were analyzed using FlowJo software.
To examine the effect of RORγt on cytokine production from Treg cells, an RORγt inhibitor, ML209 (40 μM)28 was added to the PBMC culture when they were stimulated by anti-CD3/CD28.
Isolation of Treg cells
Treg cells were isolated from PBMCs using the CD4+CD25+CD127lo/− regulatory T cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. Briefly, non-CD4+ cells and CD127high cells were first depleted using a cocktail of biotinylated antibodies and anti-biotin microbeads. CD4+CD25+CD127lo/− cells were directly labeled with CD25 microbeads and isolated over a selection column. The purity of isolated Treg cells and CD25-depleted PBMCs was examined by flow cytometry after staining with fluorochrome-conjugated antibodies to CD3, CD4, CD25, CD127, and Foxp3.
Isolation of TNF-α-producing cells and DNA methylation analysis
PBMCs were stimulated as described in the above section: ‘In vitro stimulation of T cells and intracellular cytokine staining (ICS)’. After staining, TNF-α+Foxp3+, TNF-α−Foxp3+, and Foxp3− cell populations were isolated from the gate of viable CD4+ T cells using a FACSAria II cell sorter (BD Biosciences). The purity of sorted cells was more than 95%.
The sorted cells were analyzed for DNA methylation status of the Treg-specific demethylated region (TSDR). DNA methylation analysis was performed as previously described.29 Genomic DNA was extracted from sorted cells using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction. Purified DNA was subjected to sodium bisulfite conversion using a MethylDetector kit (Active Motif, Carlsbad, CA). Following bisulfite conversion, the CpG island of TSDR was amplified by nested PCR (primer pairs used: 5′-TGT GGA GTT TGT TTG GTA TTT TTA G and 5′-AAA AAT TCT CCC CAA ACA CAT ATA A for direct round; 5′-TGG GTT AAG TTT GTT GTA GGA TAG G and 5′-TCC CTT TCT AAC TAA ATT TCT CAA AAA C) for the nested round. The final PCR product of 257 base pairs was purified and cloned into the TOPO TA cloning vector (Invitrogen), and the methylation status of CpG loci in each cell population was ascertained by automatically sequencing more than ten clones from each experiment.
Treg cell suppression assay
Treg cell suppression assays were performed as previously described.15 Briefly, Treg cells were depleted from PBMCs, and then Treg-depleted PBMCs were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen). CFSE-labeled, Treg-depleted PBMCs, serving as responder T (Tresp) cells, were stimulated by soluble anti-CD3 antibody (0.1 μg/mL) and anti-CD28 antibody (1 μg/ml) with or without re-addition of the depleted Treg cell-enriched population with various ratios. Following 96 hours of co-culture, cells were analyzed for proliferation, as assessed by the percentage of CFSElo cells under either the live CD4+ or CD8+ T cell gates using an LSR II flow cytometer. Dead cells were excluded by staining cells with 7-AAD. The percentage of suppression was calculated as [1 - (% T cell proliferation with Treg cells/% T cell proliferation without Treg cells)] × 100.
To examine the effect of TNF-α on the suppression activity of Treg cells, anti-TNF-α blocking antibody (5 μg/ml; R&D Systems) or mouse IgG1 isotype control antibody was added to the co-cultures.
Statistics
Statistical analyses were performed using the Prism software version 5.0 (GraphPad, La Jolla, CA). To compare two groups, the nonparametric Mann-Whitney U-test was performed. Comparisons of paired values were performed using the nonparametric Wilcoxon matched-pairs signed rank test. Correlations between two parameters were tested using the nonparametric Spearman’s rank correlation test. A P value of 0.05 or less was considered statistically significant.
Results
CD4+CD25+Foxp3+ Treg cells from AHA patients produce TNF-α
First, we examined inflammatory cytokine production by CD4+CD25+Foxp3+ Treg cells from AHA patients. The production of IFN-γ, IL-17A, and TNF-α was analyzed by ICS and flow cytometry in response to in vitro anti-CD3/CD28 stimulation of PBMCs (Supplementary Figure 1). We found that compared to Treg cells from healthy controls, those from AHA patients produced significantly more of all three cytokines (Figure 1A). Of the three cytokines, the production of TNF-α was most prominent, while IFN-γ and IL-17A were produced at lower levels (Figure 1A and B). In addition, mean fluorescence intensity of TNF-α was significantly higher in TNF-α-producing CD4+CD25+Foxp3+ T cells from AHA patients than in those from healthy controls (Figure 1C). Moreover, TNF-α production was increased in AHA patients compared to healthy controls even in the absence of in vitro anti-CD3/CD28 stimulation (Figure 1A). However, TNF-α production from CD4+CD25+Foxp3+ Treg cells of AHA patients was not notably observed when they were stimulated by HAV VP2 protein or OLP mix (Figure 1D), indicating that Treg cells of AHA patients produce TNF-α in a TCR-dependent manner, but not in an HAV antigen-specific manner. In the HAV VP2 stimulation assay, CD4+CD25+Foxp3+ Treg cells were minor population for the TNF-α production compared with non-Treg CD4+ T cells and CD8+ T cells in the peripheral blood of AHA patients (Figure 1E).
Figure 1. Production of inflammatory cytokines by Treg cells from AHA patients.
(A–C) PBMCs from AHA patients were stimulated with anti-CD3/CD28 antibodies, and cytokine production (TNF-α, IFN-γ, and IL-17A) was analyzed by ICS. Percentages of cells producing each cytokine among CD4+CD25+CD127lo/−Foxp3+ T cells from AHA patients were determined (n=19 for TNF-α and IFN-γ; n=11 for IL-17A) and compared to those from healthy controls (n= 19 for TNF-α and IFN-γ; n=14 for IL-17A) (A). Horizontal bars represent mean percentages. Representative flow cytometry dot plots of a patient and a healthy donor are shown in (B). Plots are gated on CD4+CD25+CD127lo/−Foxp3+ T cells. Mean fluorescence intensity of TNF-α in TNF-α-producing CD4+CD25+Foxp3+ T cells is compared between AHA patients (n=19) and healthy controls (n=19) (C). (D) PBMCs from AHA patients (n=4) were stimulated with HAV VP2 protein, HAV VP2 OLP mix or anti-CD3/CD28 antibodies, and TNF-α production was analyzed by ICS. Percentage of TNF-α-producing cells among CD4+CD25+CD127lo/−Foxp3+ Treg cells was presented. (E) PBMCs from AHA patients (n=4) were stimulated with HAV VP2 protein or HAV VP2 OLP mix, and TNF-α production was analyzed by ICS. In the gate of TNF-α+ T cells, percentages of CD4+CD25+CD127lo/−Foxp3+ Treg cells, Foxp3− non-Treg CD4+ T cells and CD8+ T cells were determined and presented.
We also examined TNF-α production by Treg cells infiltrating inflamed livers of AHA patients who underwent liver transplantation due to fulminant hepatic failure. The frequency of Treg cells was not increased in the liver compared to the peripheral blood (Figure 2A). We found that liver-infiltrating CD4+CD25+Foxp3+ Treg cells produced TNF-α following anti-CD3/CD28 stimulation (Figure 2B). Next, we isolated Treg cells from PBMCs of AHA patients with a high purity and stimulated them with anti-CD3/CD28 antibodies. The isolated Treg cells produced TNF-α (Figure 2C), indicating that TNF-α production from Treg cells can be attributed to direct TCR stimulation of Treg cells and not due to an indirect effect caused by stimulation of other T cells. Next, we investigated TNF-α production by Treg cells obtained over the course of AHA and found a dramatic reduction in TNF-α production by Treg cells during the convalescent from AHA (Figure 2D). We also examined if TNF-α-producing Treg cells are increased in patients with other liver diseases. We found that the frequency of them was significantly increased in patients with chronic hepatitis B, chronic hepatitis C, and toxic/drug-induced hepatitis (Figure 2E and F).
Figure 2. Production of TNF-α by Treg cells from AHA patients.
(A) Percentage of CD4+CD25+CD127lo/−Foxp3+ Treg cells in the gate of CD4+ T cells was examined in PBMCs and LILs of AHA patients (n=3). (B and C) LILs (B) or magnetically-isolated peripheral blood CD4+CD25hiCD127lo/− T cells (C) were stimulated with anti-CD3/CD28 antibodies, and production of TNF-α was analyzed by ICS and flow cytometry. Plots are gated on CD4+CD25+CD127lo/−Foxp3+ T cells. (D) Production of TNF-α from CD4+CD25+CD127lo/−Foxp3+ T cells in response to anti-CD3/CD28 antibodies was evaluated at three time points during the course of AHA, from the time point of initial manifestation of acute hepatitis and admission (day 0) to the time point of complete remission (day 28) (n=5). Representative flow cytometry dot plots over the course of AHA from a single patient are shown in the right panel. (E) Percentage of TNF-α+ Treg cells was determined in the peripheral blood of healthy controls (n=6) and patients with acute hepatitis B (n=6), chronic hepatitis B (n=14), acute hepatitis C (n=3) and chronic hepatitis C (n=12). (F) Percentage of TNF-α+ Treg cells was determined in the peripheral blood of age- and sex-matched healthy controls (n=4) and patients with toxic/drug-induced hepatitis (n=4). Horizontal bars represent mean percentages.
TNF-α-producing CD4+CD25+Foxp3+ T cells are bona fide Treg cells
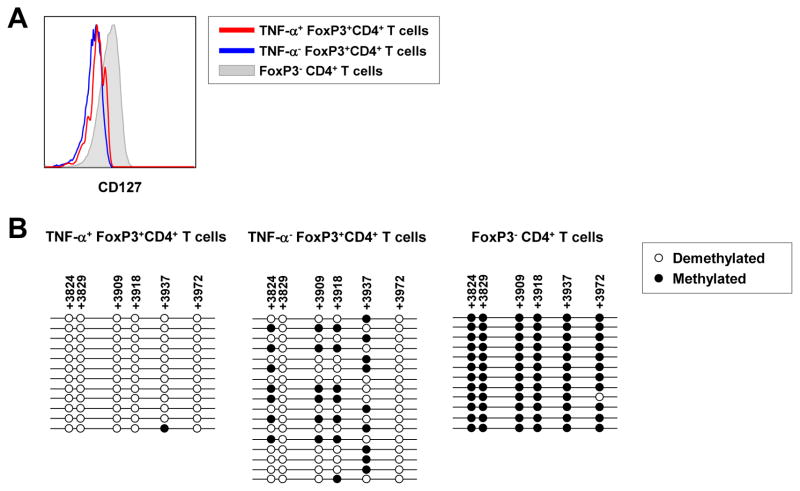
Since Foxp3 can be expressed by activated, non-Treg CD4+ T cells, we next sought to clarify if TNF-α-producing CD4+CD25+Foxp3+ T cells are bona fide Treg cells. First, we examined the expression of CD127 on TNF-α+ and TNF-α− cells within the gate of CD4+CD25+Foxp3+ T cells from AHA patients, as it has been shown that CD127 is a reliable marker to discriminate non-Treg CD4+ T cells (CD127hi) from Treg cells (CD127lo/−).30, 31 TNF-α+CD4+CD25+Foxp3+ T cells expressed CD127 at a low level, comparable to the TNF-α− counterparts, whereas CD4+CD25−Foxp3− non-Treg cells showed a higher level of CD127 expression than both populations in the gate of CD4+CD25+Foxp3+ T cells (Figure 3A). We also examined the methylation status of highly conserved CpG islands located in the TSDR, as methylation of this region controls Foxp3 expression.32, 33 For this, we sorted TNF-α+ Treg cells, TNF-α− Treg cells, and non-Treg CD4+ T cells from PBMCs of AHA patients and investigated the methylation status of TSDR in these 3 sorted populations. The results showed that the CpG loci in the TSDR of both TNF-α+ Treg cells and TNF-α− Treg cells were heavily demethylated in contrast to the non-Treg CD4+ T cells, which showed complete methylation (Figure 3B). We also performed control experiments with PBMCs of healthy donors. First, we confirmed complete demethylation and methylation of the TSDRs of Treg cells and non-Treg CD4+ T cells, respectively (Supplementary Figure 2A). These demethylation/methylation status was maintained even after anti-CD3/CD28 stimulation (Supplementary Figure 2B). As another control experiment, we first stimulated PBMCs of healthy donors with anti-CD3/CD28 antibodies for 6 hours and then isolated CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3− T cells to analyze the methylation status of the TSDRs. As a result, the isolated CD4+CD25+Foxp3+ T cells exhibited nearly complete demethylation in the TSDR, and the isolated CD4+CD25−Foxp3− T cells did complete methylation in the TSDR (Supplementary Figure 2C). This result indicates that the methylation status of the TSDR is not changed by short-term (6 hours) anti-CD3/CD28 stimulation. Taken together, we conclude that TNF-α-producing CD4+CD25+Foxp3+ T cells are bona fide Treg cells.
Figure 3. TNF-α-producing CD4+CD25+Foxp3+ T cells are CD127lo/− Treg cells with demethylated TSDRs.
(A) Shown is a representative histogram comparing the CD127 expression levels on TNF-α+ and TNF-α− CD4+CD25+Foxp3+ T cells and Foxp3− T cells. (B) The TSDR was amplified by nested PCR from bisulfite-treated genomic DNA extracted from each sorted fraction. By analyzing the DNA sequences of the PCR product, the DNA methylation patterns of the CpG islands in the TSDR were determined.
TNF-α-producing Treg cells exhibit Th17-like features
Next, we examined the expression of Th cell type-specific transcription factors in TNF-α-producing Treg cells from AHA patients. Previous studies performed in human autoimmune diseases have shown that inflammatory Treg cells express T-bet or RORγt,21, 23 which are helper 1 T (Th1) and Th17 cell-specific transcription factors, respectively. We found that TNF-α+CD4+CD25+Foxp3+ Treg cells from AHA patients expressed higher levels of T-bet and lower levels of GATA3 than the TNF-α− counterparts (Figure 4A). More strikingly, TNF-α+CD4+CD25+Foxp3+ Treg cells showed substantially higher levels of RORγt expression than did the TNF-α− cells (Figure 4A). We also examined the expression of Ki-67, a proliferation marker, and found that the percentage of Ki-67+ cells in TNF-α+CD4+CD25+Foxp3+ Treg cells was 0.6~6.8% and not higher than that in the TNF-α− counterparts or non-Treg CD4+ T cells, indicating that TNF-α+CD4+CD25+Foxp3+ Treg cells are not actively proliferating during AHA (Figure 4B).
Figure 4. Immunophenotypes of TNF-α-producing CD4+CD25+Foxp3+ Treg cells.
(A–C) Several immunophenotype markers representing the CD4+ helper T cell lineage were examined in TNF-α+ and TNF-α− CD4+CD25+Foxp3+ Treg cells from AHA patients by flow cytometry, including lineage-specific transcription factors (A), Ki-67 (B) and chemokine receptors (C). The expression of each marker in Foxp3− non-Treg CD4+ T cells is presented for comparison. All markers were examined in separate samples from more than five patients. Representative flow cytometry dot plots for RORγt and CCR6 from the gates of TNF-α+ and TNF-α− CD4+CD25+Foxp3+ Treg cells are shown in (D). (E) Comparison of the percentage of IL-17A-producing cells in TNF-α+ and TNF-α− CD4+CD25+Foxp3+ Treg cells from AHA patients (n=5). Representative flow cytometry dot plots for RORγt and IL-17A from the gates of TNF-α+ and TNF-α− CD4+CD25+Foxp3+ Treg cells and Foxp3− non-Treg CD4+ T cells are shown in (F). (G) Comparison of the percentage of RORγt+IL-17A+ cells in TNF-α+ and TNF-α− CD4+CD25+Foxp3+ Treg cells from AHA patients (n=5). Error bars represent standard deviations.
We examined the expression of chemokine receptors on TNF-α-producing Treg cells from AHA patients, as inflammatory Treg cells have been known to express Th cell type-specific chemokine receptors.20, 21, 23 TNF-α+CD4+CD25+Foxp3+ Treg cells of AHA patients expressed higher levels of CCR6 and CCR5 than did the TNF-α− counterparts, while the expression of CXCR3 and CCR4 did not differ between the two populations (Figure 4C). We confirmed the remarkable upregulation of RORγt and CCR6 in TNF-α+ Treg cells compared to TNF-α− Treg cells (Figure 4D). The overexpression of RORγt and CCR6 indicates that TNF-α+CD4+CD25+Foxp3+ Treg cells have features characteristic of Th17 cells, as RORγt and CCR6 are a Th17-specific transcription factor and chemokine receptor, respectively.34 In fact, approximately 10% of the TNF-α+CD4+CD25+Foxp3+ Treg cells in AHA patients produced IL-17A upon in vitro stimulation, whereas the TNF-α− cells did not (Figure 4E). In addition, we analyzed the frequency of RORγt+IL-17A+ cells in the each population and found that TNF-α+CD4+CD25+Foxp3+ Treg cells of AHA patients had higher frequency of RORγt+IL-17A+ cells than the TNF-α− counterparts or non-Treg CD4+ T cells (Figure 4F and G).
We also examined phenotypes of TNF-α+CD4+CD25+Foxp3+ Treg cells of healthy donors (Supplementary Figure 3) although their frequency is very low in the peripheral blood of healthy donors (typically <3%; Figure 1A and B). We found that they exhibited similar phenotypes with TNF-α+CD4+CD25+Foxp3+ Treg cells of AHA patients: higher levels of RORγt expression, CCR6 expression and IL-17A production than the TNF-α− counterparts (Supplementary Figure 3), indicating that TNF-α+CD4+CD25+Foxp3+ Treg cells of healthy donors are not different from those of AHA patients in the molecular expression although the frequency is much lower in healthy donors than in AHA patients.
We next examined whether TNF-α production by Treg cells from AHA patients is controlled by RORγt. In this experiment, we used an RORγt inhibitor, ML209,28 and evaluated the production of TNF-α by CD4+CD25+Foxp3+ Treg cells from AHA patients and found TNF-α production to be significantly, but not completely, reduced by ML209 (Figure 5A and B). The reduced production of TNF-α by ML209 was confirmed by the analysis of mean fluorescence intensity of TNF-α in TNF-α-producing Treg cells (Figure 5C). We also examine the effect of RORγt inhibition on IL-17A production and CCR6 expression in CD4+CD25+Foxp3+ Treg cells of AHA patients and found that RORγt inhibition decreased the production of IL-17A (Figure 5D), but not the expression of CCR6 (Figure 5E).
Figure 5. Attenuated production of TNF-α by Treg cells following RORγt inhibition.
PBMCs from AHA patients were stimulated with anti-CD3/CD28 antibodies in the presence or absence of ML209, an RORγt inhibitor, and production of TNF-α was analyzed by ICS and flow cytometry (n=6) (A). Representative flow cytometry plots from a single patient are shown in (B). Plots are gated on CD4+CD25+CD127lo/−Foxp3+ Treg cells. Mean fluorescence intensity of TNF-α in TNF-α-producing Treg cells is compared between in the presence and absence of ML209 (C). In the same setting, IL-17A production (D) and CCR6 expression (E) were also analyzed by flow cytometry.
Taken together, these data show that TNF-α-producing CD4+CD25+Foxp3+ Treg cells from AHA patients exhibit Th17-like features and produce TNF-α in an RORγt-dependent manner.
Suppressive activity of TNF-α-producing Treg cells
In order to determine if these altered Treg cells from AHA patients were functioning, we examined their suppressive activity. First, we assessed expression of markers known to represent the suppressive function of Treg cells, including Foxp3 itself and CD39.35, 36 TNF-α+CD4+CD25+Foxp3+ Treg cells from AHA patients expressed lower levels of Foxp3 than did the TNF-α− counterparts (Figure 6A). Similarly, the percentage of CD39+ cells was lower in TNF-α+CD4+CD25+Foxp3+ Treg cells than in the TNF-α− counterparts (Figure 6B). These findings suggest that the suppressive function of TNF-α+ Treg cells from AHA patients might be reduced compared to the TNF-α− counterparts. Therefore, we next looked at the Treg suppressive activity in AHA patients. The Treg suppressive activity in AHA patients was significantly reduced compared to that in healthy controls when non-Treg CD4+ T cells or CD8+ T cells were used as responder cells (Figure 6C).
Figure 6. Suppressive activity of TNF-α-producing CD4+CD25+Foxp3+ Treg cells.
(A and B) Expression levels of proteins known to be related with the Treg cell suppression function, including Foxp3 (A) and CD39 (B), were analyzed by flow cytometry. (C) Anti-CD3/CD28-stimulated proliferation of non-Treg CD4+ (left) and CD8+ (right) responder T cells was assessed by measuring the percentage of CFSElo cells in Treg-depleted PBMCs and Treg-reconstituted PBMCs at various ratios. Reconstitution at the original ratio of Treg and non-Treg cells in the peripheral blood is marked as ‘1:1’ and indicated by the arrow. The percentage of suppression was calculated as [1-(% Tresp proliferation in the reconstituted PBMCs/% Tresp proliferation in the Treg-depleted PBMCs)]×100 and was compared between AHA patients (n=11) and healthy controls (n=8). (D) Anti-CD3/CD28-stimulated proliferation of non-Treg CD4+ (left) and CD8+ (right) responder T cells was assessed by measuring the percentage of CFSElo cells in Treg-depleted PBMCs and Treg-reconstituted PBMCs from AHA patients (n=5) and healthy controls (n=5) in the presence or absence of anti-TNF-α blocking antibody. The percentage of suppression was calculated as shown above. Error bars represent standard deviations.
Next, we investigated the effect of TNF-α on the suppressive function of Treg cells. To this end, the suppression activity of Treg cells obtained from AHA patients or healthy controls was assessed in the absence and presence of TNF-α blocking antibody. We found no significant effect of blocking TNF-α on Treg suppressive activity against CD4+ T or CD8+ T cell proliferation in either AHA patients or healthy controls (Figure 6D). These data indicate that Treg cells of AHA patients have attenuated suppression activity independently of TNF-α, even though these cells produce TNF-α.
Clinical implications of TNF-α-producing Treg cells during AHA
Finally, we examined the clinical significance of TNF-α-producing CD4+CD25+Foxp3+ Treg cells in AHA patients. In particular, we focused on liver injury, which is known to be mediated by effector T cells during AHA. Intriguingly, the frequency of TNF-α+ cells among circulating Treg cells significantly correlated with the serum ALT level, whereas the frequency of IFN-γ+ or IL-17A+ cells did not (Figure 7A). However, the frequency of TNF-α+ cells among circulating CD8+ T cells or non-Treg CD4+ cells did not correlate with the serum ALT level (Figure 7B). These results suggest that TNF-α produced by Treg cells might not directly contribute to the liver injury. Instead, reduced suppressive activity of TNF-α-producing Treg cells might be involved in the liver injury by leading to unchecked immunopathologic T cell responses during AHA.
Figure 7. Correlation between the frequency of TNF-α-producing CD4+CD25+Foxp3+ Treg cells and serum ALT levels in AHA patients.
(A) Correlations between the percentage of peripheral blood CD4+CD25+Foxp3+ Treg cells producing either TNF-α, IFN-γ, or IL-17A and serum ALT levels were analyzed in AHA patients (n=19 for TNF-α and IFN-γ; n=11 for IL-17A). (B) Correlations between the percentage of peripheral blood CD8+ or non-Treg CD4+ T cells producing TNF-α and serum ALT levels were analyzed in AHA patients (n=16). (C and D) Percentage of TNF-α-producing Treg cells was determined at the acute stage (C, n=10) or the convalescence stage (D, n=8), and was correlated with the frequency of HAV-VP2-specific, IFN-γ-producing CD8+ or non-Treg CD4+ T cells, which was measured at the convalescence stage.
TNF-α-producing Treg cells might also contribute to the contraction of effector T cell responses as known in other acute infection models.37, 38 However, the frequency of TNF-α-producing Treg cells, which was measured at the acute stage (at the time of admission) (Figure 7C) or convalescence stage (at the time of discharge) (Figure 7D) did not correlate with the frequency of HAV VP2-specific, IFN-γ-producing CD8+ T cells or non-Treg CD4+ cells at the convalescence stage in AHA patients.
Discussion
Immune responses against HAV are successful in almost cases of AHA and eliminate the virus without chronic persistence. However, HAV infection in adult patients often results in severe liver injury that is mediated by immunopathologic mechanisms,6 and some cases are complicated by fulminant hepatic failure. In the present study, we examined the functional conversion of Treg cells during AHA in relation to the immunopathological liver injury rather than protective immune responses. Herein, we discovered that CD4+CD25+Foxp3+ Treg cells from AHA patients produce inflammatory cytokines, particularly TNF-α, in direct response to TCR stimulation. Further, TNF-α-producing Treg cells exhibit a phenotype shared by Th17 cells and reduced suppressive functions. In addition, the frequency of TNF-α-producing Treg cells in the peripheral blood is correlated with the degree of liver injury.
The production of inflammatory cytokines such as IFN-γ and IL-17A by CD4+CD25+Foxp3+ T cells has been demonstrated in human autoimmune inflammatory diseases.19–22 However, the origin of CD4+CD25+Foxp3+ T cells co-expressing features of Th cells has not been clearly elucidated. These cells might result from either promiscuous gain of Foxp3 expression by non-Treg CD4+ effector T cells or inflammatory conversion of true CD4+CD25+Foxp3+ Treg cells. Several murine studies reported that the inflammatory plasticity of Foxp3+ T cells reflects Foxp3 expression in activated conventional T cells, instead of inflammatory conversion of bona fide Foxp3+ Treg cells.39, 40
In the present study, it should be noted that TNF-α-producing CD4+CD25+Foxp3+ T cells from AHA patients exhibited demethylated CpG patterns in the TSDR. This finding indicates that these cells are true Treg cells with stable expression of Foxp3 and that they have likely been converted into TNF-α producing cells by the inflammatory milieu.
In AHA patients, the frequency of TNF-α+ cells among circulating Treg cells positively correlated with the degree of liver injury, as represented by the serum ALT (Figure 7A). However, the frequency of TNF-α+ cells among CD8+ T cells or non-Treg CD4+ cells did not correlate with the serum ALT level (Figure 7B). Considering that Treg cells were minor population for the TNF-α production in the peripheral blood of AHA patients (Figure 1E) and that Treg cells were not enriched in the liver compartment (Figure 2A), TNF-α produced by Treg cells might not directly contribute to the liver injury. It is plausible that reduced suppressive activity of TNF-α-producing Treg cells might contribute to the liver injury by leading to unchecked immunopathologic T cell responses during AHA.
In fact, TNF-α+ Treg cells expressed lower levels of Foxp3 and CD39 than the TNF-α− counterparts. Moreover, Treg cells from AHA patients displayed attenuated suppressive functions. Interestingly, our findings show that the suppressive activity of TNF-α+ Treg cells was not restored by TNF-α blockade per se, suggesting an intrinsic functional insufficiency of TNF-α+ Treg cells. Additional study will be required to determine the fundamental cause of the inflammatory conversion and functional insufficiency of Treg cells. Definition of this pathway may reveal valuable therapeutic targets for a variety of inflammatory disorders.
TNF-α-producing Treg cells might also contribute to the contraction of effector T cell responses as known in other acute infection models.37, 38 TNF-α-induced contraction of T cell responses is mediated by both TNF receptors p55 and p75.37, 38 However, the frequency of TNF-α-producing Treg cells at the acute or convalescence stage did not correlate with HAV VP2-specific, IFN-γ-producing T cell responses at the convalescence stage in AHA patients (Figure 7C and D). This result suggests that TNF-α-producing Treg cells might not be involved in the contraction of effector T cell responses during the convalescence from AHA, although more data are needed to reach a clear conclusion.
Previous studies in murine models suggested the requirement of intrinsic expression of GATA3 by Treg cells to maintain optimal Treg cell suppressive function and limit acquisition of effector phenotypes in the presence of a sustained inflammatory condition.41,42 Intriguingly, in these studies GATA3-deleted Treg cells showed phenotypes of the Th17 lineage as well as attenuated suppressive function. Likewise, we found that TNF-α+ Treg cells expressed significantly lower levels of GATA3 compared to TNF-α− Treg cells (Figure 4A). These findings might indicate a possible role of GATA3 in maintaining Treg cell stability and inhibiting the inflammatory conversion of Treg cells. However, RORγt also plays a critical role in the conversion of Treg cells to produce TNF-α, as evidenced by the reduction of TNF-α production following incubation of cells with an RORγt inhibitor. Thus, further studies are warranted to delineate the molecular mechanisms underlying the inflammatory conversion of Treg cells, particularly with respect to GATA3 and RORγt expression.
In our previous study, we showed that the frequency of Treg cells is decreased through a Fas-mediated mechanism in AHA patients and that this decrease is associated with liver injury in AHA. In addition to this quantitative change in the Treg cell population, here we demonstrate that Treg cells from AHA patients undergo qualitative changes resulting in the production of TNF-α. Moreover, TNF-α-producing Treg cells from AHA patients exhibit Th17-like features in terms of their phenotype and have an attenuated suppression function. Importantly, this study provides new insight into the functional change of Treg cells during human acute viral hepatitis by demonstrating the inflammatory conversion of Treg cells and the association of these cells with the immunopathological liver injury in AHA.
Supplementary Material
Acknowledgments
Grant support:
This work was supported by Samsung Science and Technology Foundation under Project Number SSTF-BA1402-18 (E.C.S.) and the National Institutes of Health grant R00DK091508 (J.R.H.).
Abbreviations
- AHA
acute hepatitis A
- ALT
alanine aminotransferase
- CFSE
carboxyfluorescein succinimidyl ester
- HAV
hepatitis A virus
- ICS
intracellular cytokine staining
- IFN
interferon
- IL
interleukin
- LIL
liver-infiltrating lymphocyte
- OLP
overlapping peptide
- PBMC
peripheral blood mononuclear cell
- TCR
T cell receptor
- Th
helper T cell
- TNF
tumor necrosis factor
- Treg cells
regulatory T cells
- Tresp
responder T cell
- TSDR
Treg-specific demethylated region
- 7-AAD
7-aminoactinomycin D
Footnotes
Disclosures:
The authors have nothing to disclose.
Author Contributions:
YSC, MKJ, JL, SJC, SHC, HWL, JJL, HJK, SHA, DHL, WK, SHP, JRH, and HPK were involved in the acquisition of data. YSC, JL, and JYP were involved in the analysis and interpretation of data. YSC, JYP, and ECS contributed to the conceptual design of the study and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14:38–58. doi: 10.1128/CMR.14.1.38-58.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco E, Meleleo C, Serino L, et al. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73. doi: 10.4254/wjh.v4.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koff RS. Hepatitis A. Lancet. 1998;351:1643–1649. doi: 10.1016/S0140-6736(98)01304-X. [DOI] [PubMed] [Google Scholar]
- 4.Walker CM, Feng Z, Lemon SM. Reassessing immune control of hepatitis A virus. Curr Opin Virol. 2015;11:7–13. doi: 10.1016/j.coviro.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43:S164–172. doi: 10.1002/hep.21052. [DOI] [PubMed] [Google Scholar]
- 6.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 11.Viglietta V, Baecher-Allan C, Weiner HL, et al. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M, Gorochov G, Ehrenstein M, et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Choi YS, Lee J, Lee HW, et al. Liver injury in acute hepatitis A is associated with decreased frequency of regulatory T cells caused by Fas-mediated apoptosis. Gut. 2015;64:1303–1313. doi: 10.1136/gutjnl-2013-306213. [DOI] [PubMed] [Google Scholar]
- 16.Manangeeswaran M, Jacques J, Tami C, et al. Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans. Gastroenterology. 2012;142:1516–1525. e1513. doi: 10.1053/j.gastro.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohlfert E, Belkaid Y. Plasticity of Treg at infected sites. Mucosal Immunol. 2010;3:213–215. doi: 10.1038/mi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Vignali DA, Rudensky AY, et al. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 19.Jung MK, Kwak JE, Shin EC. IL-17A-Producing Foxp3+ Regulatory T Cells and Human Diseases. Immune Netw. 2017;17:276–286. doi: 10.4110/in.2017.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovhannisyan Z, Treatman J, Littman DR, et al. Characterization of interleukin -17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Sun X, Zhao J, et al. Regulatory T cells in rheumatoid arthritis show ed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–1301. doi: 10.1136/annrheumdis-2013-204228. [DOI] [PubMed] [Google Scholar]
- 24.Koch MA, Tucker-Heard G, Perdue NR, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Sharma S, Edwards J, et al. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol. 2015;16:197–206. doi: 10.1038/ni.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang BH, Hagemann S, Mamareli P, et al. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016;9:444–457. doi: 10.1038/mi.2015.74. [DOI] [PubMed] [Google Scholar]
- 27.Oldenhove G, Bouladoux N, Wohlfert EA, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh JR, Englund EE, Wang H, et al. Identification of potent and selective dip henylpropanamide ROTγ inhibitors. ACS Med Chem Lett. 2013;4:79–84. doi: 10.1021/ml300286h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)- 2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polansky JK, Kretschmer K, Freyer J, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD 39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moncrieffe H, Nistala K, Kamhieh Y, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct population s, one regulatory and one memory T cell population. J Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zganiacz A, Santosuosso M, Wang J, et al. TNF-α is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubtsov YP, Niec RE, Josefowicz S, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyao T, Floess S, Setoguchi R, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GAT A-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlfert EA, Grainger JR, Bouladoux N, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.