Abstract
BACKGROUND
Babesiosis is a potentially life-threatening zoonotic infection most frequently caused by the intraerythrocytic parasite Babesia microti. The pathogen is usually tickborne, but may also be transfusion or vertically transmitted. Healthy persons, including blood donors, may be asymptomatic and unaware they are infected. Immunocompromised patients are at increased risk for symptomatic disease.
STUDY DESIGN AND METHODS
All reported community-acquired babesiosis cases in New York from 2004 to 2015 were evaluated, enumerated, and characterized. All potential transfusion-transmitted babesiosis (TTB) cases reported through one or more of three public health surveillance systems were investigated to determine the likelihood of transfusion transmission. In addition, host-seeking ticks were actively collected in public parks and other likely sites of human exposure to B. microti.
RESULTS
From 2004 to 2015, 3799 cases of babesiosis were found; 55 (1.4%) of these were linked to transfusion. The incidence of both community-acquired and TTB increased significantly during the 12-year study period. The geographic range of both ticks and tickborne infections also expanded. Among TTB cases, 95% of recipients had at least one risk factor for symptomatic disease. Implicated donors resided in five states, including in 10 New York counties. More than half of implicated donors resided in counties known to be B. microti endemic.
CONCLUSION
The increasing incidence of TTB correlated with increases in community-acquired babesiosis and infection of ticks with B. microti. Surveillance of ticks and community-acquired cases may aid identification of emerging areas at risk for Babesia transfusion transmission.
Keywords: Babesiosis, tickborne disease, transfusion-transmitted infection
INTRODUCTION
Babesiosis is a zoonotic infection caused by the tickborne intra-erythrocytic protozoan parasite Babesia.1 In the United States (U.S.), human babesiosis is attributed primarily to infection with B. microti, which is endemic to portions of the Northeast and upper Midwest.2-5 Seven states have historically been considered endemic: Connecticut, Rhode Island, New York, Massachusetts, New Jersey, Minnesota, and Wisconsin. In addition, we consider Maine and New Hampshire endemic based on recent published literature and Pennsylvania has seen increasing incidence and some endemic foci.6-10 Distribution is variable; endemic states have hyperendemic foci and areas at much lower risk. Babesiosis can also be caused by other Babesia species, such as B. duncani on the West Coast of the U.S.11-14
B. microti is usually transmitted to humans, as an incidental host, by the black-legged tick, Ixodes scapularis. The organism is maintained naturally through an enzootic reservoir, primarily the white-footed mouse (Peromyscus leucopus), while deer can nourish and transport adult ticks to expanding geographic areas.15,16 Tick larvae acquire the organism via a blood meal from an infected rodent host and continue to carry the parasite as they develop into nymphs (approximately the size of a poppy seed), the stage most likely to transmit the organism to humans. In addition to tickborne transmission, B. microti may be transmitted by blood transfusion from asymptomatic blood donors.17 Vertical transmission has also been reported rarely.18 The parasite invades erythrocytes, resulting in an infection that can range from asymptomatic to severe disease. Symptomatic disease presents most commonly with fever, chills, malaise, myalgia, gastrointestinal symptoms, and/or hemolytic anemia.19 Fulminant disease can manifest with disseminated intravascular coagulation, hemodynamic instability, and potentially fatal multi-organ dysfunction.19
The first case of human babesiosis in New York was identified in 1975, and the disease was designated a reportable communicable disease by the New York State Department of Health in 1986.20 Shelter Island and the South Fork of Long Island, east of the Shinnecock Canal, have been and continue to be hyperendemic foci. However, cases have been reported in larger numbers and in expanding geographic areas. Outside New York, other high-incidence areas include coastal regions of southern New England, especially all coastal regions of Connecticut, Martha’s Vineyard and Nantucket Island in Massachusetts, and Block Island in Rhode Island. Babesiosis became nationally notifiable in 2011, meaning that, in states in which babesiosis is a reportable communicable disease (31 as of 2014), the state health department is expected to report confirmed cases to the Centers for Disease Control and Prevention (CDC) in a non-identifying fashion, for surveillance purposes, using a standardized case definition.21 To be classified as confirmed, a case of babesiosis requires symptoms and supportive laboratory findings. However, a blood donor may be considered implicated even if confirmed case criteria are not met.
The incidence of transfusion-transmitted babesiosis (TTB) in the U.S. is increasing. A 2011 report identified 159 cases of TTB attributed to B. microti from 1979 to 2009, with the majority of them (77%) occurring between 2000 and 2009.17 TTB cases have been linked to red blood cells (RBCs), frozen deglycerolized RBCs, and whole blood-derived platelet concentrates (presumably from residual red cells).17,22 There have been no confirmed reports of cases associated with fresh frozen plasma, plasma frozen within 24 hours, apheresis platelets, or cryoprecipitate. B. microti is currently the most common red blood cell transfusion-transmitted pathogen reported to the U.S. Food and Drug Administration (FDA) through the Biological Product Deviations Reporting (BPDR) system, accounting for 20/26 (77%) of reports of pathogen transmission in Fiscal Year (FY) 2015.23 Babesia was linked to three of five reports of fatalities in the U.S. attributed to RBCs contaminated with an infectious agent in FY 2011 to 2015.24
MATERIALS AND METHODS
Case Finding
This is a retrospective study of reported community-acquired babesiosis and TTB cases that occurred in New York from 2004 to 2015. For TTB cases, the date of exposure was based on the date of implicated transfusion. The primary data source was public health records. Physicians are required to report babesiosis cases to the local health department of the patient’s county of residence. The public health surveillance investigation process includes two questions regarding possible transfusion or transplantation within the previous six months. Cases with an affirmative response to either question are referred to the blood and tissue oversight program for investigation. Also, laboratories must report positive Babesia-related test results to the State Department of Health using an electronic reporting system. In addition, babesiosis cases that may be transfusion transmitted are reportable directly to the blood and tissue oversight program. Case investigation included assessing timeframes and recipient medical circumstances, donor laboratory testing, and follow-up interviews. If a blood component and donor were implicated, efforts were made to identify recipients of co-components from the implicated donation and previous recipients of blood components from the implicated donor.
Case Criteria and Classification
We used the following case criteria to determine Babesia infection in patients: Diagnosed Babesia infection confirmed by laboratory testing. Parasitologic evidence included observation of Babesia organisms on peripheral blood smear or detection of Babesia deoxyribonucleic acid (DNA) by a molecular method. Serologic evidence included positive results (titer ≥ 256) by indirect fluorescent antibody (IFA) or Immunoglobin G (IgG) immunoblot. Additional criteria for TTB were: 1) receipt of a blood component within a plausible time frame and 2) absence of evidence that another route was more likely than transfusion. A TTB case was considered definite if an extant segment demonstrated evidence of infection. A TTB case was considered probable if linked, in a plausible time frame, to a donor found positive by nucleic acid testing or a titer ≥ 64 on serologic testing on a specimen collected subsequently. A TTB case was considered possible if no donor was implicated, but it was not possible to test all donors, and the patient had no other plausible risk. If all associated donors were seronegative or the patient’s infection pre-dated any transfusion(s), the case was excluded as transfusion transmitted. Cases not linked to transfusion or transplantation were considered community acquired.
Tick Surveillance
Host-seeking Ixodes scapularis ticks were collected using standardized drag surveys, conducted during peak tick activity periods in New York (May–July and October–December) as described.20 Sites included publicly accessible lands across New York, primarily parks and nature preserves, considered to be at high risk of human exposure to B. microti due to the presence of suitable tick and small mammal habitat and recreational trails. Following species confirmation, individual I. scapularis ticks were homogenized and tested for B. microti DNA by polymerase chain reaction (PCR).25
Data Collection and Analysis
Data on community-acquired cases, including geographic location, were obtained through investigation of reported cases of the disease and laboratory reporting of positive test results indicative of infection. The cases determined to be confirmed were enumerated, characterized, and stratified by county of patient residence for each year. The four highest incidence counties were identified for further analysis. The community-acquired group was stratified by age and sex.
For each reported potential TTB case, we obtained and evaluated the recipient’s clinical history and course, including outcome, and laboratory test results, including consideration of date(s) of pertinent transfusion(s) and type(s) of component(s) received. Following identification of potentially implicated transfusion(s), we determined whether any extant segment(s) from pertinent unit(s) were available for testing, along with any possible extant co-components in storage or units donated subsequently by a suspect donor. If no other sample was available, suspect donors were contacted and asked to provide a new sample for testing. Testing, performed or confirmed at the state public health laboratory, included IFA and PCR. When an implicated unit and donor were identified, the following data were determined and recorded in a database: 1) date of implicated donation, 2) type of component transfused, 3) interval between donation and transfusion, 4) interval between transfusion and development of recipient symptoms, and 5) implicated donor demographics, including the counties of residence and of donation. In our analysis, we considered cases in which transfusion transmission was excluded and those that met only possible criteria not to be transfusion transmitted. We stratified the implicated donor group by age and sex.
Ticks testing positive for B. microti were enumerated by county of collection. We calculated county-level prevalence rates by determining the mean percentage of ticks infected with B. microti. Tick density was summarized as the average number of nymphs collected per man-hour at the site and county levels. Site-and county-level Entomologic Risk Indices (ERI) were calculated as a product of nymphal tick density and B. microti prevalence in host-seeking nymphs, as described.26
Statistical Analysis
We calculated incidence rates as the number of babesiosis cases per 100,000 population per year. We employed linear regression to analyze trends over time. Sex differences between TTB patients and donors were compared using chi-square comparisons with Bonferroni adjustment. Spearman correlation tests were used to explore the relationship between ERI and incidence of babesiosis at the county level. Data analyses were performed using SAS v9.4 software (SAS Institute Inc., Cary, NC, USA). A p-value of <0.05 was considered significant in this analysis. Incidence rates and pathogen prevalence in host-seeking nymphal ticks were mapped using ArcGIS version 10.4 (ESRI, Redland, CA).
RESULTS
A total of 3799 cases of babesiosis were identified in New York State from 2004 to 2015. There was a significant increase in the number of community-acquired cases over the study period, with 91 cases reported in 2004 and 576 reported in 2015 (R2=0.8180, p<0.0001). There were 55 (1.4%) definite or probable TTB cases. TTB cases increased from two in 2004 to eight in 2015 (R2=0.4134, p=0.0241) (Figure 1). Tick surveillance data demonstrated a similar increase over time. Figure 2 displays the prevalence of B. microti in I. scapularis nymphs from 2004 to 2015, along with the incidence of human babesiosis.
Figure 1.
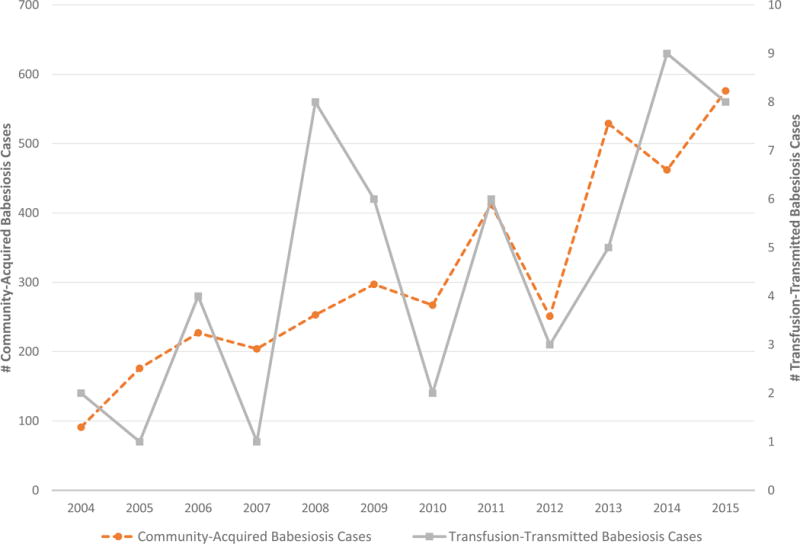
Number of community-acquired and transfusion-transmitted babesiosis cases, 2004-2015
Figure 2.
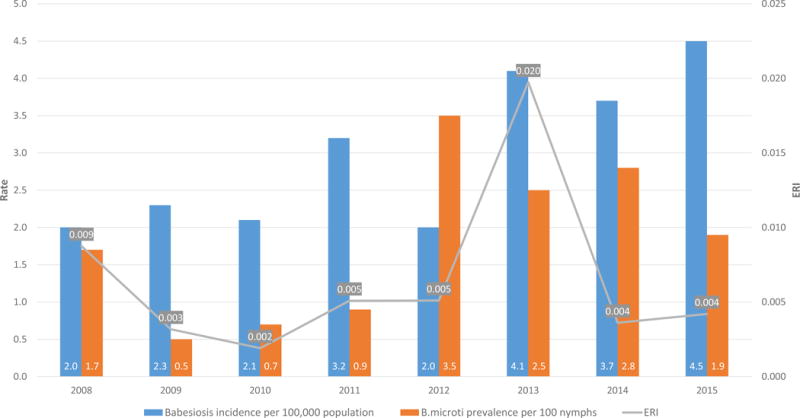
Incidence rate (per 100,000 population) of human babesiosis, prevalence of B. microti in host-seeking I. scapularis nymphs, and Entomological Risk Index (ERI) in New York State, 2008-2015
Of the 55 TTB cases included in the analysis, four met the criteria for definite TTB based on testing of an extant segment. One segment was positive by PCR; the other three were positive by IFA, with titers of 1024, 1024, and 256, respectively. The other 51 cases met criteria as probable TTB. In addition, 10 reported potential cases were excluded as TTB (most frequently when a retrospective review of blood smears demonstrated B. microti parasites prior to any transfusion) and 26 cases meeting only the definition of possible TTB were identified. These 36 were not included in the analysis of TTB cases, but were included in “all” cases.
In 54 TTB cases, the implicated component was RBCs; in the other case, a unit of frozen deglycerolized RBCs was implicated. There were two clusters; in each, blood collected from the same donor on two different occasions resulted in infection in two recipients. In one cluster, two units of RBCs donated by a single donor, 56 days apart, were each implicated in transmission of Babesia to a (different) recipient. In the other cluster, two units of RBCs donated 69 days apart were each implicated in transmission to a (different) recipient. The mean observed incubation period from date of implicated transfusion to onset of symptoms was 35.9 days (median 34, range 8-91 days). The mean maximum parasitemia, when known, in TTB cases was 6.1% (median 5%, range 0.01-29%).
For both all cases and TTB cases, the patient age and sex distributions are shown in Table 1. TTB cases accounted for 9.2% of cases among children up to age 19, and 31% of all patients less than 5 years of age. While there was an even distribution of TTB cases across the sexes (49% male, 51% female), the distribution of all reported babesiosis cases was more heavily skewed towards males (62% male, 38% female). Although the difference was not statistically significant (X2=3.8213, p=0.0506), it is meaningful in that it highlights the difference between the sex distribution of TTB versus all cases. The implicated donors in the TTB cases were more likely to be male (84% male, 16% female), compared to the overall donor population, which is more evenly split between the sexes (57% male, 43% female). Comparison of the sex distribution of the three groups specific to this study, TTB cases, community-acquired babesiosis cases, and implicated donors, showed a statistically significant difference (X2=14.77, p=0.0006).
Table 1.
Age and sex distribution of transfusion-transmitted and community-acquired babesiosis cases and sex distribution of implicated donors.
| Age (years) | All Babesiosis Cases n (%) |
TTB* Cases n (%) |
% TTB Cases |
|---|---|---|---|
| <1 | 7 (0.2) | 2 (3.6) | 28.6 |
| 1-4 | 6 (0.2) | 2 (3.6) | 33.3 |
| 5-19 | 85 (2.2) | 5 (9.1) | 5.9 |
| 20-29 | 99 (2.6) | 3 (5.5) | 3.0 |
| 30-39 | 238 (6.3) | 4 (7.3) | 1.7 |
| 40-49 | 488 (12.8) | 9 (16.4) | 1.8 |
| 50-59 | 809 (21.3) | 4 (7.3) | 0.5 |
| 60+ | 2063 (54.3) | 26 (47.3) | 1.3 |
| Unknown | 4 (0.1) | 0 (0.0) | 0.0 |
| Sex |
All Babesiosis Cases n (%) |
TTB Cases n (%) |
Implicated Donors n (%) |
| Male | 2355 (62.0) | 27 (49.1) | 46 (83.6) |
| Female | 1444 (38.0) | 28 (50.9) | 9 (16.4) |
| Total | 3799 | 55 | 55 |
Transfusion-Transmitted Babesiosis cases
Figure 3 shows all implicated donations for years 2004-2015 stratified by month of donation. Month of implicated donation was relatively evenly distributed over the 12 years studied (data not shown). A large proportion (27/55, 49%) of implicated donations took place in July, August and September, consistent with exposure occurring during the known peak nymphal I. scapularis tick activity from mid-May through mid-August.6 In contrast, little activity was seen in March and April, providing only two (4%) of the total implicated donations over the 12-year study interval. However, it is important to note that at least one implicated donation took place in each month of the year.
Figure 3.
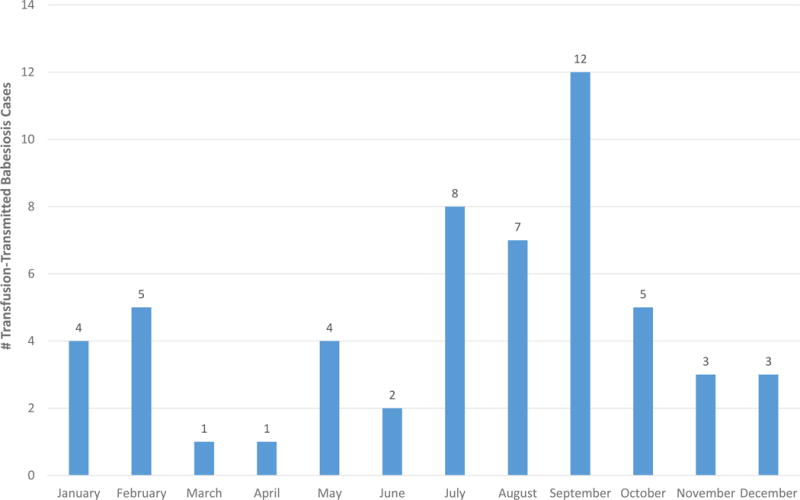
Distribution of TTB cases by month of implicated donation, 2004-2015
Entomologic Risk Index (ERI) is a measure of tickborne disease risk: the product of tick abundance and pathogen prevalence in host-seeking ticks from a given location. Babesiosis incidence rates per 100,000 population and B. microti ERI, by New York county, for years 2004, 2008, 2012, and 2015 are shown in Figure 4. The increase in incidence across the counties in the Downstate and Lower- to Mid-Hudson Valley regions can be seen in the time progression displayed, with a corresponding increase in B. microti ERI. Three counties - Columbia, Dutchess, and Suffolk - saw statistically significant increases in incidence over the 12-year study period (Figure 5): Columbia County’s incidence increased from 0.0 to 37 cases per 100,000 population from 2004 to 2015 (R2=0.6634, p=0.0013); Dutchess County’s incidence increased from 2.4 to 25.3 cases per 100,000 population (R2=0.4385, p=0.0190); Suffolk County’s incidence increased from 4.0 to 14.2 cases per 100,000 population (R2=0.7980, p<0.0001). Westchester County’s incidence increased from 1.0 to 5.8 cases per 100,000 population during this period, although this increase was not statistically significant. New York State also saw a significant increase from 0.5 to 3.0 cases per 100,000 population from 2004 to 2015 (R2=0.8136, p<0.0001). During the study period, there was a positive association between 1) nymphal tick density and human babesiosis incidence (r= 0.58, p<0.0001), 2) prevalence rates of B. microti in host-seeking nymphs and human babesiosis incidence (r= 0.57, p<0.0001), and 3) B. microti ERI and human babesiosis incidence (r= 0.58, p<0.0001) (Table 2).
Figure 4.
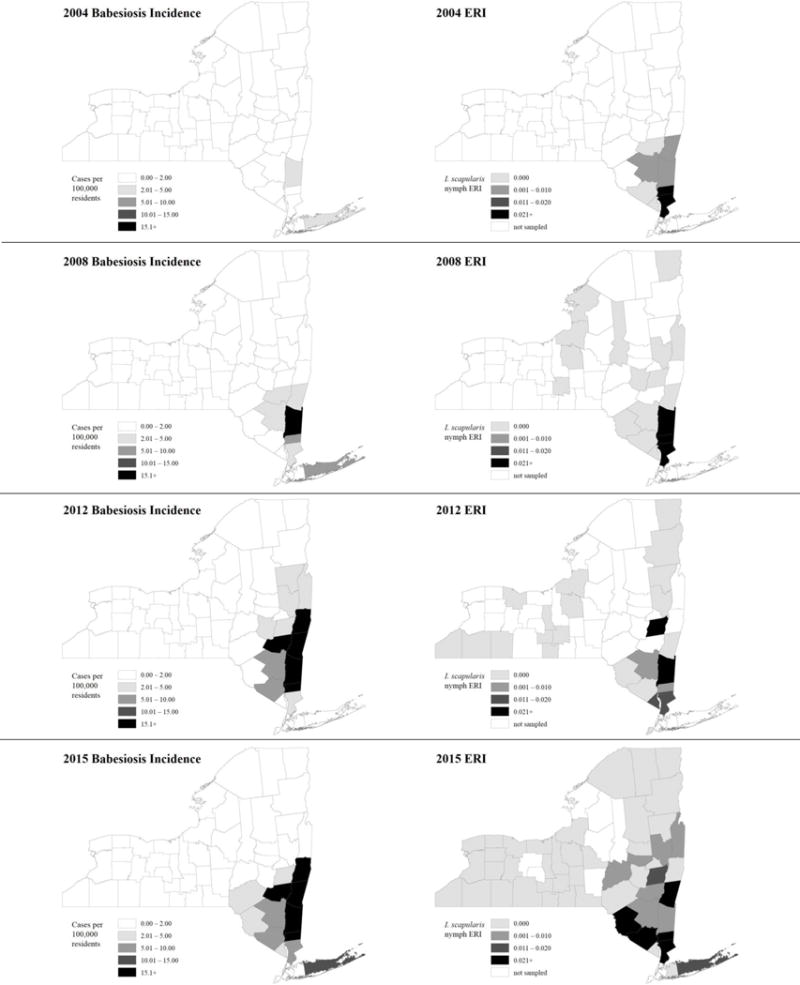
County-level incidence rates (per 100,000 population) of human babesiosis (shown left) and B. microti Entomological Risk Indices (shown right), 2004, 2008, 2012 and 2015
Figure 5.
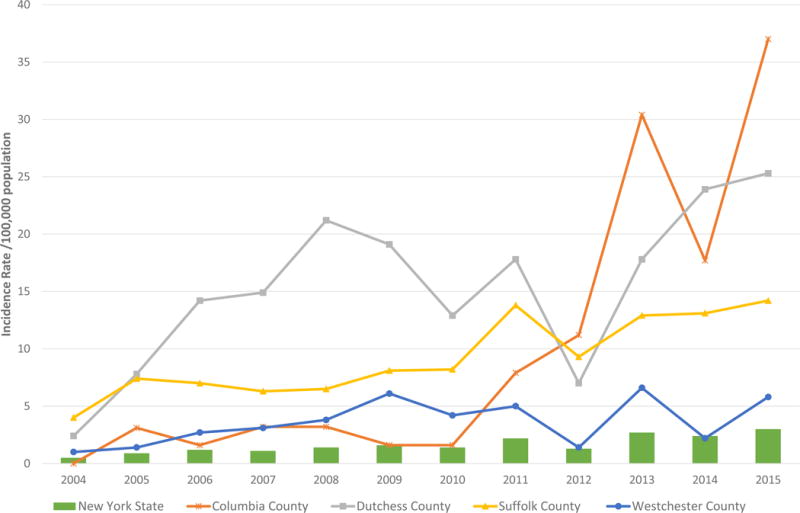
Babesiosis incidence rates (per 100,000 population) in New York State and four highest incidence counties, 2004-2015
Table 2.
Association between B. microti Entomological Risk Index (ERI) and human babesiosis incidence rates in New York State, 2008-2015.
| Year | Number of counties | Mean nymph density (ticks/m) | Mean ERI* | Mean Babesiosis incidence | Spearman correlation | P-value |
|---|---|---|---|---|---|---|
| 2008 | 18 | 0.466 | 0.009 | 2.283 | 0.681 | 0.0019 |
| 2009 | 23 | 0.428 | 0.003 | 1.530 | 0.502 | 0.0146 |
| 2010 | 21 | 0.207 | 0.002 | 1.681 | 0.472 | 0.0308 |
| 2011 | 20 | 0.424 | 0.005 | 2.590 | 0.572 | 0.0084 |
| 2012 | 24 | 0.191 | 0.005 | 1.625 | 0.699 | 0.0001 |
| 2013 | 32 | 0.426 | 0.020 | 4.281 | 0.776 | <0.0001 |
| 2014 | 54 | 0.135 | 0.004 | 2.367 | 0.612 | <0.0001 |
| 2015 | 50 | 0.189 | 0.004 | 3.532 | 0.681 | <0.0001 |
Entomological Risk Index; the product of B. microti prevalence in host-seeking ticks and tick abundance.
All implicated donors resided in the northeastern region of the U.S., with a majority (30/55, 55%) residing within one of the four known highest-incidence counties (of 62) in New York (Suffolk, Westchester, Dutchess, and Columbia Counties). In addition, one upstate donor had traveled to Connecticut, seven implicated units were collected in New Jersey, and blood units imported from out-of-state blood centers had been collected in Massachusetts (3), Rhode Island (1), or Pennsylvania (1).
All except two (5%) of the reported TTB patients had at least one established risk factor for symptomatic babesiosis among their age or underlying comorbidity(ies). Although more than half (28/55, 51%) of affected recipients were either 60 years of age or older (26, 48%) or less than one year of age (two, 4%), the most notable risk factor was asplenia (17/55, 32%). Of the 17 patients who had undergone splenectomy, 11 (65%) had a hemoglobinopathy (β thalassemia or sickle cell disease). In addition, two spleen-intact patients had sickle cell disease, in which functional hyposplenism is common.27 Other identified risk factors included treatment for a malignancy or other immunosuppression (11, 20%), and history of hematopoietic progenitor cell (HPC) or solid organ transplant (two and three patients, respectively). One patient (2%) had three risk factors, eight (15%) had two risk factors, and 44 (80%) had one risk factor. The two cases (4%) not linked to a recognized risk factor included a 58-year-old with liver disease and a 50-year-old with iron deficiency anemia. Two patients (4%) died of Babesia infection in the context of their underlying comorbidities: a woman in her 90s with gastrointestinal bleeding and a man in his 60s status post hip surgery complicated by infection.
DISCUSSION
This study identified 3799 babesiosis cases in New York from 2004 to 2015, of which 55 (1.4%) were linked to transfusion. More than 200 TTB cases have been reported in the U.S.9,10,17, 28 While the incidence per population is higher in Connecticut and Rhode Island, a significant portion of TTB cases have occurred in New York – 105 definite, probable or possible cases to date – more than twice as many as in any other state.17 In this series, all implicated donors resided in one of nine states now considered endemic or in Pennsylvania, which is recognized as having emerging B. microti.7 More than half (56%) resided in one of four known highly endemic counties in New York. Babesia seroprevalence in blood donors has been found to be as high in 4.3% in hyperendemic foci in New York29 and 3.0% in coastal Connecticut.30
Temporal and geographic changes in the incidence of community-acquired babesiosis and TTB, as well as tick activity, demonstrated oscillations between years. For example, 2012 was a low year for both tick activity and human cases in New York, as well as in the U.S.6 Annual fluctuations in human babesiosis may be due to fluctuations in I. scapularis density. Field studies have described annual oscillations of I. scapularis nymph populations, attributed to the influence of multiple biotic and abiotic factors.31,32 Such temporal fluctuations in tick density have occurred in parallel with fluctuations in annual human Lyme disease incidence,32,33 and it is likely that the incidence of babesiosis is also influenced by fluctuations in tick density. An ERI based upon the number of I. scapularis ticks infected by Borrelia burgdorferi in a 12-town area of Connecticut correlated highly with the incidence of Lyme disease in the same area.32 Site-level ERI values have been found to be a significant predictor of the total number of reported cases of babesiosis by municipality.32 In the current study, a strong positive relationship between ERI and human babesiosis incidence rates suggests that the ERI is predictive of human babesiosis risk in New York. ERI appears to be useful for assessing human risk of babesiosis, which could be an important factor in planning interventional strategies to reduce disease risk effectively.
The vast majority (95%) of TTB patients identified in this study had at least one identified risk factor for symptomatic disease; in two patients (4%), their Babesia infection proved fatal. The median incubation period from implicated transfusion to development of symptoms was 34 days, consistent with other studies.17
Limitations of the study include reliance on passive reporting of cases, although three avenues of reporting were in place. Passive reporting relies on a patient being diagnosed with clinical babesiosis, so these numbers do not reflect asymptomatic cases. In addition, collection of host-seeking I. scapularis nymphs over-selected sites of possible emerging tick activity from 2008 to 2015, rather than known hyperendemic foci sampled in 2004, and may thus underestimate abundance of infected ticks on a statewide level.
TTB is a serious problem that can result in morbidity and mortality in transfusion recipients. The data herein present a comprehensive longitudinal statewide compilation of reported babesiosis cases, and show increasing frequency and geographic expansion, corresponding with an expansion of B. microti-infected ticks, over the 12-year period in a Babesia-endemic state. Blood donor testing in some highly endemic areas has been initiated by some blood collection organizations under investigational product-release testing protocols. Efficacy of such screening in reducing the risk of TTB has been demonstrated.9,10 However, no licensed test for donor screening is available and investigational testing is not available in all areas. Also, testing cannot be relied on to detect all infectious donations, and temporal and geographic expansion may surpass testing availability. In addition, testing for evidence of B. microti infection may not detect other species of Babesia or other agents, such as Anaplasma phagocytophilum, which is carried by the same tick vector and can result in signs and symptoms very similar to those seen in babesiosis.
Public health efforts, including surveillance of community-acquired babesiosis and of tick abundance and infection, may be useful tools in identifying emerging areas of risk that could pose a hazard to transfusion recipients. Establishing communication channels with public health agencies could facilitate blood collection organizations’ abilities to tap such resources effectively to inform blood supply safety policies.
Acknowledgments
We thank Adam Rowe and Alexis Russell of the New York State Department of Health, Bureau of Communicable Disease Control for generating human incidence maps and updating statistics in Table 2 and for generating ERI maps, respectively. We also thank Dr. Erin Moritz of the American Red Cross, Biomedical Services, Scientific Affairs for initial advice on statistical analyses. This study was funded in part by U.S. National Institutes of Health, Grant AI097137.
Footnotes
COI : None
References
- 1.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 2.Krause PJ, McKay K, Gadbaw J, et al. the Tick-Borne Infection Study Group Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68:431–6. [PubMed] [Google Scholar]
- 3.Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843–7. doi: 10.3201/eid1705.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause PJ, Telford SR, III, Ryan R, et al. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29:1–4. doi: 10.1128/jcm.29.1.1-4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–54. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Surveillance for babesiosis - United States, 2014 annual summary. Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; 2016. Available at www.cdc.gov/parasites/babesiosis/resources/babesiosis_surveillance_summary_2016.pdf Accessed June 1, 2017. [Google Scholar]
- 7.Genda J, Negron EA, Lotfipour M, et al. Severe Babesia microti infection in an immunocompetent host in Pennsylvania. J Investig Med High Impact Case Rep. 2016;4 doi: 10.1177/2324709616663774. 2324709616663774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RP, Jr, Elias SP, Borelli TJ, et al. Human babesiosis, Maine, USA, 1995-2011. Emerg Infect Dis. 2014;12:1727–30. doi: 10.3201/eid2010.130938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moritz ED, Winton CS, Tonnetti L, et al. Screening for Babesia microti in the U.S. blood supply N Engl J Med. 2016;375:2236–45. doi: 10.1056/NEJMoa1600897. [DOI] [PubMed] [Google Scholar]
- 10.Moritz ED, Tonnetti L, Hewins ME, et al. Description of 15 DNA-positive and antibody-negative “window period” blood donations identified during prospective screening for Babesia microti. Transfusion. 2017;57:1781–6. doi: 10.1111/trf.14103. [DOI] [PubMed] [Google Scholar]
- 11.Quick RE, Herwaldt BL, Thomford JW, et al. Babesiosis in Washington State: a new species of Babesia? Ann Intern Med. 1993;119:284–90. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Herwaldt BL, Kjemtrup AM, Conrad PA, et al. Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J Infect Dis. 1997;175:1259–62. doi: 10.1086/593812. [DOI] [PubMed] [Google Scholar]
- 13.Kjemtrup AM, Lee B, Fritz CL, et al. Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion. 2002;42:1482–7. doi: 10.1046/j.1537-2995.2002.00245.x. [DOI] [PubMed] [Google Scholar]
- 14.Bloch EM, Herwaldt BL, Leiby DA, et al. The third described case of transfusion-transmitted Babesia duncani. Transfusion. 2012;52:1517–22. doi: 10.1111/j.1537-2995.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray J, Zintl A, Hildebrandt A, et al. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Hersh MH, Tibbetts M, Strauss M, et al. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18:1951–7. doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 18.Joseph JT, Purtill K, Wong SJ, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18:1318–21. doi: 10.3201/eid1808.110988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357–70. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kogut SJ, Thill CD, Prusinski MA, et al. Babesia microti, upstate New York. Emerg Infect Dis. 2005;11:476–8. doi: 10.3201/eid1103.040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Babesiosis (Babesia spp) 2011 case definition. Atlanta, Georgia: U.S. Department of Health and Human Services, CDC; 2011. Available at wwwn.cdc.gov/nndss/conditions/babesiosis/case-definition/2011/ Accessed June 1, 2017. [Google Scholar]
- 22.Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. FY 2015: Number of BPD reports by type of blood and plasma establishment. Available at http://FDA.gov/downloads/biologicsbloodvaccines/safetyavailability/reportaproblem/biologicalproductdeviations/ucm506090.pdf. Accessed June 12, 2017.
- 24.Food and Drug Administration. Fatalities reported to FDA following blood collection and transfusion annual summary for FY 2015. 2016 Available at FDA.gov/downloads/biologicsbloodvaccines/safetyavailability/reportaproblem/transfusiondonationfatalities/ucm518148.pdf. Accessed June 12, 2017.
- 25.Prusinski MA, Kokas JE, Hukey KT, et al. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J Med Entomol. 2014;51:226–36. doi: 10.1603/me13101. [DOI] [PubMed] [Google Scholar]
- 26.Mather TN, Nicholson MC, Donnelly EF, et al. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–9. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- 27.Nottage KA, Ware RE, Winter B, et al. Predictors of splenic function preservation in children with sickle cell anemia treated with hydroxyurea. Eur J Haematol. 2014;93:377–83. doi: 10.1111/ejh.12361. [DOI] [PubMed] [Google Scholar]
- 28.Fang DC, McCullough J. Transfusion-transmitted Babesia microti. Transfus Med Rev. 2016;30:132–8. doi: 10.1016/j.tmrv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Linden JV, Wong SJ, Chu FK, et al. Transfusion-associated transmission of babesiosis in New York State. Transfusion. 2000;40:285–9. doi: 10.1046/j.1537-2995.2000.40030285.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson ST, Cable RG, Tonnetti L, et al. Prevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through 2007. Transfusion. 2009;49:2574–82. doi: 10.1111/j.1537-2995.2009.02430.x. [DOI] [PubMed] [Google Scholar]
- 31.Falco RC, Daniels TJ, Fish D. Increase in abundance of immature Ixodes scapularis (Acari: Ixodidae) in an emergent Lyme disease endemic area. J Med Entomol. 1995;32:522–6. doi: 10.1093/jmedent/32.4.522. [DOI] [PubMed] [Google Scholar]
- 32.Stafford KC, III, Cartter ML, Magnarelli LA, et al. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Micro. 1998;36:1240–4. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White DJ, Chang HG, Benach JL, et al. The geographic spread and temporal increase of the Lyme disease epidemic. JAMA. 1991;266:1230–6. [PubMed] [Google Scholar]


