Abstract
Hypercoupling of activity in speech‐perception‐specific brain networks has been proposed to play a role in the generation of auditory‐verbal hallucinations (AVHs) in schizophrenia; however, it is unclear whether this hypercoupling extends to nonverbal auditory perception. We investigated this by comparing schizophrenia patients with and without AVHs, and healthy controls, on task‐based functional magnetic resonance imaging (fMRI) data combining verbal speech perception (SP), inner verbal thought generation (VTG), and nonverbal auditory oddball detection (AO). Data from two previously published fMRI studies were simultaneously analyzed using group constrained principal component analysis for fMRI (group fMRI‐CPCA), which allowed for comparison of task‐related functional brain networks across groups and tasks while holding the brain networks under study constant, leading to determination of the degree to which networks are common to verbal and nonverbal perception conditions, and which show coordinated hyperactivity in hallucinations. Three functional brain networks emerged: (a) auditory‐motor, (b) language processing, and (c) default‐mode (DMN) networks. Combining the AO and sentence tasks allowed the auditory‐motor and language networks to separately emerge, whereas they were aggregated when individual tasks were analyzed. AVH patients showed greater coordinated activity (deactivity for DMN regions) than non‐AVH patients during SP in all networks, but this did not extend to VTG or AO. This suggests that the hypercoupling in AVH patients in speech‐perception‐related brain networks is specific to perceived speech, and does not extend to perceived nonspeech or inner verbal thought generation.
Keywords: auditory‐verbal hallucinations, auditory oddball, connectivity, functional brain networks, speech perception
1. INTRODUCTION
Coordinated hyperactivity or hypercoupling1 in speech‐perception‐related brain regions has been implicated in the generation of auditory‐verbal hallucinations (AVHs) in schizophrenia. Studies examining functional brain activity during the experience of hallucinations have reported activation of language and auditory regions (e.g., Broca's area, middle/superior temporal gyri; Allen et al., 2012; Jardri, Pouchet, Pins, & Thomas, 2011), findings which are supported by network‐based connectivity analyses (Hoffman, Pittman, Constable, Bhagwagar, & Hampson, 2011b; Thoma et al., 2016). Trait studies, in which functional brain activity is compared between patients with and without a history of AVHs, have also demonstrated hypercoupling/coordinated hyperactivity within auditory/language networks in AVH patients at rest (Alderson‐Day et al., 2016; Shinn, Baker, Cohen, & Öngür, 2013); however, findings of task‐based trait studies are less consistent (Ćurčić‐Blake et al., 2017), with some reporting hypoactivity (Kompus, Westerhausen, & Hugdahl, 2011), suggesting interference between AVHs and external auditory processing (Hugdahl, 2015), and others reporting hyperactivity in similar regions (Hoffman, Fernandez, Pittman, & Hampson, 2011a; Lavigne et al., 2015b). These equivocal findings are likely due to differences in the tasks and statistical analysis techniques employed. In a previous study, we observed hypercoupling during speech perception (SP) in AVH patients relative to non‐AVH patients within a network of speech‐related brain regions (e.g., bilateral superior temporal gyri, left inferior frontal gyrus) consistent with symptom capture and resting‐state studies. This hypercoupling in AVH patients was not observed during inner verbal thought generation (VTG), suggesting that it is not present when control is exerted over verbal material, as with inner speech (Lavigne et al., 2015b; Rapin et al., 2012).
Several theoretical accounts of AVHs also point to hyperactivity in speech‐related brain regions as a contributing factor. Ford and Hoffman (2013) and Hoffman et al. (2011a) proposed that spontaneous activation of verbal imagery results in AVHs due to a hyperconnected corticostriatal loop (left inferior frontal gyrus, Wernicke's area and right homologue, and bilateral putamen) in combination with top–down factors in the form of efference copy (Ford, Roach, Faustman, & Mathalon, 2007). Several other theories highlight the importance of hypersensitivity of auditory cortex as a bottom–up process involved in the generation of AVHs, either as a primary feature (e.g., breakaway speech/unbidden thoughts; Hoffman, 1999, 2010), or as a factor involved in the interplay between top–down and bottom–up processes (Aleman and Vercammen, 2013; Ćurčić‐Blake et al., 2017).
Given the verbal nature of most auditory hallucinations, this hyperactivity may be specific to perception of verbal material. This should be tested by directly comparing functional brain activity between patients with and without AVHs during contrasting verbal and nonverbal auditory perception tasks. In this study, we investigated this by combining already‐available data: one set from our laboratory involving the verbal sentence task (SP and VTG conditions) described above (Lavigne et al., 2015b), and another from the publicly available Function Biomedical Informatics Research Network (fBIRN) phase II multisite study involving a nonverbal auditory oddball (AO) task (Friedman et al., 2008; Keator et al., 2008). The auditory oddball task is commonly used in schizophrenia research on attention and salience detection (Kim, 2014), and is a theoretically interesting comparison condition because it requires auditory perception in the absence of a verbal component. That is, there are two aspects of speech perception that might contribute to hyperactivity in auditory (and language) regions in AVHs, the verbal component and the auditory component. VTG includes the verbal, but not auditory, component, whereas AO includes the auditory, but not verbal, component of SP. Simultaneous investigation of these three tasks provides a means of examining the speech‐specificity of hyperactivity in auditory and language networks in AVHs.
We have previously published analyses of these two datasets separately (Lavigne, Menon, & Woodward, 2016; Lavigne et al., 2015b), and found an association with hallucinations for the verbal speech perception task (Lavigne et al., 2015b), but not the nonverbal AO task (Lavigne et al., 2016), providing preliminary support for the proposition that hyperactivity is specific to verbal material. However, comparing these two results in this indirect fashion is not conclusive because different brain networks emerged in the two studies. Ideally, the brain networks under study would be held constant, and activation in the associated hemodynamic response (HDR) shapes would be compared between tasks and groups. We have previously developed methodology to do this (Lavigne, Metzak, & Woodward, 2015a), but in that work compared two versions of the same task, not two tasks with different perceptual content.
This task‐combination methodology and patient‐group comparison involved using group constrained principal component analysis for functional magnetic resonance imaging (group fMRI‐CPCA; Hunter and Takane, 2002; Metzak et al., 2011; Takane and Shibayama, 1991; Woodward et al., 2015; Woodward, Leong, Sanford, Tipper, & Lavigne, 2016) on the combined dataset. This allows identification of the degree to which functional brain networks are involved in all task conditions, provides spatial and temporal information for each network, and allows statistically‐based group comparisons of HDR response shapes. For this study, based on our past work (Lavigne et al., 2016, 2015b), we expected to identify auditory and language processing networks, and hypothesized that schizophrenia patients with AVHs, relative to those without and healthy controls, would show hypercoupling in both of these networks for SP, but not for VTG (no overt language perception) or AO (nonverbal material), which would confirm that hyperactivity specific to perceived speech‐related brain regions plays a role in AVHs in schizophrenia.
2. METHODS
2.1. Participants
Participants were schizophrenia patients and healthy controls from two functional magnetic resonance imaging (fMRI) datasets. The first dataset consisted of 27 healthy controls, 11 non‐AVH, and 12 AVH schizophrenia patients who performed a verbal sentence task including speech perception and inner verbal thought generation conditions (Lavigne et al., 2015b). Mean illness duration was 14.05 years (SD = 10.14), and all but one patient was currently taking antipsychotic medication. Nineteen patients were taking an atypical antipsychotic, and two were taking a typical antipsychotic, as their primary medication. Fourteen patients were also taking a second antipsychotic medication (2 typical, 12 atypical), and four were taking a third atypical medication (1 missing data). Chlorpromazine equivalent dosages were available for 14 patients: mean = 1,018.57; SD = 2,402.92. Symptoms were assessed using the Signs of Symptoms of Psychotic Illness (SSPI; Liddle, Ngan, Duffield, Kho, & Warren, 2002), which is scored on a 5‐point scale corresponding to severity of hallucinations (0 = absent, 1 = vague descriptions of hallucinations, 2 = hallucinations that the patient accepts as arising from within his/her own mind, 3 = definite hallucinations occurring occasionally [e.g., < once/day], 4 = definite hallucinations that are frequent and/or influence observable behavior). Patients scoring >2 on the hallucinations item were included in the AVH group. All patients experienced AVHs (i.e., voices), though two also experienced other auditory hallucinations, such as auditory distortions, simple noises, or music. Six patients also experienced multimodal hallucinations (6 tactile, 3 visual, 1 olfactory). All participants were screened for MRI compatibility and gave written informed consent prior to participation. Experimental procedures were approved as part of a larger study by the University of British Columbia clinical research ethics board.
The second dataset was acquired from the publicly available fBIRN phase II multisite study (Friedman et al., 2008), which consists of data collected at six sites across the United States of America: Duke/UNC, Brigham and Women's Hospital, Massachusetts General Hospital, University of California – Irvine, University of New Mexico, and Yale. Data were downloaded from the fBIRN Data Repository, Project Accession Number 2007‐BDR‐6UHZ1. The quality‐controlled data set (see Lavigne et al., 2016) included 50 healthy controls, 23 non‐AVH, and 35 AVH patients diagnosed with schizophrenia or schizoaffective disorder who completed an auditory oddball task. Patients' symptoms were assessed using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), which is scored on a six‐point scale from 0 to 5 in increasing severity: 0 = none; 1 = questionable; 2 = mild (noises or single words, which only appear occasionally); 3 = moderate (clear evidence of voices occurring at least weekly); 4 = marked (clear evidence of voices occurring almost daily); 5 = severe (voices often occur daily). As with the sentence task sample, patients scoring >2 on the hallucinations item were included in the AVH group. Observation of scores on the SAPS items Voices Commenting and Voices Conversing confirmed that most hallucinating patients experienced AVHs; however, the presence of AVHs could not be confirmed in eight patients who reported auditory hallucinations, as the AVH‐specific items were not endorsed or were scored below the same cutoff value of 2. Nineteen patients also reported multimodal hallucinations (10 somatic‐tactile, 6 olfactory, 13 visual). All patients were stable and had no changes in their medications in the two months prior to testing; however, additional information regarding medication and illness history was not available, which precluded us from comparing the two samples on these measures. All fBIRN sites received local Institutional Review Board approval.
Table 1 shows the demographic information for each group, for the auditory oddball and sentence tasks separately. There were no significant differences between groups on age, gender, or handedness for either task.
Table 1.
Demographic information for each group and study
| Sentence task | Auditory oddball | |||||
|---|---|---|---|---|---|---|
| Control | Non‐AVH | AVH | Control | Non‐AVH | AVH | |
| Demographics | ||||||
| N | 27 | 11 | 12 | 50 | 23 | 35 |
| Gender (male:female) | 16:11 | 6:5 | 6:6 | 34:16 | 15:8 | 30:5 |
| Handedness (right:left) | 25:2 | 10:1 | 12 | 48:2 | 21:2 | 32:3 |
| Age (mean, SD) | 28.89 (8.98) | 35.45 (8.96) | 30.08 (9.72) | 34.88 (12.82) | 40.61 (13.43) | 35.57 (11.93) |
| Hallucinations type | ||||||
| Auditory‐Verbal | ‐ | ‐ | 12 | ‐ | ‐ | 35 |
| Somatic‐Tactile | ‐ | ‐ | 6 | ‐ | ‐ | 10 |
| Olfactory | ‐ | ‐ | 1 | ‐ | ‐ | 6 |
| Visual | ‐ | ‐ | 3 | ‐ | ‐ | 13 |
Note. Abbreviations: AVH = auditory verbal hallucinations; SD = standard deviation.
2.2. Experimental Design
2.2.1. Sentence task
Participants were presented with a noun (object) and its corresponding image (e.g., Table) and instructed to either listen to (speech perception condition; SP) or mentally generate (inner verbal thought generation condition; VTG) a simple definition of the word (e.g., Something you eat dinner on; see Figure 1a,b, respectively). The SP and VTG conditions were presented in blocks consisting of 15 trials each (30 trials total for each condition across two runs), with an intertrial interval (ITI) between stimuli, and a 60 s rest break between the two conditions. The ITI was exponential to optimize deconvolution of the blood oxygenation level dependent (BOLD) signal (Serences, 2004), and lasted from 2 to 20 s (mean = 4.46 s). Stimuli were randomly assigned to each condition for each participant separately. The conditions were cued with the words “listen…” and “something you…” presented under the images in the SP and VTG conditions, respectively, in order to ensure that at least some words were mentally generated on every trial, and to minimize any interpretational confounds between conditions. Following the experiment, participants were asked whether or not they experienced hallucinations during the scanning session. One patient reported active AVHs during the speech perception trials, and one patient reported tactile hallucinations during the thought generation trials (data were missing for two subjects).
Figure 1.
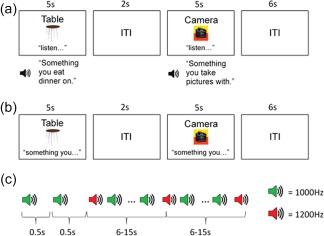
Timelines of the experimental paradigms. (a) Speech perception (SP). (b) Verbal thought generation (VTG). (c) Auditory oddball (AO) [Color figure can be viewed at http://wileyonlinelibrary.com]
2.2.2. fBIRN auditory oddball task
The two‐tone AO task (Figure 1c) involved listening to a series of tones and indicating with a button press when a target tone (i.e., a tone deviating in frequency) was presented. Four runs of the auditory oddball task were completed in each of two sessions, leading to a total of eight runs per subject that each lasted 280 s. Each run began with 15 s of silence, followed by a series of tones lasting 100 ms each, with 500 ms interstimulus intervals between them; a 15 s period of silence indicated the end of each run. The majority of the tones (standard tones; 95% occurrence) were presented at a frequency of 1,000 Hz and the remaining tones (target tones; 5% occurrence) were presented at a frequency of 1,200 Hz. The latency between two target tones varied between 6 and 15 s, allowing for deconvolution of the BOLD signal (Serences, 2004). Auditory stimuli were presented binaurally through headphones. Prior to the functional scan, participants adjusted right and left ear volumes to a test stimulus to ensure the tones could be heard over scanner noise. Participants were instructed to focus on a black fixation cross displayed on a grey screen throughout the run, and to respond with a button press when they heard the target tone. Stimuli were presented using E‐Prime software (http://www.pstnet.com/products/e-prime/). After the experiment, patients were asked whether they experienced hallucinations during the scanning session, with one patient reporting AVHs “almost constantly,” one “occasionally,” and one reporting visual hallucinations occasionally (data were missing for 11 subjects).
2.3. Image acquisition and processing
Imaging for the sentence task data was performed at the University of British Columbia MRI Research Centre using a Philips Achieva 3.0 Tesla (T) MRI scanner with quasar dual gradients (maximum gradient amplitude, 80 mT/m; maximum slew rate, 200 mT/m/s). The participant's head was firmly secured using a customized head holder. Functional image volumes were collected using a T2*‐weighted gradient‐echo spin pulse sequence with 36 axial slices; thickness/gap, 3/1 mm; matrix, 80 × 80; repetition time (TR), 2,500 ms; echo time (TE), 30 ms; flip angle (FA), 90°, field of view (FOV), 240 × 240 mm, effectively covering the whole brain. 352 images were acquired over two runs of ∼7 min and 30 s each.
Imaging for the fBIRN data was performed at five sites (one site was excluded during quality control; Lavigne et al., 2016) across the United States of America, for which imaging parameters were matched as closely as possible based on preliminary testing (Friedman et al., 2008; Magnotta and Friedman, 2006): 27 slices if possible; thickness/gap = 4 mm/1 mm; matrix = 64 × 64; repetition time (TR) = 2,000 ms; echo time (TE) = 30 ms (3 T)/40 ms (1.5 T); flip angle (FA) = 90°; field of view (FOV) = 22 cm; voxel dimensions = 3.4375 × 3.4375 × 4 mm. One site (Duke/UNC) employed a spiral echo sequence, while all other sites used a single‐shot EPI sequence. 140 volumes were collected in each run lasting 280 s.
Functional volumes for both datasets were pre‐processed using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging, UK). For each participant, each functional run was realigned, normalized to the Montreal Neurological Institute (MNI) EPI brain template (voxel size = 2 mm3), and spatially smoothed with an 8 mm full width at half maximum Gaussian filter. Following preprocessing, the SPM realignment parameters were examined, and any runs exceeding 3 mm translation or 3° rotation were excluded from further analysis. This led to the removal of 29 runs in the fBIRN sample. Additional quality control procedures for the fBIRN sample included removing runs for images that showed artifacts and/or led to errors during preprocessing, and for runs in which there were few responses or a high false positive rate, suggesting participants were not engaged in the task. Further information on this quality control can be found in previous work (Lavigne et al., 2016).
2.4. Data analysis
2.4.1. Group fMRI‐CPCA
fMRI data analysis was carried out using constrained principal component analysis for fMRI to compare groups (group fMRI‐CPCA) with orthogonal rotation (Metzak et al., 2011; Metzak et al., 2012; Woodward et al., 2006; Woodward, Feredoes, Metzak, Takane, & Manoach, 2013). The theory and proofs for CPCA are detailed in previously published work (Hunter and Takane, 2002; Takane and Hunter, 2001; Takane and Shibayama, 1991). The fMRI‐CPCA application is available on‐line, free of charge (http://www.nitrc.org/projects/fmricpca). fMRI‐CPCA computes (via PCA) components representing functional brain networks on blood‐oxygen level‐dependent (BOLD) signal for which variance has been constrained (via multivariate multiple regression) to that predictable from task timing, and provides both spatial (dominant brain regions) and temporal (hemodynamic response shapes) information for each task‐based functional brain network. When applied to multiple datasets, group fMRI‐CPCA allows for visualization of task‐common and task‐specific networks, through observation of spatial and temporal replication across tasks within each network (Lavigne et al., 2015a; Ribary et al., 2017).
Group fMRI‐CPCA involved the preparation of two matrices: (a) a data matrix (Z), containing the BOLD time series of each voxel, with one column per voxel and one row per whole brain scan; and (b) the design matrix (G), containing finite impulse response (FIR) models of the expected BOLD response to the timing onsets of stimulus presentations (i.e., pictures for the sentence task, target tones for the oddball task). To compare functional brain networks across experiments, data from all tasks (SP, VTG, AO) were included in Z, leading to a matrix of 115,040 rows (158 subjects × up to 11 runs × up to 176 volumes) and 585,930 columns (voxels in 2 mm3 resolution). Each column contained normalized and smoothed activations over all scans, with subjects, runs, and scans stacked vertically to produce Z. In G, a value 1 was placed in rows for which BOLD signal amplitude was to be estimated, and the value 0 in all other rows, creating “mini boxcar” functions. The columns of G code 7 poststimulus time points for each condition (SP, VTG, AO) for each of the (combined) 158 subjects, totaling 3,318 columns (7 × 3 × 158 = 3,318). These time points reflect different poststimulus time points in each study due to the difference in TR across studies, and are converted to seconds in the figures and results section to facilitate interpretation. Group fMRI‐CPCA proceeds in two steps. First, the data matrix, Z, is regressed onto the design matrix, G, which partitions the overall variance into predicted and residual scores. The matrix predicted scores, which reflects variance in BOLD signal that is predictable from task timing, is then submitted to a principal component analysis (PCA), resulting in task‐specific functional brain networks.
2.4.2. Relation to experimental conditions
Group fMRI‐CPCA produces predictor weights for each combination of subject, task condition, and poststimulus time. These predictor weights, which provide estimates of the engagement of functional networks, can be analyzed statistically to determine whether or not they reflect reliable and biologically plausible HDR shapes, and whether differences between groups and/or task conditions exist within each network. These analyses were carried out for each task condition separately as three 3 × 7 × 3 mixed‐model ANOVAs (one each for SP, VTG, and AO), with the within‐subjects factors of Component (3 components were extracted) and Poststimulus Time (7 whole brain scans after stimulus onset), and the between‐subjects factor of Group (control, non‐AVH, AVH). Significant three‐way interactions were followed up with separate 7 × 3 ANOVAs for each component, and significant two‐way interactions were followed up with simple contrasts comparing each Group pair at each level of Poststimulus Time. Tests of sphericity were carried out, and Greenhouse‐Geisser adjustment in degrees of freedom for any analyses in which the assumption was violated did not affect interpretation of the results; therefore, the original degrees of freedom are reported below. Effect sizes (partial eta squared, η2 p) are displayed.
3. RESULTS
3.1. Group fMRI‐CPCA
Inspection of the scree plot (Cattell, 1966; Cattell and Vogelmann, 1977) of singular values suggested three components should be extracted. The percentages of task‐related variance accounted for by each component were 23.27%, 7.34%, and 4.60%, for Components 1 to 3, respectively. All components/tasks showed a significant effect of Poststimulus Time (all ps < .001; see Figures 2, 3, 4). Visual inspection of the predictor weights for each task condition confirmed a biologically plausible hemodynamic response shape for all components for SP and VTG task conditions, and for AO Component 1. Although AO Components 2 and 3 were reliable, they were not clearly valid with respect to a standard fMRI BOLD signal, opening the possibility that subtle but reliable coordinated (de)activations, uncorrected movement, or task‐timing‐predictable blood flow changes could contribute to this pattern.
Figure 2.

Mean finite impulse response (FIR)‐based predictor weights for speech perception (SP) plotted as a function of poststimulus time. (a) Auditory‐Motor Network. (b) Language Processing Network. (c) Default‐Mode Network. AVH = auditory‐verbal hallucinations [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.

Mean finite impulse response (FIR)‐based predictor weights for Verbal Thought Generation (VTG) plotted as a function of poststimulus time. (a) Auditory‐Motor Network. (b) Language Processing Network. (c) Default‐Mode Network. AVH = auditory‐verbal hallucinations [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.

Mean finite impulse response (FIR)‐based predictor weights for Auditory Oddball (AO) plotted as a function of poststimulus time. (a) Auditory‐Motor Network. (b) Language Processing Network. (c) Default‐Mode Network. AVH = auditory‐verbal hallucinations [Color figure can be viewed at http://wileyonlinelibrary.com]
3.1.1. Anatomical descriptions
The cortical regions associated with Components 1–3 are displayed in Figure 5a–c, with anatomical descriptions in Tables 2, 3, 4. Component 1 was characterized by activations in bilateral temporal pole (Brodmann Area (BA) 38), superior temporal gyrus (STG; BA 22), supplementary motor area (SMA; BA 6)/dorsal anterior cingulate cortex (dACC; BA 24), visual cortex (BA 17), left precentral gyrus (BA 6), bilateral insula, thalamus, and cerebellum. This network included regions described as part of the auditory network described in Ćurčić‐Blake et al. (2017)'s review on AVHs in schizophrenia, and regions comprising the somatosensory network from Yeo et al. (2011) resting‐state fMRI parcellation analysis, and was labelled the Auditory‐Motor Network. Component 2 was characterized by activations in left posterior middle temporal gyrus (BA 21), inferior frontal gyrus (IFG; BAs 44, 45), orbitofrontal cortex (BA 47), dorsolateral prefrontal cortex (BA 46), and bilateral visual cortex (BAs 17, 18, 19). These regions correspond to Broca's and Wernicke's areas, as well as other regions involved in language processing, and are similar to the language network described in previous research (Ćurčić‐Blake et al., 2017); this network was, therefore, called the Language Processing Network. Component 3 was characterized by activations in bilateral superior temporal gyrus (BA 22), visual cortex (BAs 18, 19), left precentral gyrus/SMA (BAs 4, 6) and primary auditory cortex (BA 41), and deactivations in bilateral ventromedial prefrontal cortex (BAs 9, 10, 11), precuneus (BAs 5, 7), posterior cingulate cortex (BA 30), and lateral occipital cortex (BAs 39/40), regions comprising the default‐mode network (DMN; Buckner, Andrews‐Hanna, & Schacter, 2008; Raichle and MacLeod, 2001).
Figure 5.

Dominant 10% of component loadings for (a) Auditory‐Motor Network (Component 1, red/yellow = positive loadings, threshold = 0.11, max = 0.18; no negative loadings passed threshold), (b) Language Processing Network (Component 2, red/yellow = positive loadings, threshold = 0.07, max = 0.15; blue/green = negative loadings, threshold = −0.07, min = −0.07), and (c) Default‐Mode Network (Component 3, red/yellow = positive loadings, threshold = 0.05, max = 0.11; blue/green = negative loadings, threshold = −0.05, min = −0.09). Montreal Neurological Institute Z‐axis coordinates are displayed [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Cluster volumes for the most extreme 10% of auditory‐motor network (Component 1) loadings, with anatomical descriptions, Montreal Neurological Institute (MNI) coordinates, and Brodmann's area (BA) for peaks within each cluster
| Brain regions | Cluster volume (voxels) | BAs for peak locations | MNI coordinate for peak locations | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive loadings | |||||
| Cluster 1: left hemisphere | 11,850 | ||||
| Planum temporale | 22 | −60 | −24 | 10 | |
| Precentral gyrus | 4 | −40 | −20 | 60 | |
| Planum polare | 38 | −56 | 2 | −4 | |
| Insular cortex | 48 | −36 | −22 | 2 | |
| Precentral gyrus | 6 | −58 | 4 | 16 | |
| Cluster 2: right hemisphere | 7,100 | ||||
| Planum temporale | 22 | 62 | −18 | 6 | |
| Superior temporal gyrus, anterior division | 21 | 58 | 0 | −6 | |
| Temporal pole | 38 | 56 | 6 | −8 | |
| Insular cortex | 48 | 40 | 10 | 0 | |
| Planum polare | 48 | 40 | −8 | −14 | |
| Cluster 3: bilateral | 3,854 | ||||
| Supplementary motor area/Dorsal anterior cingulate cortex | 6/24 | −2 | 4 | 46 | |
| Cluster 4: right hemisphere | 1,483 | ||||
| Cerebellum VI | n/a | 20 | −54 | −22 | |
| Cerebellum V | n/a | 8 | −62 | −14 | |
| Cluster 5: bilateral | 1,113 | ||||
| Intracalcarine cortex | 17 | −6 | −78 | 10 | |
| Intracalcarine cortex | 17 | 14 | −72 | 12 | |
| Cluster 6: left hemisphere | 485 | ||||
| Thalamus | n/a | −10 | −18 | 6 | |
| Cluster 7: left hemisphere | 341 | ||||
| Cerebellum VI | n/a | −24 | −60 | −24 | |
| Cluster 8: right hemisphere | 324 | ||||
| Thalamus | n/a | 12 | −16 | 6 | |
| Cluster 9: right hemisphere | 14 | ||||
| Supramarginal gyrus, anterior division | 40 | 52 | −34 | 54 | |
| Cluster 10: right hemisphere | 12 | ||||
| Middle frontal gyrus | 6 | 42 | 0 | 58 | |
Note. Negative loadings: no negative loadings passed threshold.
Table 3.
Cluster volumes for the most extreme 10% of language processing network (Component 2) loadings, with anatomical descriptions, Montreal Neurological Institute (MNI) coordinates, and Brodmann's area (BA) for peaks within each cluster
| Brain regions | Cluster volume (voxels) | BAs for peak locations | MNI coordinate for peak locations | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive loadings | |||||
| Cluster 1: bilateral | 21,500 | ||||
| Occipital fusiform gyrus | 19 | 40 | −72 | −18 | |
| Occipital fusiform gyrus | 19 | −44 | −66 | −20 | |
| Lateral occipital cortex, inferior division | 19 | −42 | −76 | −16 | |
| Lateral occipital cortex, inferior division | 18 | 40 | −90 | −8 | |
| Occipital fusiform gyrus | 18 | −28 | −84 | −20 | |
| Occipital pole | 18 | −28 | −98 | 6 | |
| Occipital pole | 18 | 32 | −94 | 8 | |
| Occipital pole | 17 | 16 | −104 | 4 | |
| Lingual gyrus | 18 | −4 | −90 | −16 | |
| Occipital pole | 17 | −4 | −102 | −12 | |
| Cerebellum crus II | n/a | 26 | −76 | −48 | |
| Temporal fusiform cortex | 20 | 34 | −24 | −32 | |
| Cluster 2: left hemisphere | 3,646 | ||||
| Frontal orbital cortex | 45 | −48 | 24 | −6 | |
| Inferior frontal gyrus, pars triangularis | 45 | −52 | 22 | 16 | |
| Frontal pole | 11 | −28 | 64 | −16 | |
| Middle frontal gyrus | 6 | −48 | 6 | 48 | |
| Frontal pole | 46 | −42 | 56 | 10 | |
| Inferior frontal gyrus, pars opercularis | 44 | −38 | 8 | 30 | |
| Middle frontal gyrus | 44 | −50 | 16 | 36 | |
| Cluster 3: left hemisphere | 1,029 | ||||
| Middle temporal gyrus, posterior division | 21 | −64 | −38 | −2 | |
| Cluster 4: right hemisphere | 201 | ||||
| Frontal pole | 47 | 30 | 66 | −18 | |
| Cluster 5: left hemisphere | 117 | ||||
| Lateral occipital cortex, superior division | 7 | −28 | −76 | 48 | |
| Cluster 6: left hemisphere | 17 | ||||
| Superior frontal gyrus | 6 | −4 | 18 | 70 | |
| Cluster 7: left hemisphere | 16 | ||||
| Superior frontal gyrus | 6 | −4 | 18 | 56 | |
| Cluster 8: left hemisphere | 15 | ||||
| Hippocampus | 20 | −20 | −32 | −6 | |
| Negative loadings | |||||
| Cluster 1: right hemisphere | 22 | ||||
| Supramarginal gyrus, anterior division | 48 | 60 | −24 | 24 | |
Table 4.
Cluster volumes for the most extreme 10% of default‐mode network (Component 3) loadings, with anatomical descriptions, Montreal Neurological Institute (MNI) coordinates, and Brodmann's area (BA) for peaks within each cluster
| Brain regions | Cluster volume (voxels) | BAs for peak locations | MNI coordinate for peak locations | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive loadings | |||||
| Cluster 1: left hemisphere | 2,457 | ||||
| Superior temporal gyrus, posterior division | 22 | −58 | −18 | 0 | |
| Planum temporale | 41 | −50 | −40 | 20 | |
| Cluster 2: right hemisphere | 2,154 | ||||
| Superior temporal gyrus, posterior division | 22 | 62 | −16 | −2 | |
| Cluster 3: bilateral | 405 | ||||
| Supplementary motor area | 6 | −4 | 4 | 56 | |
| Cluster 4: right hemisphere | 220 | ||||
| Lateral occipital cortex, inferior division | 19 | 32 | −88 | −4 | |
| Occipital fusiform gyrus | 19 | 34 | −72 | −12 | |
| Cluster 5: left hemisphere | 91 | ||||
| Precentral gyrus | 6 | −52 | −6 | 48 | |
| Cluster 6: right hemisphere | 66 | ||||
| Temporal occipital fusiform cortex | 37 | 34 | −50 | −22 | |
| Cluster 7: left hemisphere | 58 | ||||
| Precentral gyrus | 4 | −38 | −24 | 56 | |
| Cluster 8: left hemisphere | 28 | ||||
| Occipital pole | 18 | −26 | −90 | −8 | |
| Cluster 9: left hemisphere | 14 | ||||
| Occipital fusiform gyrus | 19 | −36 | −66 | −14 | |
| Negative loadings | |||||
| Cluster 1: bilateral | 10,692 | ||||
| Precuneus cortex | 7 | 2 | −62 | 48 | |
| Lateral occipital cortex, superior division | 39 | 44 | −76 | 38 | |
| Lateral occipital cortex, superior division | 7 | 8 | −64 | 68 | |
| Precuneus cortex | 5 | −6 | −54 | 72 | |
| Cuneal cortex | 19 | 4 | −92 | 44 | |
| Cingulate cortex, posterior division | 30 | 4 | −46 | 8 | |
| Precuneus cortex | 5 | 6 | −46 | 76 | |
| Superior parietal lobule | 7 | 24 | −54 | 72 | |
| Lateral occipital cortex, superior division | 19 | 22 | −86 | 48 | |
| Angular gyrus | 40 | 46 | −50 | 60 | |
| Occipital pole | 18 | 2 | −102 | 26 | |
| Cluster 2: bilateral | 7,221 | ||||
| Middle frontal gyrus | 9 | 28 | 32 | 48 | |
| Frontal pole | 10 | 6 | 58 | −4 | |
| Paracingulate gyrus | 32 | 4 | 50 | 12 | |
| Frontal pole | 9 | 6 | 50 | 46 | |
| Frontal pole | 8 | 6 | 42 | 54 | |
| Frontal pole | 10 | −22 | 58 | 8 | |
| Superior frontal gyrus | 8 | 6 | 32 | 60 | |
| Frontal pole | 11 | −24 | 56 | 0 | |
| Cluster 3: left hemisphere | 1,584 | ||||
| Lateral occipital cortex, superior division | 19 | −42 | −84 | 32 | |
| Lateral occipital cortex, superior division | 39 | −50 | −78 | 26 | |
| Cluster 4: bilateral | 1,172 | ||||
| Cerebellum crus I | n/a | −36 | −78 | −38 | |
| Cerebellum crus II | n/a | −46 | −64 | −46 | |
| Cluster 5: left hemisphere | 345 | ||||
| Frontal pole | 9 | −26 | 38 | 42 | |
| Cluster 6: right hemisphere | 35 | ||||
| Cerebellum crus II | n/a | 44 | −66 | −48 | |
| Cluster 7: left hemisphere | 11 | ||||
| Lateral occipital cortex, inferior division | 37 | −64 | −66 | −6 | |
3.1.2. Relations to experimental conditions
The three‐way interactions were not significant for SP or VTG (ps > .63). Therefore, for verbal conditions, the components were combined and 7 (Poststimulus Time) × 3 (Group) ANOVAs were computed for each task condition separately. For SP (Figure 2), there was a significant Poststimulus Time × Group interaction, F(12,282) = 2.72, p < .05, η2 p = .10, and a main effect of Group, F(2,47) = 7.83, p < .005, η2 p = .25. The Poststimulus Time × Group interaction was interpreted by investigating simple main effects of group at each time point. This showed significantly increased activity in (a) AVH patients relative to controls at 3.75, 6.25, and 8.75 s; (b) AVH relative to non‐AVH patients at 6.25 and 8.75 s; and (c) non‐AVH relative to both controls and AVH patients at 13.75 s. For VTG (Figure 3), a significant Poststimulus Time × Group interaction, F(12,282) = 2.94, p < .05, η2 p = .11, and a significant main effect of Group, F(2,47) = 3.43 p < .05, η2 p = .13, were observed. Simple contrasts of group at each time point revealed that this interaction was due to increased activity in AVH patients relative to controls at 6.25 and 8.75 s. Although no significant differences emerged between AVH and non‐AVH patients nor between controls and non‐AVH patients on VTG, activity in the non‐AVH group was situated midway between controls and AVH patients at peak, which also contributed to the significant interaction effect.
For AO (Figure 4), there was a significant Component × Poststimulus Time × Group interaction, F(24,1260) = 3.36, p < .001, η2 p = .06. This was followed up by three 7 (Poststimulus Time) × 3 (Group) ANOVAs, one for each component. All three networks showed a significant interaction between Poststimulus Time and Group, F (12,630) = 3.77, p < .005, η2 p = .07, F(12,630) = 2.22, p < .05, η2 p = .04, and F(12,630) = 3.98, p < .005, η2 p = .07, for Components 1–3, respectively. This was interpreted using simple contrasts. For the Auditory‐Motor network (Component 1), this revealed increased activity in controls relative to both non‐AVH, at 7 s, and AVH patients at 5 and 7 s. For the Language Processing Network (Component 2), controls showed greater activity than both patient groups at 7 and 9 s. In contrast, controls showed decreased intensity (i.e., both lesser activations and deactivations) relative to non‐AVH patients at 7, 9, and 11 s and AVH patients at 7 and 9 s on the DMN (Component 3). There were no significant differences between the patient groups on any of the three components.
4. DISCUSSION
In this study, we investigated whether hypercoupling in speech‐related brain networks in schizophrenia patients with AVHs is specific to verbal material by combining previously published data from verbal (SP, VTG) and nonverbal (AO) auditory fMRI datasets in schizophrenia patients with and without AVHs and healthy controls. Using a statistical analysis technique allowing for comparison of coordinated activity in task‐related brain networks across groups and tasks while holding the network under study constant, we identified separate auditory‐motor, language processing, and default‐mode networks. During SP, AVH patients showed hypercoupling across all networks relative to the other groups; during VTG, AVH patients showed hypercoupling relative to controls, but did not differ from non‐AVH patients, replicating our previous study (Lavigne et al., 2015b). Finally, diagnosis‐specific rather than symptom‐specific differences were observed for all three components during AO, suggesting that hypercoupling in speech‐related brain networks (and the DMN) in AVH patients is specific to verbal perceived stimuli. These findings are consistent with symptom capture and resting state studies pointing to hyperactivity in speech and auditory networks as an important factor in the generation of AVHs in schizophrenia (Ćurčić‐Blake et al., 2017), and support the notion that this hypercoupling is a core feature of AVHs in schizophrenia, and is not present when control is exerted over verbal material, such as during inner speech.
By combining data from separate studies, and using group fMRI‐CPCA to extract common networks, we were able to identify common and distinct functional brain networks elicited by each experiment. Much like including multiple conditions in a single study, this method provides a means of comparing network‐level HDR shapes for different tasks on the same networks, overcoming the confounding factor of comparing different brain networks across tasks (Lavigne et al., 2015a; Ribary et al., 2017). Thus, the auditory‐motor network showed strong coordinated activations for SP and AO, both of which involved auditory stimuli, but not for VTG, for which sentences were internally generated. In contrast, the language processing network showed strong coordinated activations for the verbal tasks (VTG and SP), but not for the nonverbal AO task. Importantly, combining the AO and sentence tasks allowed the auditory‐motor and language networks to separately emerge, whereas they were aggregated onto one network in our previous sentence task study (Lavigne et al., 2015b). These differential patterns of coordinated activity across tasks, along with the networks' spatial configurations, allow us to interpret the function of these networks with increased accuracy, and provide a more fine‐grained understanding of the networks (and subnetworks) underlying AVHs in schizophrenia.
4.1. Auditory oddball (AO)
Although the auditory‐motor network showed the greatest degree of activity during AO, AO showed no differences between the hallucination‐based patient groups on this (or the other two) networks. These findings provide evidence that the hypercoupling in speech‐related brain networks observed in SP is not present during presentation of nonverbal auditory stimuli in schizophrenia patients with AVHs. Instead, patients showed hypoactivity, or reduced coupling, in this network regardless of hallucination status during oddball processing, which is in line with previous research on auditory oddball processing in schizophrenia (Kim, 2014; Wynn et al., 2015), and is also consistent with our previously published study (Lavigne et al., 2016). Moreover, in addition to auditory‐motor regions, this network included nodes of the ventral attention network (i.e., bilateral insula and anterior cingulate cortex), which has been strongly implicated in auditory oddball deficits in schizophrenia (Kim, 2014).
AO produced a reliable but not clearly biologically plausible HDR shape on the language processing network, suggesting that, as expected given the nonverbal nature of the task, this task does not elicit standard linguistic processes. This supports our selection of AO as a suitable comparison task condition for the current study. In fact, AO showed evidence of deactivity on this network during target detection, a finding suggesting suppression of language regions (relative to baseline) during nonverbal auditory processing. Despite the reduced activation observed on this network in AO relative to the other tasks, we were still able to detect group differences. As with the auditory‐motor network, these were diagnosis‐ rather than symptom‐based, and showed a similar pattern of decreased activity in patients relative to controls, with no differences between hallucination‐based patient groups.
4.2. Verbal thought generation (VTG)
No significant differences between hallucination‐based groups were observed during VTG. Although AVH patients demonstrated increased activity in all three networks relative to controls, this activity did not differ from that of non‐AVH patients, which, in turn, did not differ from controls. These findings differ slightly from our previous study, in which both patient groups showed significantly greater activation than controls on VTG, and was interpreted as reflecting top–down processes in terms of expectation of control over verbal material. Although the sentence task samples were identical between the two studies, these between‐study differences could be explained by the novel networks that emerged from the new analysis, and/or the reduced cutoff for hallucination severity used in order to equate the groups across studies, which may have obscured the previously reported effect in this study. However, this absence of difference between groups should be interpreted with caution, as they may become significant with increased power.
4.3. Default‐mode network (DMN)
The finding of STG activations coordinating with DMN deactivations on Component 3 (dominated by SP) is likely due to STG peaking at both 6.25 and 8.75 s on Component 3 (and at 8.75 s on Component 1). Component 3 demonstrates that the STG 6.25/8.75 s peaks increase simultaneously with the DMN decreases, but this does not imply that they are causally related, which cannot be determined with functional connectivity analysis methods. Thus, we interpret Component 3 as being driven primarily by DMN deactivations, but also coordinating activation in task‐positive regions occurring at 6.25/8.75 s (with the 8.75 s STG peak captured on Component 1).
As was the case with the other two networks, only diagnosis‐based differences emerged on the DMN for AO and VTG, with differences emerging between the AVH and non‐AVH groups during SP. The DMN is commonly associated with self‐referential processing and recollection of autobiographical memories (Buckner et al., 2008), but also shows increased deactivation during reality monitoring, which involves distinguishing between information that is self‐ or other‐generated (Metzak, Lavigne, & Woodward, 2015). Since the DMN consists of negative loadings, increased deactivations can also be interpreted as decreased activations for the DMN; therefore, decreased coordinated activation for the DMN network in hallucinations may contribute to a loss of self‐source tags (alienation) for verbal material, leading to inner, verbal thought generated events being experienced as not inner or not self‐generated, or as neither (Larøi and Woodward, 2007; Woodward and Menon, 2013), contributing to the formation of hallucinations. This interpretation is in line with previous findings reported an anticorrelation between the DMN and AVH‐related regions during the resting state (Alderson‐Day et al., 2016; Jardri, Thomas, Delmaire, Delion, & Pins, 2012).
4.4. Other literature
Using a larger sample of the fBIRN AO dataset, Ford et al. (2009) reported hypoactivity in left primary auditory cortex in AVH relative to non‐AVH patients, interpreting this as oddball tones competing for neural resources with voices. Although these findings appear to contradict the current results, it is difficult to directly compare them due to differences in the subsamples used, as well as the use of different analysis techniques. Particularly, our use of a network‐based connectivity method may not detect a region‐specific effect, although a region‐specific effect should still emerge on a distinct network. Research using other nonverbal auditory tasks has also reported differences between hallucinating and nonhallucinating patients, for example, in pitch discrimination and melodic streaming (McLachlan, Phillips, Rossell, & Wilson, 2013) and spoken nonverbal sounds (Rossell and Boundy, 2005). There is also evidence that auditory imagery recruits similar regions to AVHs, with one study finding that the timing of activation in the SMA distinguished between the two conditions (Linden et al., 2010). Our VTG condition shows similarities to auditory imagery; however, due to the network‐based nature of the analysis, we were not able to distinguish between timing of activation in the SMA and auditory regions, as these emerged on the same network. From the current findings, we can conclude that the networks showing hypercoupling in AVH patients during SP do not show the same pattern during the presentation of nonverbal auditory oddball stimuli. Future research will be necessary to tease apart these seemingly contradictory findings.
Although there is robust evidence that auditory and language networks are more active during hallucination‐on than hallucination‐off periods (Jardri et al., 2011), the trait‐based literature is more equivocal. For example, a meta‐analysis comparing brain activations during the experience of hallucinations versus external auditory stimuli in AVH patients suggested that external sounds led to hypoactivation in auditory and language regions (Kompus et al., 2011); however, several of these studies included nonverbal auditory stimuli. One possibility for these equivocal findings concerns the lack of reporting whether AVHs occurred during the scanning session in trait‐based studies. Hyperactivity to external (verbal) stimuli may occur in the absence of AVHs, and hypoactivity in the presence of AVHs, but it is not possible to determine this without knowledge of whether participants were hallucinating during the session. Another interpretation is that an optimal level of connectivity is required, such that either hyperactivity or hypoactivity results in AVHs (Ćurčić‐Blake et al., 2017). Finally, differences in experimental design, analysis methodology (especially network versus region of interest studies), and clinical status of patients (phenomenology of hallucinations, course of illness, etc.) may also contribute to these equivocal findings.
4.5. Limitations
One limitation of this study is that the verbal and nonverbal tasks used independent samples and study sites, leading to the possibility that site differences contributed to our interpretation of some of the results. This also prevented us from directly comparing the conditions statistically; therefore, our comparisons of significant to nonsignificant results across tests should be explicitly tested in future research with a within‐subjects design. Although neuroimaging research is increasingly combining data from separate studies, a within‐subjects design including experimentally controlled verbal and nonverbal auditory tasks would be more definitive. Another disadvantage of combining separate studies is the use of different assessment measures, which was the case in the current study for symptoms, though we attempted to equate the AVH group across tasks as closely as possible by including patients endorsing any degree of auditory hallucinations in the AVH groups. Moreover, although all patients who completed the sentence task were confirmed to be experiencing auditory verbal hallucinations in the past week, this could not be definitively confirmed for eight fBIRN subjects due to the nature of using publicly available data. Future research examining the phenomenology of hallucinations in more depth would speak to the generalizability of these findings. Finally, the auditory oddball paradigm involves cognitive processes in addition to perception of nonverbal auditory stimuli (e.g., monitoring, vigilance), and is more commonly used in research on attention and salience detection, and not often in the context of AVHs. While a nonverbal auditory task with certain perceptual qualities matched to verbal material would be better suited to address our research question, the current secondary analysis leverages immediately available, publicly available resources to provide strong preliminary support for the notion that hypercoupling in speech‐related brain networks is specific to verbal material.
4.6. Conclusion
The current findings provide evidence that hypercoupling in speech‐specific brain networks in schizophrenia patients with hallucinations is specific to verbal material, an underlying assumption of several theories of AVHs in schizophrenia. It also supports previous research (Lavigne et al., 2015b) suggesting that, for schizophrenia patients with hallucinations, the expectation of exerting cognitive control attenuated both increased activation of networks involving temporal‐frontal regions and increased reduction of DMN. From this, we can speculate that, clinically, expecting to control inner verbal thought processes may reduce hypercoupling in speech‐related functional networks and reduce the likelihood of hallucinations. However, future research should attempt to replicate these findings using a within‐subjects design with a dedicated nonverbal auditory condition, to determine whether this hypercoupling is a core feature of AVHs in schizophrenia.
ACKNOWLEDGMENTS
TSW was supported by a scholar award from the Michael Smith Foundation for Health Research (CI‐SCH‐00073) and a New Investigator Award from the Canadian Institutes of Health Research (MMS8770). Data were downloaded from the Function Biomedical Informatics Research Network (BIRN) Data Repository (http://fbirn.birncommunity.org:8080/BDR/), supported by grants to the Function BIRN (U24‐RR021992) Testbed funded by the National Center for Research Resources at the National Institutes of Health, USA (MOI RR 000827). The authors acknowledge Jan Bölts and Florian Sandhäger for their contributions to data analysis and quality control, thank the University of British Columbia MRI Research Centre, and report no conflicts of interest.
Lavigne KM, Woodward TS. Hallucination‐ and speech‐specific hypercoupling in frontotemporal auditory and language networks in schizophrenia using combined task‐based fMRI data: An fBIRN study. Hum Brain Mapp. 2018;39:1582–1595. 10.1002/hbm.23934
Funding information Michael Smith Foundation for Health Research, Grant/Award Number: CI‐SCH‐00073; Canadian Institutes of Health Research, Grant/Award Number: MMS8770; Function BIRN, Grant/Award Number: U24‐RR021992; National Center for Research Resources at the National Institutes of Health, USA, Grant/Award Number: MOI RR 000827
Footnote
A clear distinction between coordinated hyperactivity and hypercoupling is not possible with functional connectivity analyses. Brain regions with correlated and strong activations over time, which emerge on the same functional network (e.g., as a result of singular value decomposition or component analysis), can be considered coupled, and do so because they increase and reduce activation in a coordinated fashion over time. Highly coordinated and strong increases/decreases in activity lead to higher intercorrelations between regions, and can be interpreted as coordinated hyperactivity and/or hypercoupling. These terms are, therefore, used interchangeably.
REFERENCES
- Alderson‐Day, B. , Diederen, K. , Fernyhough, C. , Ford, J. M. , Horga, G. , Margulies, D. S. , … Jardri, R. (2016). Auditory hallucinations and the brain's resting‐state networks: Findings and methodological observations. Schizophrenia Bulletin, 42, 1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman, A. , & Vercammen, A. (2013). The “bottom‐up” and “top‐down” components of the hallucinatory phenomenon. The neuroscience of hallucinations (pp. 107–121). Springer. [Google Scholar]
- Allen, P. , Modinos, G. , Hubl, D. , Shields, G. , Cachia, A. , Jardri, R. , … Plaze, M. (2012). Neuroimaging auditory hallucinations in schizophrenia: From neuroanatomy to neurochemistry and beyond. Schizophrenia Bulletin, sbs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, N. C. (1984). Scale for the assessment of positive symptoms (SAPS). Iowa City, IA: University of Iowa. [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cattell, R. B. (1966). The screen test for the number of factors. Multivariate Behavioral Research, 1, 245–276. [DOI] [PubMed] [Google Scholar]
- Cattell, R. B. , & Vogelmann, S. (1977). A comprehensive trial of the scree and kg criteria for determining the number of factors. Multivariate Behavioral Research, 12, 289–325. [DOI] [PubMed] [Google Scholar]
- Ćurčić‐Blake, B. , Ford, J. M. , Hubl, D. , Orlov, N. D. , Sommer, I. E. , Waters, F. , … Aleman, A. (2017). Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Progress in Neurobiology, 148, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, J. M. , & Hoffman, R. E. (2013). Functional brain imaging of auditory hallucinations: From self‐monitoring deficits to co‐opted neural resources. The neuroscience of hallucinations (pp. 359–373). Springer. [Google Scholar]
- Ford, J. M. , Roach, B. J. , Faustman, W. O. , & Mathalon, D. H. (2007). Synch before you speak: Auditory hallucinations in schizophrenia. American Journal of Psychiatry, 164, 458–466. [DOI] [PubMed] [Google Scholar]
- Ford, J. M. , Roach, B. J. , Jorgensen, K. W. , Turner, J. A. , Brown, G. G. , Notestine, R. , … Mathalon, D. H. (2009). Tuning in to the voices: A multisite FMRI study of auditory hallucinations. Schizophrenia Bulletin, 35, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. , Stern, H. , Brown, G. G. , Mathalon, D. H. , Turner, J. , Glover, G. H. , … Potkin, S. G. (2008). Test‐retest and between‐site reliability in a multicenter fMRI study. Human Brain Mapping, 29, 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. (1999). New methods for studying hallucinated ‘voices’ in schizophrenia. Acta Psychiatrica Scandinavica, 99, 89–94. [DOI] [PubMed] [Google Scholar]
- Hoffman, R. (2010). Apr 29, Comment on Vercammen, Knegtering, den Boer, Liemburg, & Aleman. Schizophrenia Research Forum [Comment].
- Hoffman, R. E. , Fernandez, T. , Pittman, B. , & Hampson, M. (2011a). Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biological Psychiatry, 69, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. E. , Pittman, B. , Constable, R. T. , Bhagwagar, Z. , & Hampson, M. (2011b). Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. British Journal of Psychiatry, 198, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl, K. (2015). Auditory hallucinations: A review of the ERC “VOICE” project. World Journal of Psychiatry, 5, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, M. A. , & Takane, Y. (2002). Constrained principal component analysis: Various applications. Journal of Educational and Behavioral Statistics, 27, 105–145. [Google Scholar]
- Jardri, R. , Pouchet, A. , Pins, D. , & Thomas, P. (2011). Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate‐based meta‐analysis. American Journal of Psychiatry, 168, 73–81. [DOI] [PubMed] [Google Scholar]
- Jardri, R. , Thomas, P. , Delmaire, C. , Delion, P. , & Pins, D. (2012). The neurodynamic organization of modality‐dependent hallucinations. Cerebral Cortex, 23, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Keator, D. B. , Grethe, J. S. , Marcus, D. , Ozyurt, B. , Gadde, S. , Murphy, S. , … Papadopoulos, P. (2008). A National Human Neuroimaging Collaboratory Enabled by the Biomedical Informatics Research Network (BIRN). IEEE Transactions on Information Technology in Biomedicine, 12, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. (2014). Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta‐analysis. Human Brain Mapping, 35, 2265–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus, K. , Westerhausen, R. , & Hugdahl, K. (2011). The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: A meta‐analysis of functional neuroimaging studies. Neuropsychologia, 49, 3361–3369. [DOI] [PubMed] [Google Scholar]
- Larøi, F. , & Woodward, T. S. (2007). Hallucinations from a cognitive perspective. Harvard Review of Psychiatry, 15, 109–117. [DOI] [PubMed] [Google Scholar]
- Lavigne, K. M. , Menon, M. , & Woodward, T. S. (2016). Impairment in subcortical suppression in schizophrenia: Evidence from the fBIRN Oddball Task. Human Brain Mapping, 37, 4640–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne, K. M. , Metzak, P. D. , & Woodward, T. S. (2015a). Functional brain networks underlying detection and integration of disconfirmatory evidence. NeuroImage, 112, 138–151. [DOI] [PubMed] [Google Scholar]
- Lavigne, K. M. , Rapin, L. A. , Metzak, P. D. , Whitman, J. C. , Jung, K. , Dohen, M. , … Woodward, T. S. (2015b). Left‐dominant temporal‐frontal hypercoupling in schizophrenia patients with hallucinations during speech perception. Schizophrenia Bulletin, 41, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle, P. F. , Ngan, E. T. , Duffield, G. , Kho, K. , & Warren, A. J. (2002). Signs and Symptoms of Psychotic Illness (SSPI): A rating scale. The British Journal of Psychiatry: The Journal of Mental Science, 180, 45–50. [DOI] [PubMed] [Google Scholar]
- Linden, D. E. , Thornton, K. , Kuswanto, C. N. , Johnston, S. J. , van de Ven, V. , & Jackson, M. C. (2010). The brain's voices: Comparing nonclinical auditory hallucinations and imagery. Cerebral Cortex, 21, 330–337. [DOI] [PubMed] [Google Scholar]
- Magnotta, V. A. , & Friedman, L. (2006). Measurement of signal‐to‐noise and contrast‐to‐noise in the fBIRN multicenter imaging study. Journal of Digital Imaging, 19, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan, N. M. , Phillips, D. S. , Rossell, S. L. , & Wilson, S. J. (2013). Auditory processing and hallucinations in schizophrenia. Schizophrenia Research, 150, 380–385. [DOI] [PubMed] [Google Scholar]
- Metzak, P. D. , Feredoes, E. , Takane, Y. , Wang, L. , Weinstein, S. , Cairo, T. , … Woodward, T. S. (2011). Constrained principal component analysis reveals functionally connected load‐dependent networks involved in multiple stages of working memory. Human Brain Mapping, 32, 856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzak, P. D. , Lavigne, K. M. , & Woodward, T. S. (2015). Functional brain networks involved in reality monitoring. Neuropsychologia, 75, 50–60. [DOI] [PubMed] [Google Scholar]
- Metzak, P. D. , Riley, J. D. , Wang, L. , Whitman, J. C. , Ngan, E. T. C. , & Woodward, T. S. (2012). Decreased efficiency of task‐positive and task‐negative networks during working memory in schizophrenia. Schizophrenia Bulletin, 38, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , & MacLeod, A. M. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin, L. A. , Dohen, M. , Lœvenbruck, H. , Whitman, J. C. , Metzak, P. D. , & Woodward, T. S. (2012). Hyperintensity of functional networks involving voice‐selective cortical regions during silent thought in schizophrenia. Psychiatry Research: Neuroimaging, 202, 110–117. [DOI] [PubMed] [Google Scholar]
- Ribary, U. , Mackay, A. L. , Rauscher, A. , Tipper, C. M. , Giaschi, D. , Woodward, T. S. , … Moiseey, A. (2017). Emerging neuroimaging technologies: Towards future personalized diagnostics, prognosis, targeted intervention and ethical challenges In Illes J., Hossain S. (Eds.), Neuroethics: Defining the issues in theory, practice and policy ‐ Looking to the future. Oxford University Press. [Google Scholar]
- Rossell, S. L. , & Boundy, C. L. (2005). Are auditory–verbal hallucinations associated with auditory affective processing deficits? Schizophrenia Research, 78, 95–106. [DOI] [PubMed] [Google Scholar]
- Serences, J. T. (2004). A comparison of methods for characterizing the event‐related BOLD timeseries in rapid fMRI. NeuroImage, 21, 1690. [DOI] [PubMed] [Google Scholar]
- Shinn, A. K. , Baker, J. T. , Cohen, B. M. , & Öngür, D. (2013). Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophrenia Research, 143, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takane, Y. , & Hunter, M. A. (2001). Constrained principal component analysis: A comprehensive theory. Applicable Algebra in Engineering, Communication and Computing, 12, 391–419. [Google Scholar]
- Takane, Y. , & Shibayama, T. (1991). Principal component analysis with external information on both subjects and variables. Psychometrika, 56, 97–120. [Google Scholar]
- Thoma, R. J. , Chaze, C. , Lewine, J. D. , Calhoun, V. D. , Clark, V. P. , Bustillo, J. , … Turner, J. A. (2016). Functional MRI evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Frontiers in Psychiatry, 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, T. S. , Cairo, T. A. , Ruff, C. C. , Takane, Y. , Hunter, M. A. , & Ngan, E. T. C. (2006). Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience, 139, 317–325. [DOI] [PubMed] [Google Scholar]
- Woodward, T. S. , Feredoes, E. , Metzak, P. D. , Takane, Y. , & Manoach, D. S. (2013). Epoch‐specific functional networks involved in working memory. NeuroImage, 65, 529–539. [DOI] [PubMed] [Google Scholar]
- Woodward, T. S. , Leong, K. , Sanford, N. , Tipper, C. M. , & Lavigne, K. M. (2016). Altered balance of functional brain networks in Schizophrenia. Psychiatry Research: Neuroimaging Section, 248, 94–104. [DOI] [PubMed] [Google Scholar]
- Woodward, T. S. , & Menon, M. (2013). Misattribution models (II): Source monitoring in hallucinating schizophrenia subjects In Jardri R., Cachia A., Thomas P., Pins D. (Eds.), The neuroscience of hallucinations (pp. 169–184). New York, NY: Springer New York. [Google Scholar]
- Woodward, T. S. , Tipper, C. M. , Leung, A. L. , Lavigne, K. M. , Sanford, N. , & Metzak, P. D. (2015). Reduced functional connectivity during controlled semantic integration in schizophrenia: A multivariate approach. Human Brain Mapping, 36, 2948–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn, J. K. , Jimenez, A. M. , Roach, B. J. , Korb, A. , Lee, J. , Horan, W. P. , … Green, M. F. (2015). Impaired target detection in schizophrenia and the ventral attentional network: Findings from a joint event‐related potential‐functional MRI analysis. NeuroImage. Clinical, 9, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]


