Abstract
Background
Previous research has shown relatively diminished medial prefrontal cortex activation and heightened psychophysiological responses during the recollection of personal events in posttraumatic stress disorder (PTSD), but the origin of these abnormalities is unknown. Twin studies provide the opportunity to determine whether such abnormalities reflect familial vulnerabilities, result from trauma exposure, or are acquired characteristics of PTSD.
Methods
In this case-control twin study, 26 male identical twin pairs (12 PTSD; 14 non-PTSD) discordant for PTSD and combat exposure recalled and imagined trauma-unrelated stressful and neutral life events using a standard script-driven imagery paradigm during functional magnetic resonance imaging (fMRI) and concurrent skin conductance (SC) measurement.
Results
Diminished activation in the medial prefrontal cortex during stressful versus neutral script-driven imagery was observed in the individuals with PTSD, relative to other groups.
Conclusions
Diminished medial prefrontal cortex activation during stressful versus neutral script-driven imagery may be an acquired characteristic of PTSD. If replicated, this finding could be used prospectively to inform diagnosis and the assessment of treatment response.
Keywords: posttraumatic stress disorder, script-driven imagery, twin study, functional neuroimaging, fMRI, skin conductance
Introduction
Posttraumatic stress disorder (PTSD) is a serious mental health condition that may occur following exposure to traumatic events such as combat, serious accidents, abuse, or violent crime. Characteristic symptoms of PTSD include increased distress and physiological reactivity to reminders of the traumatic event (American Psychiatric Association [APA], 2013). The biological correlates of these symptoms can be studied using the script-driven imagery (SDI) paradigm, a standard symptom provocation task (Pitman et al., 1987). During SDI, participants recall and imagine personal life events while psychophysiological and brain responses are measured. An important advantage of SDI over other symptom provocation tasks is that it employs autobiographical stimuli, which allows it to more accurately represent each individual's unique experiences (Pitman et al., 1987).
Neuroimaging studies have consistently reported decreased activation of the rostral regions of medial prefrontal cortex (mPFC), including the anterior cingulate cortex (ACC) and medial frontal gyrus (MFG), during traumatic versus neutral SDI in individuals with PTSD, compared to control participants (Bremner et al., 1999; Britton et al., 2005; Lanius et al., 2001; 2003; Liberzon et al., 2003; Lindauer et al., 2004; Shin et al., 1999; 2004; reviewed in Hayes et al., 2012). Furthermore, PTSD symptom severity has been found to be inversely correlated with mPFC activation during SDI in some (Osuch et al., 2001; Shin et al., 2004), but not all (Lanius et al., 2002; Gold et al., 2011) studies.
Individuals with PTSD also exhibit increased psychophysiological (e.g., heart rate, skin conductance, and facial electromyographic) responses to trauma-related SDI, compared to trauma-exposed participants without PTSD (Orr et al., 1993; 1998; Pitman et al., 1987; 1990; Shalev et al., 1993; Shin et al., 2004; reviewed in Orr et al., 2004). Elevated psychophysiological responses in PTSD are associated with increased PTSD symptom severity (reviewed in Orr & Roth, 2000).
Recent studies have shown that abnormal brain activation and psychophysiological responses to imagery of traumatic events in PTSD may extend to imagery of trauma-unrelated, stressful events. The use of trauma-unrelated stressful events as stimuli allows for the inclusion of trauma-unexposed comparison groups; scripts describing trauma-unrelated stressful events (e.g., divorce, job loss) can be used for all participants, regardless of whether they've been exposed to traumatic events severe enough to meet the Criterion A requirements for PTSD diagnosis. For example, Britton and colleagues (2005) measured regional cerebral blood flow (rCBF) during stressful versus neutral imagery in combat veterans with and without PTSD and in combat-unexposed control participants. They found that the PTSD group had significantly greater deactivation in the rostral ACC (rACC) than the two comparison groups. Similarly, Gold and colleagues (2011) found relatively diminished rACC responses and elevated skin conductance (SC) responses to stressful versus neutral imagery in PTSD compared to trauma-exposed control participants without PTSD.
Although previous research has provided evidence for diminished mPFC activation and increased psychophysiological responses during the recollection and imagery of stressful life events in PTSD, the origin of these abnormalities remains unclear. They may reflect familial vulnerability factors that increase the risk of PTSD after trauma exposure, or result from trauma exposure, or be acquired characteristics of PTSD. Determining the origin of these abnormalities could have important clinical implications. For example, an abnormality that reflects a familial vulnerability for PTSD could be identified before potential exposure to traumatic events and hence guide primary or secondary prevention efforts. An abnormality that is an acquired characteristic could potentially assist in the diagnosis of PTSD or in the assessment of treatment response.
The origin of these abnormalities can be clarified with twin studies (e.g., Gilbertson et al., 2002; Shin et al., 2009, 2011). The present study examined identical twin pairs discordant for combat exposure; within each pair, one twin was exposed (Ex) and the other was unexposed (Ux) to combat. The Ux co-twin served as a proxy of what the Ex twin would be like if combat had not been experienced. Two types of twin pairs were included in the current design: PTSD (P+) twin pairs, in which the Ex twin had a current diagnosis of PTSD, and non-PTSD (P-) twin pairs, in which the Ex twin did not have a history of PTSD (Supplemental Figure 1). Thus, our design included four distinct participant groups: combat-exposed participants with PTSD (ExP+) and their combat-unexposed identical co-twins without PTSD (UxP+), and combat-exposed participants without PTSD (ExP-) and their combat-unexposed identical co-twins without PTSD (UxP-). In this design, any abnormalities demonstrated in the P+ twin pairs (both ExP+ and UxP+participants) would indicate a familial vulnerability for PTSD; abnormalities demonstrated in the Ex participants (both ExP+ and ExP- participants) would reflect combat exposure; and abnormalities demonstrated in only the ExP+ participants (the only participants diagnosed with PTSD) would indicate an acquired characteristic of PTSD (for review of the twin study design see Pitman et al., 2006).
In an attempt to resolve the origin of mPFC and psychophysiological abnormalities in PTSD, we examined functional magnetic resonance imaging (fMRI) and SC responses during SDI in these twins. Based on previous findings, we hypothesized that during (trauma-unrelated) Stressful versus Neutral SDI, combat-exposed individuals with PTSD (ExP+) would show diminished mPFC activation and exaggerated SC responses relative to combat-exposed individuals without PTSD (ExP-). Due to a lack of prior research, we had no basis for predicting whether these abnormalities would represent familial vulnerabilities (observed in both twins of the P+ pairs) or acquired characteristics of PTSD (observed in only the ExP+ participants). In the event that familial vulnerability factors were identified, we planned to examine the relationship between Ex participants' PTSD symptom severity and their Ux co-twins' mPFC activation and SC responses; significant inverse correlations would provide further evidence of a familial vulnerability for PTSD. In the event that acquired characteristics were identified, we planned to examine the relationship between the ExP+ participants' PTSD symptom severity and their own mPFC activation and SC responses.
Methods and Materials
Participants
Participants were male identical twins recruited from the Vietnam Era Twin (VET) Registry (Henderson et al., 1990), the University of Washington Twin Registry (Strachan et al., 2013), letters sent through the Veterans Benefits Administration (Washington, DC; Orr et al., 2003), or by advertisements on electronic media. There were four distinct participant groups: ExP+ (n=12), UxP+ (n=12), ExP- (n=14), and UxP- (n=14). ExP+ participants were exposed to combat during the Vietnam War (n=11) or a serious accident (n=1). (Analyses were completed with and without the latter P+ twin pair; because these analyses yielded similar results, we included this twin pair.) ExP- participants were exposed to combat during the Vietnam War (n=13) or Operation Desert Storm/Shield (n=1). No participant reported neurological disorders or major head trauma involving loss of consciousness for more than 10 minutes. A complete description of the study was provided to the participants, and written informed consent was obtained. This research was approved by the Institutional Review Boards of the Partners Healthcare System at Massachusetts General Hospital and the VET registry.
Demographic and Clinical Characteristics
A trained clinician (N.B.L.) administered the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) and the Structured Clinical Interview for the DSM-IV (SCID; First et al., 2002) to all participants in order to determine PTSD diagnostic status/symptom severity and comorbidity, respectively. Four of the ExP+ participants reported partial remission of PTSD symptoms; however, all of these individuals reported at least mild to moderate current PTSD symptoms (as defined by Weathers et al., 2001) and were therefore included in the analyses. According to the SCID, ExP+ participants met criteria for the following current comorbid diagnoses: dysthymia (n=3), major depression (n=2), specific phobia (n=2), substance abuse/dependence (n=2), alcohol abuse/dependence (n=1), social phobia (n=1), generalized anxiety disorder (n=1), panic disorder (n=1), and eating disorders (n=1). UxP+ participants met criteria for the following current diagnoses: alcohol abuse/dependence (n=3), PTSD (n=1), dysthymia (n=1), specific phobia (n=1), and panic disorders (n=1). Among ExP- participants, current diagnoses included: alcohol abuse/dependence (n=2), substance abuse/dependence (n=1), and paranoid/delusion disorders (n=1). One UxP- participant met criteria for current alcohol abuse/dependence (n=1). Analyses were run with and without the pair in which the UxP+ twin had PTSD; the results were the same, so we retained this pair in the final analyses.
Participants also completed the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994), Beck Depression Inventory (BDI; Beck & Steer, 1987), Beck Anxiety Inventory (BAI; Beck & Steer, 1993), the Michigan Alcoholism Screening Test (MAST; Selzer, 1971), and a combat exposure severity index for Vietnam era veterans (combat-exposed participants only; Janes et al., 1991).
Script-Driven Imagery Task Procedures
One day before the fMRI session, participants completed an interview in which they provided detailed descriptions of two neutral and two trauma-unrelated, stressful personal events. Additionally, the Ex participants (ExP+ and ExP-) provided descriptions of two combat-related personal events (results not reported here). After describing each event, participants examined a list of bodily responses (e.g. “heart races,” “labored breathing”) and circled those that they recalled having experienced during the event. Immediately after this interview, the investigators wrote scripts (i.e. brief narratives describing each event) in the second person, present tense, including an average of about four (but no more than five) of the bodily response cues that each participant selected. The scripts were audio-recorded in an emotionally-neutral, male voice for playback during fMRI scanning the next day.
Each participant was scanned during two Neutral, two Stressful, and two Combat script blocks across two functional runs. (Because the Ux participants did not experience combat, they heard standardized Combat scripts. Given the differing personal relevance of the Combat scripts across Ex and Ux participants, the Combat condition was not included in the analyses.) Before each scan, participants were instructed to close their eyes, listen carefully to each script, and imagine the described event as vividly as possible, as if they were actually back in the situation. Functional MRI and SC data were collected at five different epochs for each script: 1) Baseline (30s), when participants focused on a fixation point; 2) Read (∼50s), when they listened to the recorded scripts; 3) Imagery (30s), when they recalled and imagined the event as if reliving the experience; 4) Recovery (30s), when they opened their eyes, stopped imagining the event, and relaxed, and 5) Rating (60s), when they used a button box to rate each script on valence, arousal, and imagery vividness.
To be consistent with previous SDI studies, although all of the above-described epochs were included in the statistical model, our contrasts included only the Imagery and Baseline epochs. (Contrasts involving the Imagery and Read epochs yielded similar findings.)
Magnetic Resonance Imaging Parameters
All MRI scans were completed using a Siemens Trio Tim 3 Tesla MRI with a 12-channel head coil at the Massachusetts General Hospital (MGH) Martinos Center for Biomedical Imaging (Charlestown, MA). High-resolution, three-dimensional structural MRI scanning was completed for each participant using a multi-echo magnetization-prepared rapid gradient echo (MEMPRAGE) sequence in 176 sagittal slices (repetition time (TR)=2530ms, echo time (TE)1=1.64ms, TE2=3.5ms, TE3=5.36ms, TE4=7.22ms, flip angle=12.50°, thickness=1.00mm). Functional MRI blood-oxygen-level dependent (BOLD) images were acquired using a gradient echo T2-weighted sequence (TR=2500ms, TE=30ms, flip angle=90°) in 46 coronal slices (thickness=2.5mm, 20% distance factor, 0.5mm skip). Total scan time was approximately 10 minutes per run, 20 minutes total.
Skin Conductance Parameters
Participants' SC levels (microsiemens, μS) were measured at a sampling rate of 10Hz by an isolated SC coupler (Coulbourn Instruments LLC, Whitehall, PA) during fMRI, according to established procedures (Orr et al., 1998; Pitman et al., 1987; 1990; Shin et al., 1999; 2004). In Vivo Metric (Healdsburg, CA) Ag/AgCl electrodes filled with an isotonic conductive paste were placed on the hypothenar surface of participants' non-dominant hand, in accordance with published guidelines (Fowles et al., 1981).
SC levels (SCL) were calculated by averaging the SC data within each individual Baseline and Imagery period for each script. The mean SCL during the Baseline period was subtracted from the mean SCL during the Imagery period for each script condition to yield a SC response (SCR) score. Additionally, a script condition difference score (Stressful SCR-Neutral SCR) was calculated to assess the difference between script conditions. Given that two scripts were presented for each condition, SC data from both scripts were averaged and used in the final analyses. SC data were not usable from 3 of the P+ twin pairs (adjusted n=9) and 2 of the P- twin pairs (adjusted n=12).
Data Analysis
For the SC data, we performed 2 (PTSD diagnosis: P+, P-) by 2 (exposure: Ex, Ux) mixed-model analyses of variance (ANOVAs) using the Statistical Package for the Social Sciences (SPSS) program, version 22 (IBM, Armonk, NY). A significant between-subjects main effect of PTSD diagnosis (P+ versus P-twin pairs) would indicate a familial vulnerability to the development of PTSD. A significant within-subjects main effect of exposure (Ex versus Ux) would indicate a consequence of combat exposure, independent of PTSD. A significant PTSD diagnosis by exposure interaction in which the ExP+ participants differed from all the other groups would indicate an acquired characteristic of PTSD. Pearson correlations were used to assess the relationships among PTSD symptom severity, fMRI, and SC data.
For the fMRI data, whole-brain voxelwise comparisons were performed using the statistical parametric mapping (SPM8) software package (www.fil.ion.ucl.ac.uk/spm/software/spm8). Each participant's functional images were co-registered to his MEMPRAGE image, spatially normalized in standard stereotactic space (Montreal Neurological Institute, MNI), and smoothed (8mm). We used an approach that consisted of hierarchical levels of analysis in which each level's random-effects analysis absorbs the random effects from the level beneath it. The first level required contrasting two conditions (e.g. Stressful Imagery versus Neutral Imagery) to generate a contrast map per participant. Movement greater than 5mm translation or 3° of rotation was exclusionary for this study. However, no participant exceeded these movement thresholds; most participants' movement was 1-2mm with <1.5° rotation. Additionally, ANOVAs on movement (translation and rotation) data revealed no significant main effects or interactions.
In the first set of analyses, which were an attempt to replicate findings from previous cross-sectional studies of PTSD (Bremner et al., 1999; Lanius et al., 2001; 2003; Liberzon et al., 2003; Lindauer et al., 2004; Shin et al., 1999; 2004), the contrast images of the ExP+ and ExP- groups were compared by independent-samples t-test. The second set of analyses utilized the twin design. To assess the main effect of exposure, the Stressful Imagery versus Neutral Imagery contrast images of the Ex twins were compared to their Ux co-twins using a paired t-test. To assess the main effect of PTSD diagnosis (P+ pairs versus P- pairs), contrast images of the Ex and Ux co-twins within each diagnostic group were averaged, and the P+ and P- groups were compared by independent-samples t-test. To assess the PTSD diagnosis by exposure interaction, Ex versus Ux contrast images were generated, and the P+ and P- groups were then compared by independent-samples t-test. To verify results, we repeated the fMRI analyses using a 2 (PTSD diagnosis: P+ versus P-) by 2 (Exposure: Ex versus Ux) ANOVA.
For the replication analyses comparing ExP+ versus ExP-, given our directional a priori hypotheses, we applied a significance threshold of p<0.001, one-tailed, uncorrected (z≥3.09) to activations in the mPFC, including the ACC and MFG. This is the same threshold used in the previous studies we are attempting to replicate (Brohawn et al., 2010; Shin et al., 2004; 2005). We did not have directional hypotheses for the twin-design analyses; therefore, we applied a significance threshold of p<0.0005, two-tailed, uncorrected (z≥3.29) for activations in the mPFC. For all other regions about which we had no a priori predictions, we applied a more conservative significance threshold of p<0.00002, two-tailed, uncorrected (z≥4.27) in accordance with previous studies (Brohawn et al., 2010; Shin et al., 2005). Activations in the mPFC were identified by their highest local maximum, and their location was verified using the Mai et al., (2015) and Talairach and Tournoux (1988) brain atlases, as well as the SPM Anatomy Toolbox. Following the whole-brain voxelwise analyses, we extracted parameter estimates for individual participants from identified functional regions of interest (ROI; spherical, 4mm radius) using the MarsBaR SPM toolbox (Brett et al., 2002) and further analyzed these data using SPSS.
Results
The demographic and clinical characteristics of the study participants are summarized in Table 1. All groups were similar in terms of age, years of education, current depressed mood (BDI), magnitude of childhood trauma (CTQ), and alcohol use (MAST). Consistent with their PTSD diagnosis, ExP+ participants reported significantly greater PTSD symptom severity (CAPS) compared to ExP- participants. Additionally, the ExP+ participants reported higher levels of combat severity than the ExP- participants. A PTSD diagnosis by exposure interaction was observed for anxiety ratings (measured by the BAI) and reflected relatively high anxiety scores in the ExP+group.
Table 1. Demographic and Clinical Characteristics for Combat-Exposed Participants with (P+) and Without (P-) PTSD and Their Combat-Unexposed Identical Co-Twins.
| PTSD Pairs (P+) | Non-PTSD Pairs (P-) | Mixed-Model Analysis of Variancea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Exposed (n=12) | Unexposed (n=12) | Exposed (n=14) | Unexposed (n=14) | Diagnosis | Trauma Exposure | Interaction | ||||||||
|
|
||||||||||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p(η2) | F | p(η2) | F | p(η2) |
| Age (years) | 60.75 | 6.37 | 60.75 | 6.37 | 63.07 | 3.93 | 63.07 | 3.93 | 1.29 | .27(.05) | - | - | - | - |
| Education (years) | 14.00 | 2.80 | 13.58 | 3.78 | 15.89 | 3.56 | 15.00 | 2.72 | 1.87 | .18(.07) | 2.63 | .12(.10) | 0.35 | .56(.01) |
| CAPS | ||||||||||||||
| Re-Experiencing | 14.50 | 9.71 | - | - | 1.29 | 2.33 | - | - | 24.44 | <.001(.51) | - | - | - | - |
| Avoidance | 19.58 | 10.82 | - | - | 1.36 | 2.53 | - | - | 37.54 | <.001(.61) | - | - | - | - |
| Hyper-Arousal | 18.00 | 7.78 | - | - | 1.57 | 2.88 | - | - | 54.12 | <.001(.69) | - | - | - | - |
| Total | 52.08 | 24.11 | - | - | 4.21 | 6.89 | - | - | 50.70 | <.001(.68) | - | - | - | - |
| Combat Severityb | 8.00 | 1.83 | - | - | 2.63 | 1.92 | - | - | 36.77 | <.001(.70) | - | - | - | - |
| CTQc | 39.60 | 11.79 | 36.77 | 10.72 | 38.72 | 7.82 | 39.00 | 11.52 | 0.03 | .86(<.01) | 0.38 | .54(.02) | 0.57 | .46(.03) |
| BDI | 10.00 | 10.65 | 4.25 | 8.06 | 4.64 | 4.18 | 2.93 | 2.70 | 2.32 | .14(.09) | 5.43 | .03(.19) | 1.59 | .22(.06) |
| BAI | 9.25 | 9.68 | 2.00 | 4.22 | 2.86 | 1.66 | 3.57 | 5.54 | 1.96 | .18(.08) | 4.47 | .05(.16) | 6.64 | .02(.22) |
| MAST | 4.58 | 5.65 | 2.83 | 4.90 | 2.14 | 2.66 | 2.71 | 3.85 | 0.65 | .43(.03) | 0.92 | .35(.04) | 3.58 | .07(.13) |
| SSRI Medication | Frequencye | Frequency | Frequency | Frequencyf | - | - | - | - | - | - | ||||
| Currently Taking | 9 | 1 | 1 | 2 | - | - | - | - | - | - | ||||
| Not Currently Taking | 2 | 11 | 13 | 11 | - | - | - | - | - | - | ||||
| SCID Diagnoses | Frequency | Frequency | Frequency | Frequency | - | - | - | - | - | - | ||||
| Major Depression | 2 | 0 | 0 | 0 | - | - | - | - | - | - | ||||
| Dysthymia | 3 | 1 | 0 | 0 | - | - | - | - | - | - | ||||
| PTSD | 12 | 1 | 0 | 0 | - | - | - | - | - | - | ||||
| Specific Phobia | 2 | 1 | 0 | 0 | - | - | - | - | - | - | ||||
| Social Phobia | 1 | 0 | 0 | 0 | - | - | - | - | - | - | ||||
| Generalized Anxiety Disorder | 1 | 0 | 0 | 0 | - | - | - | - | - | - | ||||
| Panic Disorder | 1 | 1 | 0 | 0 | - | - | - | - | - | - | ||||
| Paranoid/Delusion Disorder | 0 | 0 | 1 | 0 | - | - | - | - | - | - | ||||
| Substance Abuse/Dependence | 2 | 3 | 1 | 0 | - | - | - | - | - | - | ||||
| Alcohol Abuse/Dependence | 1 | 0 | 2 | 1 | - | - | - | - | - | - | ||||
| Eating Disorder | 1 | 0 | 0 | 0 | - | - | - | - | - | - | ||||
Significant effects in bold
df=1,24 unless noted otherwise
df=1,16
df=1,21
One participant did not report current medications
Two participants did not report current medications
Abbreviations: BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CAPS, Clinician-Administered PTSD Scale; CTQ, Childhood Trauma Questionnaire; MAST; Michigan Alcoholism Screening Test; PTSD, posttraumatic stress disorder; SCID, Structured Clinical Interview for DSM; SSRI, selective serotonin reuptake inhibitor
Functional MRI Results
Replication Analyses: ExP+ versus ExP-
The ExP+ group, relative to the ExP- group, showed less activation in the rACC (MNI 14, 38, 24) in the Stressful versus Neutral Imagery contrast, replicating previous findings (Table 2). Inspection of the means (Figure 1A) revealed that the ExP+ group showed rACC deactivation whereas the ExP- group showed rACC activation.
Table 2. ExP+ versus ExP-: Voxelwise Analyses of Stressful Versus Neutral Imagery in Combat-Exposed Participants Only.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Comparison | Region | z Score | k | x | y | z | |
| ExP+ > ExP- | Within Exposed Twins (Ex) | ||||||
| none | - | - | - | - | - | ||
| ExP- > ExP+ | a priori | rACC | 3.18 | 2 | 14 | 38 | 24 |
| non-a priori | superior frontal gyrus | 4.38 | 5 | -16 | 42 | 46 | |
Participant Groups: ExP+, participants exposed to combat with PTSD; ExP-, participants exposed to combat without PTSD
Abbreviations: MNI, Montreal Neurological Institute; k = cluster size at the significance threshold (see below); rACC, rostral anterior cingulate cortex.
For a priori regions, a significance threshold was applied at z≥3.09, p≤.001, 1-tailed.
For non-a priori regions, the applied significance threshold was z≥4.27, p≤.00002, 2-tailed. Significant a priori regions are listed in bold print.
Figure 1.
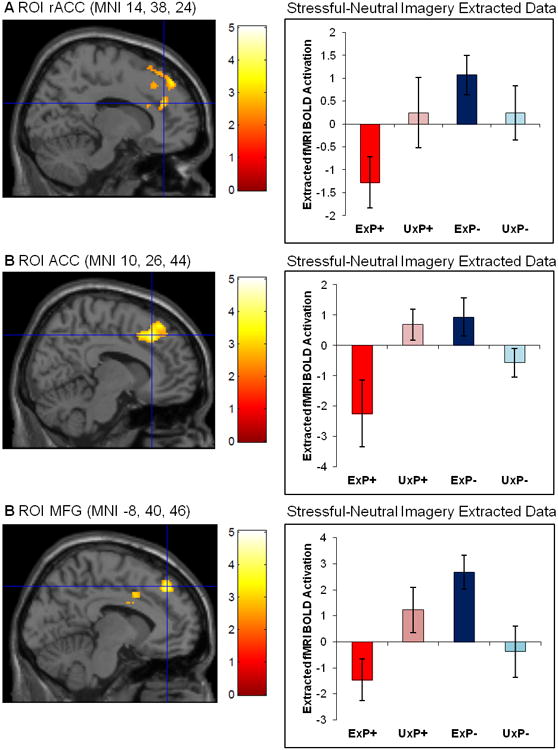
Significant PTSD diagnosis by exposure interactions during Stressful versus Neutral Imagery in A) the rACC (MNI 14, 38, 34; z=3.18), B) the ACC (MNI 10, 26, 44; z=3.71), and C) the MFG (MNI -8, 40, 46; z=3.58). Error bars represent standard error of the mean. ROI masked by cluster for display purposes only.
Abbreviations: ACC, anterior cingulate cortex; BOLD, blood-oxygen-level dependent; fMRI, functional magnetic resonance imaging; MFG; medial frontal gyrus; MNI, Montreal Neurological Institute; PTSD, posttraumatic stress disorder; rACC, rostral anterior cingulate cortex; ROI, region of interest
Group Abbreviations: Ex, combat-exposed; Ux, combat-unexposed; P+, PTSD twin pair; P-, Control twin pair
Twin-Design Analyses
First, using the rACC (MNI 14, 38, 24) functional ROI that was identified in the preceding ExP+ versus ExP- analysis, we extracted data from all four subject groups. An ANOVA demonstrated a significant PTSD diagnosis by exposure interaction; ExP+ participants showed lower rACC activation during Stressful versus Neutral Imagery, relative to all other groups, F(1,24)=5.447, p=.028, η2=.185 (Figure 1A). The main effects of PTSD diagnosis and exposure were both non-significant (p≥.09).
Second, we conducted whole-brain voxelwise twin-design analyses of the Stressful versus Neutral Imagery contrast images. These also yielded significant PTSD diagnosis by exposure interactions in the mPFC, which included a region located on the boundary of the dorsal ACC and rACC (ACC; MNI 10, 26, 44) and a region in the MFG (MNI -8, 40, 46). There were no significant main effects of exposure (Table 3). A main effect of PTSD diagnosis, in which the P+ pairs showed significantly less activation compared to P- pairs, was found in one non-a priori region (postcentral gyrus). The 2 (PTSD diagnosis: P+ versus P-) by 2 (Exposure: Ex versus Ux) ANOVA yielded nearly identical findings, although the z-scores were lower in the ANOVA (z=3.09 for the ACC; z = 3.14 for the MFG; and z=3.76 for the postcentral gyrus.)
Table 3. Twin Analyses: Voxelwise Analyses of Stressful Versus Neutral Imagery in All Participants.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Comparison | Region | z Score | k | x | y | z | |
| Main Effect of PTSD Diagnosis | |||||||
| P+ > P- | none | - | - | - | - | - | |
| P- > P+ | a priori | none | - | - | - | - | - |
| non-a priori | postcentral gyrus | 4.36 | 12 | -48 | -32 | 42 | |
|
| |||||||
| Main Effect of Exposure | |||||||
| Ex > Ux | none | - | - | - | - | - | |
| Ux > Ex | none | - | - | - | - | - | |
|
| |||||||
| PTSD Diagnosis x Exposure Interaction | |||||||
| ExP+ < all other groups | a priori | ACC | 3.71 | 22 | 10 | 26 | 44 |
| MFG | 3.58 | 31 | -8 | 40 | 46 | ||
| non-a priori | none | - | - | - | - | - | |
Participant Groups:P+ twin pairs in which the exposed twin has PTSD; P- twin pairs in which the exposed twin does not have PTSD; Ex, combat-exposed twins; Ux, combat-unexposed twins
Abbreviations: MNI, Montreal Neurological Institute; k = cluster size at the significance threshold (see below); ACC, anterior cingulate cortex; MFG, medial frontal gyrus.
For a priori regions, a significance threshold was applied at z≥3.29, p≤.0005, 2-tailed.
For non-a priori regions, the applied significance threshold was z≥4.27, p≤.00002, 2-tailed. Significant a priori regions are listed in bold print.
In order to fully examine the PTSD diagnosis by exposure interactions, values for each participant were extracted from each functional ROI using the MarsBaR SPM toolbox. Analyses of the data extracted from the ACC (MNI 10, 26, 44) confirmed a significant PTSD diagnosis by exposure interaction; ExP+ participants showed less ACC activation during Stressful versus Neutral Imagery relative to all other groups, F(1,24)=12.690, p=.002, η2=.346 (Figure 1B). Similarly, analyses of the extracted data from the MFG (MNI -8, 40, 36) confirmed a significant PTSD diagnosis by exposure interaction; ExP+ participants showed less MFG activation during Stressful versus Neutral Imagery, relative to all other groups, F(1,24)=12.395, p=.002, η2=.341 (Figure 1C). An examination of the extracted fMRI data further broken down by Stressful Imagery versus Baseline and Neutral Imagery versus Baseline confirmed that the results from the Stressful versus Neutral Imagery contrast were due to mPFC deactivation in the ExP+ group during Stressful Imagery (Supplemental Figure 2).
Correlations with mPFC Activation
The ExP+ participants' Stressful versus Neutral SDI activation in the rACC (MNI 14, 38, 24), ACC (MNI 10, 26, 44), and MFG (MNI -8, 40, 46) did not significantly correlate with their own total CAPS scores (ps≥.074, 1-tailed).
Skin Conductance Results
Results for the SC data are presented in Table 4. No significant effects were found for average SCL during the Imagery and Baseline periods of the Stressful and Neutral script conditions. Additionally, no significant effects were found for average SCR (Imagery SCL-Baseline SCL) during the Stressful or Neutral script conditions. When the Stressful SCR-Neutral SCR difference score was assessed, a significant PTSD diagnosis by exposure interaction emerged, F(1,19)=4.531, p=.047, η2=.193. ExP+ individuals had significantly smaller average Stressful SCR-Neutral SCR difference scores relative to all other groups, which appears to be due to higher average SCLs (although not significantly so) in ExP+ participants throughout the SDI paradigm, particularly during the both the Baseline and Imagery periods of the Stressful condition (Supplemental Figure 3). Stressful versus Neutral SCR difference scores were not significantly correlated with PTSD symptom severity (r(19)<.065, p>.355).
Table 4. Skin Conductance (μS) during the Stressful and Neutral Imagery Conditions.
| PTSD Pairs (P+) | Control Pairs (P-) | Mixed-Model Analysis of Variancea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Exposed n=9 | Unexposed n=9 | Exposed n=12 | Unexposed n=12 | Diagnosis | Trauma Exposure | Interaction | ||||||||
|
|
||||||||||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | P | F | p | F | p |
| Stressful Condition | ||||||||||||||
| Baseline SCL | 4.19 | 4.22 | 3.82 | 1.75 | 3.92 | 2.49 | 3.54 | 2.33 | 0.07 | .788 | 0.27 | .609 | 0.00 | .993 |
| Imagery SCL | 4.17 | 4.31 | 3.97 | 1.75 | 4.03 | 2.51 | 3.68 | 2.26 | 0.05 | .831 | 0.15 | .708 | 0.01 | .918 |
| SCR (Imagery-Baseline) | -0.02 | 0.13 | 0.14 | 0.34 | 0.11 | 0.28 | 0.13 | 0.43 | 0.23 | .638 | 1.50 | .235 | 0.92 | .350 |
|
| ||||||||||||||
| Neutral Condition | ||||||||||||||
| Baseline SCL | 3.94 | 4.09 | 3.73 | 1.79 | 3.64 | 2.37 | 3.10 | 2.13 | 0.23 | .637 | 0.30 | .588 | 0.06 | .810 |
| Imagery SCL | 4.08 | 4.17 | 3.78 | 1.77 | 3.81 | 2.59 | 3.33 | 2.26 | 0.13 | .726 | 0.31 | .583 | 0.02 | .900 |
| SCR (Imagery-Baseline) | 0.15 | 0.14 | 0.05 | 0.11 | 0.18 | 0.32 | 0.23 | 0.30 | 1.83 | .192 | 0.12 | .728 | 1.04 | .320 |
|
| ||||||||||||||
| Difference Score: Stressful SCR-Neutral SCR | -0.16 | 0.11 | 0.10 | 0.27 | -0.07 | 0.42 | -0.10 | 0.38 | 0.16 | .695 | 2.92 | .104 | 4.53 | .047 |
Significant effects in bold
df=1,19
Abbreviations: PTSD, posttraumatic stress disorder; SCL, skin conductance level; SCR, skin conductance response
Covariate Analyses
Assessment of Potential Confounders
Using a method we have previously employed (e.g. Gilbertson et al., 2002; Kasai et al., 2008; Shin et al., 2009; 2011), the following variables were tested as potential confounders: age, years of education, left-handedness, birth weight, BDI, BAI, MAST, CTQ, and selective serotonin reuptake inhibitor (SSRI) use. Specifically, we examined their associations with the fMRI and SC dependent measures using correlation analyses with a screening threshold of p≤.20. Any variable that met this criterion was subsequently assessed using analysis of covariance (ANCOVA).
rACC (MNI 14, 38, 24)
fMRI data extracted from this rACC ROI identified in the ExP+ versus ExP- contrast did not correlate with any of the potential confounders below the p≤.20 screening threshold.
ACC (MNI 10, 26, 44)
fMRI data extracted from this ROI in the PTSD diagnosis by exposure interaction correlated with BDI and SSRI use below the p≤.20 screening threshold. Separate ANCOVAs controlling for Ex participants' BDI and SSRI use showed that the previously significant PTSD diagnosis by exposure interactions remained significant (F(1,23)=11.283, p=.003, η2=.329 and F(1,22)=7.614, p=.011, η2=.257, respectively).
MFG (MNI -8, 40, 46)
fMRI data extracted from this ROI in the PTSD diagnosis by exposure interaction correlated with age and SSRI use below the p ≤.20 screening threshold. A separate ANCOVA controlling for Ex participants' age showed that the previously significant PTSD diagnosis by exposure interaction remained significant (F(1,23)=10.536, p=.004, η2=.314). A separate ANCOVA controlling for Ex participants' SSRI use reduced the previously significant PTSD diagnosis by exposure interaction to a trend level (F(1,22)=14.003, p=.058, η2=.154).
Stressful versus Neutral SC Response Difference Scores
These difference scores were correlated with age below the p≤.20 threshold. An ANCOVA controlling for age showed that the previously significant PTSD diagnosis by exposure interaction was reduced to a trend level (F(1,18)=3.513, p=.077, η2=.163).
Discussion
Our analyses confirmed previous findings of relatively diminished mPFC activation during stressful versus neutral imagery in individuals with PTSD compared to trauma-exposed individuals without PTSD (Bremner et al., 1999; Britton et al., 2005; Gold et al., 2011; Lanius et al., 2001; 2003; Liberzon et al., 2003; Lindauer et al., 2004; Shin et al., 1999; 2004). Furthermore, we found reduced mPFC activation in ExP+ relative to other groups, providing evidence that this abnormality is an acquired characteristic of PTSD. Contrary to our predictions, mPFC activation was not inversely correlated with PTSD symptom severity in this study. However, previous evidence supporting this relationship has been inconsistent (e.g., Gold et al., 2011; Lanius et al., 2002).
Controlling for the use of SSRI medications did not affect the PTSD diagnosis by exposure interaction observed for the rACC and ACC, but reduced it to a trend (p=.058) for the MFG (MNI -8, 40, 36). Thus, SSRI use may affect MFG activation during SDI, but have little or no impact in other mPFC regions. No other potential confounders changed the significance of the PTSD diagnosis by exposure interactions for the mPFC.
There is significant evidence that the mPFC plays a critical role in fear extinction learning and extinction recall; mPFC malfunction may be related to the development of PTSD and maintenance of symptoms by impairing the extinction of fear (reviewed in Yehuda & LeDoux, 2007; VanElzakker et al., 2014). Our imaging finding of reduced mPFC activation in trauma-exposed individuals with PTSD is consistent with previous studies, particularly Kasai and colleagues (2008), who reported that diminished gray matter density in the rACC is an acquired characteristic of PTSD. However, we acknowledge that the current twin study finding could alternatively reflect an acquired vulnerability factor (e.g. due to early non-shared life experience or stochastic variation during neurodevelopment) rather than an acquired characteristic of PTSD itself. Our study design cannot eliminate this possibility.
Previous findings have typically demonstrated increased psychophysiological reactivity to trauma-related scripts in PTSD (e.g. Gold et al., 2011; Orr et al., 1993; 1998; Pitman et al., 1987; 1990; Shalev et al., 1993; Shin et al., 2004; reviewed in Orr et al., 2004). In contrast, our results indicate that ExP+ individuals show smaller average Stressful SCR-Neutral SCR difference scores, which may be due to higher average SCLs throughout the SDI paradigm, particularly during the both the Baseline and Imagery periods of the Stressful condition. ExP+ participants' SCL may have already been high during the Baseline period leading to ceiling effects and lack of upwards modulation during the Imagery period, which could be interpreted as higher arousal as an acquired characteristic of PTSD. However, it is important to note that only the SCR difference scores showed a significant interaction, and that comparisons of the SCL levels, which were non-significant, did not provide a clear interpretation of the data; therefore, the SCR results should be interpreted with caution. Additionally, the average SCL during Stressful Imagery in the ExP+ participants (M=4.17μS) did not represent a large magnitude SCL (for review see Orr et al., 2004). In fact, all groups showed relatively low magnitude SCLs, which could be due to a variety of factors including older age, time since the events, etc. Our assessment of potential confounding variables suggests that age may affect SCL. Indeed, when age was controlled for, the previously significant PTSD diagnosis by exposure interaction of the Stressful SCR-Neutral SCR difference score was no longer significant.
If the current findings are replicated in future twin or longitudinal studies, these acquired, objectively measured biological characteristics could potentially assist in the diagnosis of PTSD or in the assessment of treatment response. In fact, recent research has shown that SCR during SDI has good convergent validity with PTSD symptom severity as measured by the CAPS total score (Bauer et al., 2013). Additionally, decreases in psychophysiological reactivity during trauma-related imagery have been observed in successful treatment of PTSD (e.g. Boudewyns & Hyer, 1990; Shalev et al., 1992) and increased mPFC activation has been shown following successful SSRI treatment of PTSD (Fani et al., 2011) providing further evidence of their potential clinical utility. More recently, a pilot study indicated that deep transcranial magnetic stimulation of the mPFC can successfully reduce PTSD symptoms (Isserles et al., 2013).
Limitations and Future Directions
The small sample size of the present study provided limited statistical power. Data lost due to unmeasurable SC response further reduced our sample size. The repeated-measures analyses techniques employed for this twin study design helped maximize statistical power, but the sample size may have contributed to Type II errors. The nature of our sample, with its unique entry criteria, made it challenging to acquire, but also made the sample uniquely suited to address the question of whether a given PTSD characteristic is acquired or a familial vulnerability factor.
This study is also limited by the characteristics of the study sample; for example, participants were all men. However, similar findings in the mPFC have been observed in all-male (Britton et al., 2005), all-female (Bremner et al., 1999; Shin et al., 1999), as well as mixed-sex (Gold et al., 2011; Lanius et al., 2001; 2003; Lindauer et al., 2004; Osuch et al., 2001; Shin et al., 2004) studies. In addition, almost all Ex participants reported exposure from combat that had occurred decades earlier; consequently the results may not be generalizable to individuals with more recent or other types of traumatic experiences.
This study required participants to travel to Boston for two full days of testing, necessitating that participants be relatively high-functioning. This may have unintentionally excluded more severely symptomatic participants. Orr and Roth (2000) suggested that less severe PTSD symptoms reduce the size of psychophysiological effects, increasing the likelihood of Type II errors. However, our sample of ExP+ individuals all presented with at least moderate PTSD symptom severity (as defined by Weathers and colleagues (2001), which lessens the impact of this limitation.
Conclusions
This study provides further evidence of reduced mPFC activation in PTSD and provides evidence that this abnormality is an acquired characteristic. These findings have important clinical implications because acquired characteristics of PTSD could potentially assist in diagnosis or the assessment of treatment response
Supplementary Material
Supplemental Figure 1. A schematic of the twin study design, which examines identical twin pairs discordant for combat exposure. Within each pair, one twin was exposed (Ex) and the other was unexposed (Ux) to combat. The Ux co-twin served as a proxy of what the Ex twin would be like if a traumatic event had not been experienced. Two types of twin pairs were included in this design: PTSD (P+) twin pairs, in which the Ex twin had a current diagnosis of PTSD, and non-PTSD (P-) twin pairs, in which the Ex twin did not have any history of PTSD. This resulted in four distinct participant groups: combat-exposed participants with PTSD (ExP+) and their combat-unexposed identical co-twins without PTSD (UxP+), and combat-exposed participants without PTSD (ExP-) and their combat-unexposed identical co-twins without PTSD (UxP-).
Supplemental Figure 2. Extracted fMRI data from script-driven imagery further broken down by Stressful-Baseline and Neutral-Baseline activations from our regions of interest: A) rACC (14, 38, 24); B) ACC (10, 26, 44); and C) MFG (-8, 40, 46)
Abbreviations: ACC, anterior cingulate cortex; BOLD, blood-oxygen-level dependent; fMRI, functional magnetic resonance imaging; MFG; medial frontal gyrus; PTSD, posttraumatic stress disorder; rACC, rostral anterior cingulate cortex
Group Abbreviations: Ex, trauma-exposed; Ux, trauma-unexposed; P+, PTSD twin pair; P-, Control twin pair
Supplemental Figure 3. Average skin conductance levels during the script-driven imagery (SDI) task for the Stressful and Neutral script conditions at Baseline and during the Imagery periods of each condition.
Group Abbreviations: Ex, combat-exposed; Ux, combat-unexposed; P+, PTSD twin pair; P-, Control twin pair
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) grant R01MH054636. The Massachusetts General Hospital (MGH) Clinical Research Center was supported by Harvard Catalyst grants 1UL1RR025758, 8UL1TR000170, 1UL1TR001102. The MGH Athinoula A. Martinos Imaging Center was supported by the Center for Functional Neuroimaging Technologies grant P41RR14075. M.B.V. was supported by a National Defense Science & Engineering Graduate fellowship from the U.S. Department of Defense. L.M.S. received additional support from the Faculty Research Awards Committee at Tufts University. The Cooperative Studies Program (CSP) of the Office of Research & Development of the United States Department of Veterans Affairs (VA) has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry.
All statements, opinions, or views are solely of the authors and do not necessarily reflect the position or policy of the NIMH, VA, or United States Government. M.K.D. previously presented preliminary analyses of these data in her Master's Thesis.
The authors would like to thank Mary Foley and Lawrence White for neuroimaging technical assistance, and the current and past members of the Tufts University Posttraumatic Stress Disorder Neuroimaging Lab, especially Navneet Kaur, Rachel Korus, Lisa Sangermano, Julia Russell, Kelsey Keyser, Phil Panic, Belal Hakim, Tyler Chang, Eliza White, Nikki Chen, Tiffany Tu, and Nicole Carter. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET and University of Washington Twin Registries and their families. Without their contribution this research would not have been possible.
Footnotes
Financial Disclosures: R.O. is employed at Glooko (Mountain View, CA), and reports having received teaching honoraria from Metis Data Science Bootcamp and Springboard Data Science Intensive. He has also received data science consulting fees from 1010 Data, Columbia University, the Massachusetts Institute of Technology, Irrational Games, and the Fletcher School.
All other authors do not have any financial conflicts of interest to disclose.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. American Psychiatric Association; Washington, D.C: 2013. [Google Scholar]
- Bauer MR, Reuf AM, Pineles SL, Japuntich SJ, Macklin ML, Lasko NB, Orr SP. Psychological assessment of PTSD: a potential research domain criteria construct. Psychological Assessment. 2013;25(3):1037–1034. doi: 10.1037/a0033432. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. Psychological Corp; San Antonio, TX: 1987. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. Harcourt Brace; San Antonio, TX: 1993. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Boudewyns PA, Hyer L. Physiological response to combat memories and preliminary treatment outcome in Vietnam veteran PTSD patients treated with direct therapeutic exposure. Behavior Therapy. 1990;21(1):63–87. [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Presented at: 8th International Conference on Functional Mapping of the Human Brain; June 2-6, 2002; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage 16(2) [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biological Psychiatry. 2010;68(11):1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Fani N, Ashraf A, Afzal N, Jawed F, Kitayama N, Reed L, Bremner JD. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: A pilot study. Neuroscience Letters. 2011;491(3):196–201. doi: 10.1016/j.neulet.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Metzger LJ, Dougherty DD, Alpert NM, Fischman AJ, Pitman RK. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychological Medicine. 2011;41:2563–2572. doi: 10.1017/S0033291711000730. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood& Anxiety Disorders. 2012;2(9) doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam era twin registry: a resource for medical research. Public Health Reports. 1990;105(4):368–373. [PMC free article] [PubMed] [Google Scholar]
- Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, Zangen A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimulation. 2013;6(3):377–383. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. Journal of Clinical Psychology. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RWJ, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Britton JC, Phan KL. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress: the International Journal on the Biology of Stress. 2003;6(3):151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habreken JB, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BLF, Gersons BPR. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biological Psychiatry. 2004;56(11):853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Mai J, Majtanik M, Paxinos G. Atlas of the Human Brain (4th Edition) Elsevier Inc; London: 2015. [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiologic assessment of posttraumatic stress disorder imagery in World War II and Korean Combat Veterans. Journal of Abnormal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting & Clinical Psychology. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. Journal of Affective Disorders. 2000;61(3):225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK Harvard/Veterans Affairs Post-traumatic Stress Disorder Twin Study Investigators. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Archives of General Psychiatry. 2003;60(3):283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Orr SP, McNally RJ, Rosen GM, Shalev A. Psychophysiological reactivity: implications for conceptualizing PTSD. In: Rosen GM, editor. Posttraumatic stress disorder: issues and controversies. Wiley; Chichester, England: 2004. pp. 101–126. [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biological Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. Journal of Abnormal Psychology. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Annals of the New York Academy of Sciences. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. Journal of Clinical Psychiatry. 1992;53(9):324–326. [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. American Journal of Psychiatry. 1993;150(4):620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman MD, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Archives of General Psychiatry. 2009;66(10):1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, Macklin ML, Gold AL, Karpf RD, Orr SP, Rauch SL, Pitman RK. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: A monozygotic twin study of posttraumatic stress disorder. American Journal of Psychiatry. 2011;168(9):979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, Watson N, Goldberg J, Buchwald D. University of Washington twin registry: poised for the next generation of twin research. Twin Research & Human Genetics. 2013;16(1):455–462. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-planar stereotaxic atlas of the human brain 3-dimensional proportional system: an approach to cerebral imaging. Rayport M, translator. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning & Memory. 2014;133:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depression & Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A schematic of the twin study design, which examines identical twin pairs discordant for combat exposure. Within each pair, one twin was exposed (Ex) and the other was unexposed (Ux) to combat. The Ux co-twin served as a proxy of what the Ex twin would be like if a traumatic event had not been experienced. Two types of twin pairs were included in this design: PTSD (P+) twin pairs, in which the Ex twin had a current diagnosis of PTSD, and non-PTSD (P-) twin pairs, in which the Ex twin did not have any history of PTSD. This resulted in four distinct participant groups: combat-exposed participants with PTSD (ExP+) and their combat-unexposed identical co-twins without PTSD (UxP+), and combat-exposed participants without PTSD (ExP-) and their combat-unexposed identical co-twins without PTSD (UxP-).
Supplemental Figure 2. Extracted fMRI data from script-driven imagery further broken down by Stressful-Baseline and Neutral-Baseline activations from our regions of interest: A) rACC (14, 38, 24); B) ACC (10, 26, 44); and C) MFG (-8, 40, 46)
Abbreviations: ACC, anterior cingulate cortex; BOLD, blood-oxygen-level dependent; fMRI, functional magnetic resonance imaging; MFG; medial frontal gyrus; PTSD, posttraumatic stress disorder; rACC, rostral anterior cingulate cortex
Group Abbreviations: Ex, trauma-exposed; Ux, trauma-unexposed; P+, PTSD twin pair; P-, Control twin pair
Supplemental Figure 3. Average skin conductance levels during the script-driven imagery (SDI) task for the Stressful and Neutral script conditions at Baseline and during the Imagery periods of each condition.
Group Abbreviations: Ex, combat-exposed; Ux, combat-unexposed; P+, PTSD twin pair; P-, Control twin pair


