Abstract
Bisphenol S (BPS) is an endocrine disrupting chemical with multiple potential mechanisms of action, including as an estrogen receptor agonist. BPS is increasingly used in plastics and thermal receipts as a substitute for bisphenol A, which has been phased out due to concerns about human health implications. The ability of BPS to alter female reproductive function in mammals has not been widely studied, despite the importance of normal hormone signaling for female reproduction. The aim of this study was to investigate how BPS (in a wide range of doses, including very low doses) affects granulosa cell and theca cell steroid hormone production and cell viability in the bovine. Granulosa cell estradiol production was stimulated when cells were exposed to 100 µM BPS under basal conditions, but there was no effect of BPS when cells were stimulated with follicle stimulating hormone (FSH). Additionally, there was no effect of BPS on granulosa cell progesterone production or cell viability under basal or FSH-stimulated conditions. BPS did not affect theca cell androstenedione or progesterone production, or theca cell viability under basal or luteinizing hormone-stimulated conditions. This study suggests for the first time that BPS may alter estradiol production by bovine granulosa cells, albeit at a concentration that is unlikely to be physiologically relevant. Further studies are needed to determine the effects of BPS on the bovine oocyte and on other functions of follicular cells.
Keywords: granulosa cells, theca cells, bisphenol S, endocrine disruptors, steroid hormones, ovarian follicle
Introduction
Endocrine disrupting chemicals (EDCs) are compounds that have the ability to alter steroid hormone signaling (Kavlock et al., 1996), thereby disrupting normal endocrine function and often resulting in deleterious health outcomes. Although the primary focus of EDC research has been on effects on humans and aquatic species, agricultural species also have the potential to be negatively affected. Fertility in dairy cattle has been in decline in recent decades (Lucy, 2001), and although the exact cause is unknown some have suggested that exposure to EDCs may be at least partially responsible (Sweeney, 2002). There are multiple potential routes of EDC exposure for farm animals (Rhind, 2005). Contamination of water sources, including drinking water and standing water, appears to be the most likely source of exposure of farm animals to EDCs (Fromme et al., 2002), although soil and sediment contamination are also known to contribute to overall exposure levels (Ingelido et al., 2009).
Arguably the best studied EDC is bisphenol A (BPA) which is used in polycarbonate plastics, epoxy lined tin cans, and in thermal receipts (Vandenberg et al., 2007). Due to increasing regulation surrounding the use of BPA amid concern about the human health implications, manufacturers have begun using substitute chemicals, such as BPS, which have been found to be present in food and personal care products (Liao and Kannan, 2013, 2014; Ye et al., 2015). Liao et al (2012) reported that, at least in humans, daily exposure levels of BPS is similar or higher than BPA exposure levels. Like BPA, BPS has been reported to interact with the estrogen receptor (Kuruto-Niwa et al., 2005; Molina-Molina et al., 2013), albeit with 3–10 fold lower potency than BPA (Chen et al., 2002; Teng et al., 2013). Further, BPS has been reported to have additional modes of action not reported for BPA, including alteration of progestogen production in human adrenal cortico-carcinoma cells (Rosenmai et al., 2014). Therefore, the biological effects of the rising EDC BPS should be investigated independently of its structural relationship with BPA.
Although a recent study found that BPS was not measurable in the follicular fluid of abattoir derived pigs (Žalmanová et al., 2017), it is possible that the BPS burden of farm animals will increase over time, reflecting the increasing human burden (Ye et al., 2015). Due to the herbivorous diet of cattle, it is likely that they would be exposed to relatively low levels of endocrine disruptors, as these tend to bioaccumulate in animals at higher trophic levels (Magnusson and Persson, 2015). Little is known about the levels of BPS that cattle may be exposed to, although these are likely to be similar to BPA concentrations, which were 229–305 ng/L (1–1.3 nM) in urine from replacement cows (Zhang et al., 2014). Although EDC levels may be low in cattle, low dose effects are well documented to occur in the absence of effects at higher doses (Vandenberg and Prins, 2016), and thus endocrine disruption in cattle may still be of concern.
The effects of BPS on the ovary are largely unknown. In zebrafish, BPS altered plasma estradiol levels and reduced the number of eggs produced (Ji et al., 2013; Naderi et al., 2014). In the pig, BPS disrupted oocyte meiotic progression and spindle formation, and altered cumulus cell gene expression and hyaluronic acid production (Žalmanová et al., 2017). However, to the best of our knowledge, there is no information about whether BPS affects follicular cell function in mammals. BPA is well established to affect female fertility (Ziv-Gal and Flaws, 2016), including altering the function of granulosa cells (Mansur et al., 2016; Mlynarčíková et al., 2005; Xu et al., 2002), and theca cells (Zhou et al., 2008) in a variety of species. Although BPS and BPA are structurally related, the two compounds exhibit different potencies for steroid hormone receptors (Rochester and Bolden, 2015) demonstrating that the effects of BPS on female reproduction cannot be inferred from what is known about BPA.
The aim of this study was to determine how BPS influences bovine granulosa and theca cell steroidogenesis and cell viability. To address this aim, the effects of a wide range of BPS doses on granulosa and theca cell were tested in the presence or absence of gonadotropin hormones. The inclusion of basal and gonadotropin-stimulated culture conditions allowed determination of potential interactions with follicle stimulating hormone (FSH) and luteinizing hormone (LH).
Materials and Methods
All reagents were purchased from Sigma Aldrich (St Louis, MO) unless otherwise stated.
Isolation of granulosa and theca cells
Ovaries were sourced from a local slaughterhouse (Champlain Beef; Whitehall, NY) and transported back to the laboratory in 0.9% NaCl, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 µg/ml amphotericin B. Ethical approval was not required as this study used slaughterhouse discard material. Antral follicles (3–7 mm in diameter) were dissected from ovaries with an active corpus luteum into Minimum Essential Medium, Hanks’ Balanced Salts (HMEM (Lonza; Walkersville, MD) supplemented with 25 mM HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 0.4% w/v bovine serum albumin, 100 µg/ml penicillin, 100 µg/ml streptomycin, and 50 µg/ml heparin). Each replicate usually pooled cells from 2–3 animals to generate enough material. To collect the granulosa cells, the follicles were aspirated, bisected and the halves were scraped with an inoculating loop, releasing the granulosa cells into the medium.
To collect the theca cells, the follicle halves were vortexed in dissection medium, and the dislodged granulosa cells were discarded. This was repeated three times. The theca interna layer was carefully peeled from the basement membrane using fine forceps under a dissecting microscope. The theca layers were pooled and incubated for with 1 mg/ml collagenase type IV and 100 µg/ml trypsin inhibitor for 1 h at 37°C with shaking. The cells were triturated for 3 min with a Pasteur pipet four times during the incubation period.
In order to lyse the red blood cells, the granulosa or theca cells were resuspended in one part 1× phosphate buffered saline (PBS), followed by two parts double distilled water. The cells were inverted for 10 seconds to lyse any red blood cells before two parts 2× PBS was added to restore isotonicity. The cells were resuspended in either: granulosa cell culture medium (Phenol red-free McCoy’s 5A medium (GE Healthcare Life Sciences; Logan, UT) supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 µg/ml amphotericin B, 2 mM L-glutamine, 20 mM HEPES, 0.1% w/v bovine serum albumin, 5 µg/ml transferrin, 5 ng/ml sodium selenite, 10 ng/ml insulin, and 100 nM androstenedione (Steraloids; Newport, RI)) or theca cell culture medium (phenol red-free McCoy’s 5A medium supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 µg/ml amphotericin B, 2 mM L-glutamine, 20 mM HEPES, 0.1% w/v bovine serum albumin, 5 µg/ml transferrin, 5 ng/ml sodium selenite, and 10 µg/ml insulin).
Culture of granulosa and theca cells
Cells were counted using a hemocytometer, and viability was determined using the trypan blue exclusion method. Average granulosa cell viability was 56% and average theca cell viability was 92%. Cells were seeded into wells of Primaria™ coated 96-well tissue culture plates (Corning; Corning, NY) at a density of 75 × 103 cells/well. The appropriate pre-warmed culture medium with or without treatments was added to each well so that the final volume in each well was 250 µl. Cells were treated with the vehicle only control (0.05% DMSO) or BPS (Sigma Aldrich catalog number 103039) at concentrations from 1 fM increasing in 10-fold increments up to 100 µM (12 concentrations total). A control in which the cells were incubated with medium alone (i.e. without treatment or vehicle) was also included. Treatment of the granulosa cells with BPS was performed in the presence or absence of 0.33 ng/mL FSH (oFSH-19-SIAFP; National Hormone & Peptide Program, Torrance, CA). This concentration of FSH was chosen to approximate the plasma levels of FSH during the final 6 days of follicle growth (Butler et al., 2008). Treatment of the theca cells with BPS was performed in the presence or absence of 100 pg/ml LH (oLH-26; National Hormone & Peptide Program, Torrance, CA). This concentration of LH was chosen as it resulted in maximal androstenedione production by theca cells (Glister et al., 2005) and approximated the follicular fluid concentration in the cow at certain times of the estrous cycle (Dieleman et al., 1983). The cells were cultured at 38.5°C with 5% CO2 in air for 6 days. Every 48 h, 80% of conditioned medium was removed, and replaced with fresh medium and treatments.
Determination of cell viability
After 6 days culture, 175 µl of conditioned medium was removed and stored at −80°C for measurement of steroid hormones. Cells were incubated with 200 µl neutral red dye (0.05 µg/ml) in the appropriate culture medium for 3 h at 38.5°C. Following centrifugation and removal of the dye, the cells were fixed with 4% paraformaldehyde. The paraformaldehyde was replaced with acidified ethanol (1% acetic acid, 50% ethanol, 49% water). The plate was incubated at 4°C overnight prior to the absorbance being measured at 540 nm.
Enzyme-linked immunosorbent assays
Concentrations of estradiol were measured in the conditioned medium collected from granulosa cell cultures using an ELISA kit (Cayman Chemicals; Ann Arbor, MI) according to the manufacturers’ instructions. The interassay coefficient of variation was 14% and the intraassay coefficient of variation was 7%.
Concentrations of androstenedione were measured in conditioned medium collected from theca cell cultures using an ELISA kit (Eagle Biosciences; Nashua, NH). The interassay coefficient of variation was 8% and the intraassay coefficient of variation was 4%.
Concentrations of progesterone were measured in conditioned medium collected from either granulosa or theca cell cultures using an ELISA kit (Cayman Chemicals; Ann Arbor, MI). The interassay coefficient of variation was 9% and the intraassay coefficient of variation was 5%.
Statistical analysis
Data are expressed relative to the untreated control. Data represent the mean ± standard error of the mean. Each experiment was independently repeated 5–9 times. The data were analyzed by a Kruskal-Wallis test with a Dunn-Bonferroni post-hoc test. Differences were considered significant when P<0.05.
Results
Granulosa cells
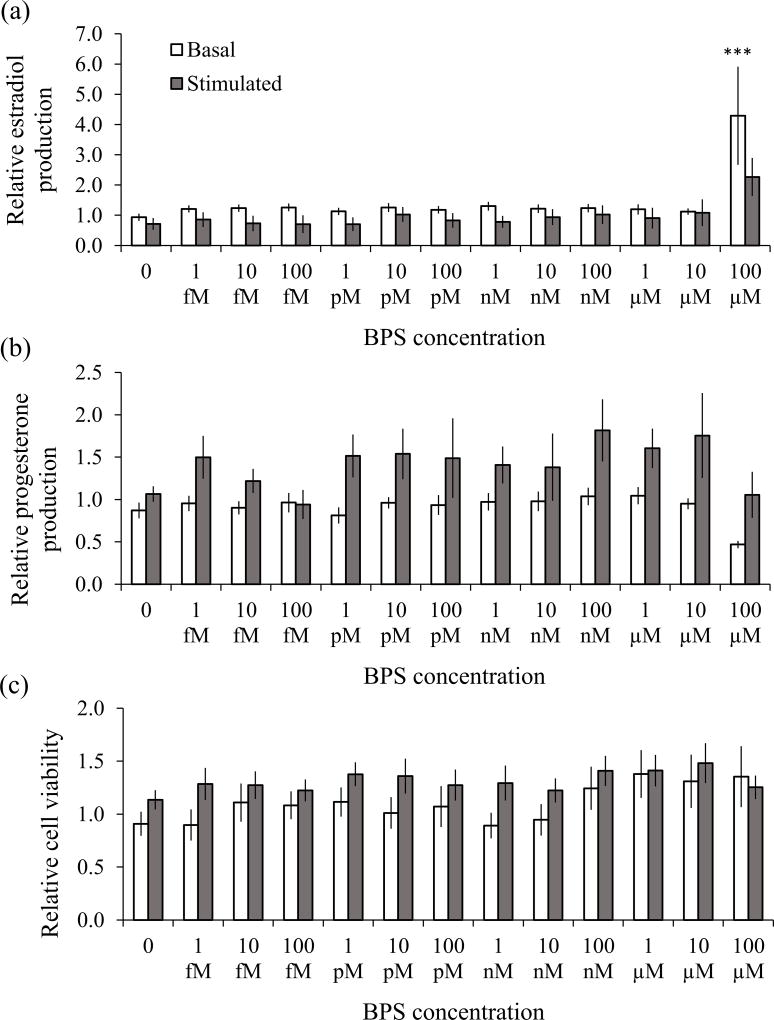
Estradiol concentrations in the untreated controls were 0.9 ± 0.3 and 2.0 ± 0.6 ng/ml under basal and FSH-stimulated conditions respectively. Basal secretion of estradiol was increased by BPS at a concentration of 100 µM (P<0.0001), as depicted in Fig 1A (unfilled bars). None of the lower concentrations of BPS had an effect on granulosa cell estradiol production under basal conditions. FSH induced a 2-fold increase in estradiol production in untreated cells. Under FSH-stimulated conditions, there was no effect of BPS on granulosa cell estradiol production (Fig 1A, filled bars). Progesterone concentrations in the untreated controls were 36.2 ± 7.3 and 103.1 ± 34.0 ng/ml under basal and FSH-stimulated conditions respectively. Secretion of progesterone was not affected by any concentration of BPS under basal or FSH-stimulated conditions (Fig 1B). FSH induced a 3-fold increase in progesterone production in untreated granulosa cells. There was no effect of BPS on granulosa cell viability under basal or FSH-stimulated conditions (Fig 1C).
Fig 1. Effect of BPS on granulosa cell estradiol (a) and progesterone (b) production and cell viability (c).
Cells were incubated with various concentrations of BPS or the vehicle alone under basal (unfilled bars) and FSH-stimulated (filled bars) conditions for 6 days. Estradiol and progesterone concentrations were measured in the conditioned medium, and are expressed relative to the untreated control. Values represent the mean ± SEM (9–10 independent experiments for basal conditions, and 5–7 independent experiments for stimulated conditions). Asterisks indicate significant differences compared to the vehicle alone control group (*** P<0.0001).
Theca cells
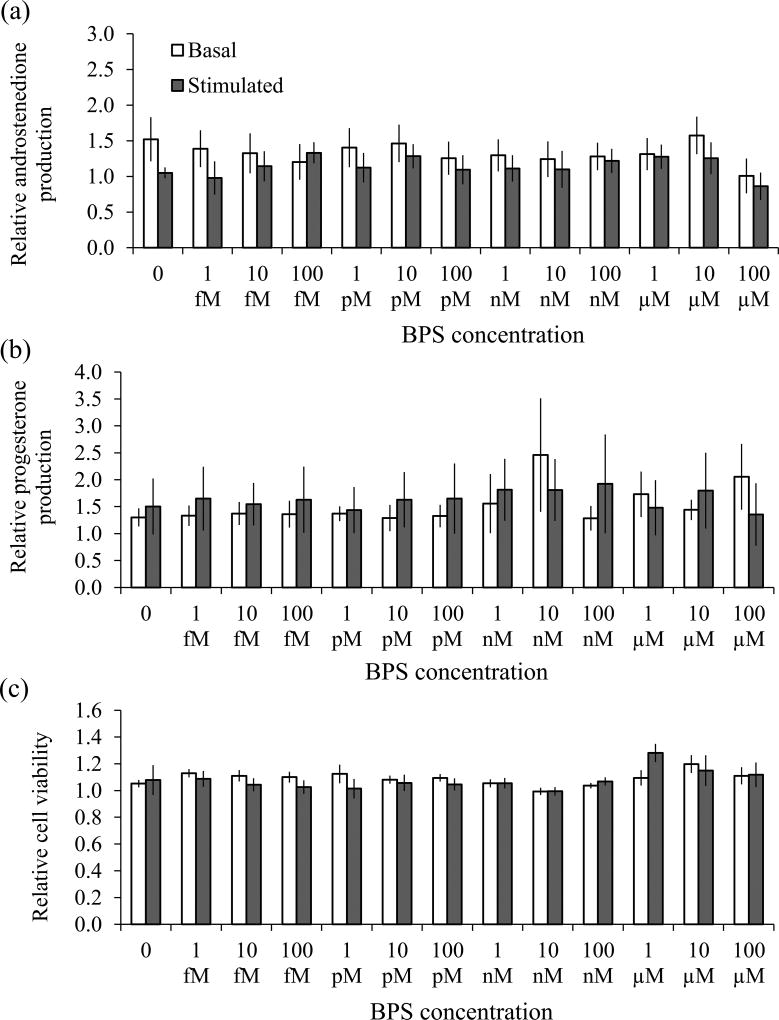
Androstenedione concentrations in the untreated controls were 2.7 ± 1.2 and 84.1 ± 17.9 ng/ml under basal and LH-stimulated conditions respectively. Secretion of androstenedione by theca cells was unaffected by BPS under basal or LH-stimulated conditions (Fig 2A). LH induced a ~30-fold increase in androstenedione production in untreated theca cells. Progesterone concentrations in the untreated controls were 7.1 ± 1.0 and 88.5 ± 20.5 ng/ml under basal and FSH-stimulated conditions respectively. Secretion of progesterone was also unaffected by BPS under basal or LH-stimulated conditions (Fig 2B). LH induced a 12-fold increase in progesterone production in untreated theca cells. There was no effect of BPS on theca cell viability under basal or LH-stimulated conditions (Fig 2C).
Fig 2. Effect of BPS on theca cell androstenedione (a) and progesterone (b) production and cell viability (c).
Cells were incubated with various concentrations of BPS or the vehicle alone under basal (unfilled bars) and LH-stimulated (filled bars) conditions for 6 days. Androstenedione and progesterone concentrations were measured in the conditioned medium, and are expressed relative to the untreated control. Values represent the mean ± SEM (6–7 independent experiments for basal conditions, and 5 independent experiments for stimulated conditions). There were no significant differences between any groups (P>0.05).
Discussion
The current study revealed that 100 µM BPS induced an increase in basal estradiol secretion by bovine granulosa cells. There was no effect of BPS on granulosa cell function under FSH-stimulated conditions, nor on theca cells under basal or LH-stimulated conditions. These results are the first to examine the effects of BPS on granulosa and theca cells in a mammalian species.
Estradiol produced by the granulosa cells is a modulator of oocyte developmental competence and is also an important component of the hypothalamic-pituitary-gonadal (HPG) axis. Therefore alterations in granulosa cell estradiol production by BPS may disrupt the HPG axis, as previously described with regards to BPA (Xi et al., 2011). The intraovarian roles of estradiol are less defined. For example, estradiol caused reductions in oocyte maturation and increased spindle abnormalities (Beker et al., 2002; Woudenberg et al., 2004) but also enhanced oocyte cytoplasmic maturation and improved fertilization and cleavage rates (Tesarik and Mendoza, 1995). A recent study demonstrated that BPS induced abnormalities in porcine oocytes, including in vitro maturation rates, spindle morphology, and cumulus expansion (Žalmanová et al., 2017). Investigation into the effects of BPS in the whole follicle is necessary to elucidate the modulating impact that intrafollicular interactions between cell types may have.
Our finding that BPS increased estradiol production by bovine granulosa cells is consistent with previous reports in the zebrafish and mice where BPS upregulated plasma estradiol levels (Ji et al., 2013; Naderi et al., 2014; Shi et al., 2017). Although beyond the scope of this study, further investigation will reveal whether BPS alters estradiol production by affecting aromatase expression, such as in porcine cumulus cells and oocytes (Žalmanová et al., 2017), or via an alternate mechanism, such as changes in the aromatase inhibitor PPARγ, as is the case in murine preadipocytes (Ahmed and Atlas, 2016). In addition to altering aromatase expression in porcine cumulus cells and oocytes, BPS also altered estrogen receptor α and β expression (Žalmanová et al., 2017). In prepubertal mice, BPS also prevented follicle activation in response to an estrogen challenge (Hill et al., 2017). These data suggest that BPS causes dysregulation of the estrogen hormone synthesis and signaling pathway at multiple levels.
Interestingly, our finding that BPS stimulates granulosa cell estradiol production is in contrast to the reported effects of BPA. These disparities demonstrate that the effects of BPA cannot be extrapolated to make conclusions about BPS or other structurally related substitutes, and that thorough testing is required for each individual chemical. Previous studies have reported that µM concentrations of BPA reduce estradiol production in rat and human granulosa cells (Mansur et al., 2016; Zhou et al., 2008). All of these studies also detected BPA-mediated alterations in granulosa cell progesterone production, whereas no effects of BPS on progesterone production were found in the current study. Further testing to provide a direct comparison within species is needed.
There was no effect of BPS on theca cell steroid hormone production or on granulosa cell progesterone production in our study. This suggests that BPS specifically targets the estradiol pathway as theca cells have little to no capacity for aromatizing androgens to produce estrogens, in contrast to granulosa cells (Fortune and Armstrong, 1978). With regards to the effects of BPS on progesterone production, a previous study by Feng and colleagues (2016) reported that BPS altered progesterone secretion, but had no effect on estradiol production. However, that study was undertaken in the human adrenocortical carcinoma cell line H295R whereas the current study utilized primary granulosa and theca cells, suggesting that the effects of BPS are cell type specific.
Although we did see an increase in granulosa cell estradiol production under basal conditions after exposure to 100 µM, this concentration is unlikely to occur naturally in cattle. Many EDCs are generally thought to bioaccumulate, due to their small size and lipophilic nature (Diamanti-Kandarakis et al., 2009), and thus cattle are likely to be exposed to low levels due to their lower trophic levels (Magnusson and Persson, 2015). To the best of our knowledge, no data are available for the concentrations of BPS in cattle or other agricultural species, with the exception of Žalmanová et al (2017) who reported that BPS was not detectable in porcine follicular fluid. Further studies are needed to determine follicular fluid levels of EDCs which would reveal the local burden for cells of the developing follicle. The current study saw no effects of physiologically relevant low dose concentrations (likely to be in the pM or nM concentration range) of BPS on granulosa or theca cell steroidogenesis or viability. Our study also did not detect a non-monotonic dose response (NMDR) curve, described as when the sign of the curve changes producing a U or inverse-U shaped curve. NMDRs have been reported for many endocrine disrupting chemicals (Lagarde et al., 2015) including BPS (Eladak et al., 2015; Nadal et al., 2017; Žalmanová et al., 2017).
Interestingly, our finding that BPS increased granulosa cell estradiol production under basal but not FSH-stimulated conditions suggests that FSH may inhibit the actions of BPS on estradiol synthesis. Little is known about the potential interactions between gonadotropins and endocrine disruptors (Kwintkiewicz and Giudice, 2009), despite the important role of changing concentrations of FSH and LH throughout the estrous cycle on granulosa and theca cell steroid hormone production (Hillier et al., 1994). BPS is converted into glucuronide and sulfate conjugated forms by a number of zebrafish and human cell lines and these conjugated forms have significantly lower estrogenic activity than the unconjugated form (Le Fol et al., 2015). Levels of glucuronosyltransferase enzymes have been reported to be upregulated in response to gonadotropins in rats (Becedas et al., 1998; Magnanti et al., 2000). Therefore, further investigation into whether FSH alters the ratio of conjugated/unconjugated BPS in bovine granulosa cells is warranted. Furthermore, given the importance of changing concentrations of gonadotropins, it will be pertinent to test a range of FSH and LH concentrations to determine whether ovarian cells are more or less susceptible to BPS at different stages of the estrous cycle.
In summary, we report for the first time that at a high dose, BPS alters estradiol production by bovine granulosa cells. Furthermore, we discovered that FSH had a protective effect, thereby attenuating the effect of BPS on granulosa cell estradiol production. Under our experimental conditions, other measures (cell viability, androstenedione production, and/or progesterone production) were not affected in either granulosa or theca cells. Future investigations will reveal whether BPS alters other granulosa cell and theca cell functions that ultimately may impair oocyte quality.
Acknowledgments
This work was supported by the National Institutes of Health [grant number 1R15ES024520-01 to CMHC]
Footnotes
KAC performed the research, analysed and interpreted the data, and drafted and edited the manuscript. ML performed the research and edited the manuscript. CMHC designed the study, and edited the manuscript.
Conflict of interest statement
The authors do not have an conflicts of interest to declare.
References
- Ahmed S, Atlas E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes. 2016;40:1566–1573. doi: 10.1038/ijo.2016.95. [DOI] [PubMed] [Google Scholar]
- Becedas L, Lundgren B, Pierre JWD. Characterization of the UDP-glucuronosyltransferase isoenzyme expressed in rat ovary and its regulation by gonadotropins. Biochem J. 1998;332:51–55. doi: 10.1042/bj3320051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beker ARCL, Colenbrander B, Bevers MM. Effect of 17β-estradiol on the in vitro maturation of bovine oocytes. Theriogenology. 2002;58:1663–1673. doi: 10.1016/s0093-691x(02)01082-8. [DOI] [PubMed] [Google Scholar]
- Butler ST, Pelton SH, Knight PG, Butler WR. Follicle-stimulating hormone isoforms and plasma concentrations of estradiol and inhibin A in dairy cows with ovulatory and non-ovulatory follicles during the first postpartum follicle wave. Domest Anim Endocrinol. 2008;35:112–119. doi: 10.1016/j.domaniend.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Chen M-Y, Ike M, Fujita M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol. 2002;17:80–86. doi: 10.1002/tox.10035. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J, Giudice L, Hauser R, Prins G, Soto A, Zoeller R, Gore A. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman SJ, Bevers MM, Poortman J, Tol HTM van. Steroid and pituitary hormone concentrations in the fluid of preovulatory bovine follicles relative to the peak of LH in the peripheral blood. J Reprod Fertil. 1983;69:641–649. doi: 10.1530/jrf.0.0690641. [DOI] [PubMed] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin M-J, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103:11–21. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Feng Y, Jiao Z, Shi J, Li M, Guo Q, Shao B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere. 2016;147:9–19. doi: 10.1016/j.chemosphere.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Armstrong DT. Hormonal Control of 17β-Estradiol Biosynthesis in Proestrous Rat Follicles: Estradiol Production by Isolated Theca Versus Granulosa. Endocrinology. 1978;102:227–235. doi: 10.1210/endo-102-1-227. [DOI] [PubMed] [Google Scholar]
- Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002;36:1429–1438. doi: 10.1016/s0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP)-4, -6, and-7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- Hill CE, Sapouckey SA, Suvorov A, Vandenberg LN. Developmental exposures to bisphenolm S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: A pilot study. Cogent Med. 2017;4:1317690. [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Ingelido AM, Abballe A, Domenico A di, Fochi I, Iacovella N, Saragosa A, Spagnesi M, Valentini S, Felip ED. Levels and Profiles of Polychlorinated Dibenzo-p-Dioxins, Polychlorinated Dibenzofurans, and Polychlorinated Biphenyls in Feedstuffs and Milk From Farms in the Vicinity of Incineration Plants in Tuscany, Italy. Arch Environ Contam Toxicol. 2009;57:397–404. doi: 10.1007/s00244-008-9262-y. [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K. Effects of Bisphenol S Exposure on Endocrine Functions and Reproduction of Zebrafish. Environ Sci Technol. 2013;47:8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104:715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y. Estrogenic activity of alkylphenols, bisphenol S their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol. 2005;19:121–130. doi: 10.1016/j.etap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health. 2015;14:13. doi: 10.1186/1476-069X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fol V, Aït-Aïssa S, Cabaton N, Dolo L, Grimaldi M, Balaguer P, Perdu E, Debrauwer L, Brion F, Zalko D. Cell-Specific Biotransformation of Benzophenone-2 and Bisphenol-S in Zebrafish and Human in Vitro Models Used for Toxicity and Estrogenicity Screening. Environ Sci Technol. 2015;49:3860–3868. doi: 10.1021/es505302c. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and Their Implications for Human Exposure. J Agric Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit Contam Part A. 2014;31:319–329. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon H-B, Nakata H, Kannan K. Bisphenol S in Urine from the United States and Seven Asian Countries: Occurrence and Human Exposures. Environ Sci Technol. 2012;46:6860–6866. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- Lucy MC. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J Dairy Sci. 2001;84:1277–1293. doi: 10.3168/jds.S0022-0302(01)70158-0. [DOI] [PubMed] [Google Scholar]
- Magnanti M, Giuliani L, Gandini O, Gazzaniga P, Santiemma V, Ciotti M, Saccani G, Frati L, Aglianò AM. Follicle-stimulating hormone, testosterone, and hypoxia differentially regulate UDP-glucuronosyltransferase 1 isoforms expression in rat Sertoli and peritubular myoid cells. J Steroid Biochem Mol Biol. 2000;74:149–155. doi: 10.1016/s0960-0760(00)00095-9. [DOI] [PubMed] [Google Scholar]
- Magnusson U, Persson S. Endocrine Disruptors in Domestic Animal Reproduction: A Clinical Issue? Reprod Domest Anim Zuchthyg. 2015;50:15–19. doi: 10.1111/rda.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur A, Adir M, Yerushalmi G, Hourvitz A, Gitman H, Yung Y, Orvieto R, Machtinger R. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum Reprod. 2016;31:1562–1569. doi: 10.1093/humrep/dew088. [DOI] [PubMed] [Google Scholar]
- Mlynarčíková A, Kolena J, Ficková M, Scsuková S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Molina-Molina J-M, Amaya E, Grimaldi M, Sáenz J-M, Real M, Fernández MF, Balaguer P, Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol. 2013;272:127–136. doi: 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Ripoll C, Villar-Pazos S, Castellano-Muñoz M, Soriano S, Martinez-Pinna J, Quesada I, Alonso-Magdalena P. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? J Steroid Biochem Mol Biol. 2017 doi: 10.1016/j.jsbmb.2017.01.014. http://www.sciencedirect.com/science/article/pii/S0960076017300146. [DOI] [PubMed]
- Naderi M, Wong MYL, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Rhind S. Are Endocrine Disrupting Compounds a Threat to Farm Animal Health, Welfare and Productivity? Reprod Domest Anim. 2005;40:282–290. doi: 10.1111/j.1439-0531.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, Vugt-Lussenburg BMA van, Wedebye EB, Taxvig C, Vinggaard AM. Are Structural Analogues to Bisphenol A Safe Alternatives? Toxicol Sci. 2014;139:35–47. doi: 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- Shi M, Sekulovski N, MacLean JA, Hayashi K. Effects of bisphenol A analogues on reproductive functions in mice. Reprod Toxicol. 2017;73:280–291. doi: 10.1016/j.reprotox.2017.06.134. [DOI] [PubMed] [Google Scholar]
- Sweeney T. Is exposure to endocrine disrupting compounds during fetal/post-natal development affecting the reproductive potential of farm animals? Domest Anim Endocrinol. 2002;23:203–209. doi: 10.1016/s0739-7240(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, Merrick BA, Jetten AM, Austin CP, Tice RR. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact. 2013;203:556–564. doi: 10.1016/j.cbi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Prins GS. Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA) Andrology. 2016;4:561–564. doi: 10.1111/andr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg BRA, Tol V, Ta H, Roelen BAJ, Colenbrander B, Bevers MM. Estradiol and Its Membrane-Impermeable Conjugate (Estradiol-Bovine Serum Albumin) During In Vitro Maturation of Bovine Oocytes: Effects on Nuclear and Cytoplasmic Maturation, Cytoskeleton, and Embryo Quality. Biol Reprod. 2004;70:1465–1474. doi: 10.1095/biolreprod.103.025684. [DOI] [PubMed] [Google Scholar]
- Xi W, Lee CKF, Yeung WSB, Giesy JP, Wong MH, Zhang X, Hecker M, Wong CKC. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31:409–417. doi: 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A Induces Apoptosis and G2-to-M Arrest of Ovarian Granulosa Cells. Biochem Biophys Res Commun. 2002;292:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ Sci Technol. 2015;49:11834–11839. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žalmanová T, Hošková K, Nevoral J, Adámková K, Kott T, Šulc M, Kotíková Z, Prokešová Š, Jílek F, Králíčková M, Petr J. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci Rep. 2017;7:485. doi: 10.1038/s41598-017-00570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Shi J, Liu X, Zhan X, Chen Q. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Res. 2014;58:248–257. doi: 10.1016/j.watres.2014.03.074. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]