Abstract
This study aimed to determine whether functional disturbances in fronto‐striatal control circuits characterize adolescents with Bulimia Nervosa (BN) spectrum eating disorders regardless of clinical severity. FMRI was used to assess conflict‐related brain activations during performance of a Simon task in two samples of adolescents with BN symptoms compared with healthy adolescents. The BN samples differed in the severity of their clinical presentation, illness duration and age. Multi‐voxel pattern analyses (MVPAs) based on machine learning were used to determine whether patterns of fronto‐striatal activation characterized adolescents with BN spectrum disorders regardless of clinical severity, and whether accurate classification of less symptomatic adolescents (subthreshold BN; SBN) could be achieved based on patterns of activation in adolescents who met DSM5 criteria for BN. MVPA classification analyses revealed that both BN and SBN adolescents could be accurately discriminated from healthy adolescents based on fronto‐striatal activation. Notably, the patterns detected in more severely ill BN compared with healthy adolescents accurately discriminated less symptomatic SBN from healthy adolescents. Deficient activation of fronto‐striatal circuits can characterize BN early in its course, when clinical presentations are less severe, perhaps pointing to circuit‐based disturbances as useful biomarker or risk factor for the disorder, and a tool for understanding its developmental trajectory, as well as the development of early interventions.
Keywords: adolescents, Bulimia Nervosa, conflict resolution, fronto‐striatal circuits, functional magnetic resonance imaging, self‐regulatory control, Simon task, machine learning, multi‐voxel pattern analysis
1. INTRODUCTION
Bulimia Nervosa (BN) is an eating disorder that typically begins in adolescence, is more prevalent in females, and associated with an increased risk for a range of future physical and mental health problems (Stice , Marti, Spoor, Presnell, & Shaw, 2008). Individuals with BN present with recurrent cycles of binge eating episodes and compensatory behaviors to avoid weight gain. These cycles are typically accompanied by obsessive thoughts related to food, weight and body image, as well as a sense of loss of control (LOC) (Kaye, Strober, & Jimerson, 2004; Klein & Walsh, 2003).
BN is associated with deficits in self‐regulatory control, a construct that encompasses executive control, emotional regulation, and the ability to delay gratification (Mischel, Shoda, & Rodriguez, 1989). Findings from studies of healthy (Casey et al., 2011; Diamond, 1988) and ill (Emond, Joyal, & Poissant, 2009; Marsh, Zhu, Wang, Skudlarski, & Peterson, 2007; Raz et al., 2009) individuals indicate that fronto‐striatal circuits underlie the capacity for self‐regulatory control. Previous MRI findings suggest that fronto‐striatal circuits are structurally (Cyr et al., 2017; He, Stefan, Terranova, Steinglass, & Marsh, 2015; Marsh et al., 2015) and functionally (Cyr et al., 2016; Lock, Garrett, Beenhakker, & Reiss, 2011; Marsh et al., 2011, 2009; Skunde et al., 2016) abnormal in BN. These disturbances likely contribute to the LOC over eating behaviors that characterize the disorder.
Our previous fMRI findings point to deficient engagement of fronto‐striatal circuits, in adult (Marsh et al., 2009) and adolescent (Marsh et al., 2011) females with BN during their performance of the Simon Spatial Incompatibility task (Simon, 1969). This task requires ignoring task‐irrelevant feature of a stimulus (i.e., the side of the screen on which an arrow appears) when it conflicts with a more task‐relevant one (the direction towards which the arrow points). In healthy individuals, activation of regions within fronto‐striatal circuits is associated with correct responses to incongruent stimuli (i.e., the resolution of cognitive conflict, Marsh et al., 2011; Marsh et al., 2009). In contrast to their healthy counterparts, adults with BN show reduced activation in bilateral inferior frontal gyrus (IFG), dorsal striatum and anterior cingulate cortex (ACC) during correct responding to incongruent stimuli, and greater reductions with more severe BN symptoms (Marsh et al., 2009). In addition, adolescents with BN also show reduced conflict‐related activation in these fronto‐striatal regions during the resolution of conflict and, particularly, during the resolution of maximal conflict (i.e., post‐congruent conflict: incongruent stimuli preceded by congruent stimuli; Marsh et al, 2011). Unclear is whether these functional deficits within fronto‐striatal circuits are markers of BN that characterize less severe clinical presentations of BN during adolescence.
Using the abovementioned fMRI paradigm, we assessed whether specific patterns of fronto‐striatal disturbances during the engagement of control and conflict resolution characterized BN adolescents regardless of their clinical presentations. BN adolescents were thus divided into those who met all DSM‐5 criteria for BN, and those who engaged LOC eating episodes and compensatory behaviors to avoid weight gain, but met DSM‐5 criteria for Other Specified Feeding or Eating Disorder (OSFED) rather than BN. Studying adolescents with this latter presentation (subthreshold BN; SBN) is novel but clinically relevant, given that the LOC over eating is more characteristic of binge‐eating behavior than the amount of food consumed in adolescents (Fitzsimmons‐Craft et al., 2014). Furthermore, given that a substantial portion of adolescents with SBN eventually progress to BN (Stice, Marti, & Rohde, 2013), identifying markers common to threshold and SBN may provide a window for early intervention.
Given the limitations of mass‐univariate approaches, which assume that activity in a given brain region occurs independently from activity in other regions (Mahmoudi, Takerkart, Regragui, Boussaoud, & Brovelli, 2012), we used multivariate pattern analyses (MVPAs) to test the following hypotheses: (a) spatially distributed patterns of activations within fronto‐striatal areas during the resolution of post‐congruent conflict will accurately discriminate BN from healthy adolescents, regardless of clinical presentation (DSM5 criteria for BN or SBN), and that (b) the patterns of fronto‐striatal disturbances detected in the more severely ill (BN) relative to healthy adolescents would accurately classify and therefore characterize the less severely ill adolescents (SBN) as BN spectrum relative to healthy.
2. METHODS
2.1. Participants
Participants were recruited through advertisement in the community or online, and included: 44 female adolescents with BN symptoms and 40 healthy control (HC) female adolescents, matched on age, BMI, race and ethnicity. Demographics are shown in Table 1, along with clinical ratings for the clinical groups. Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, developmental disorder, or current Axis I disorders (other than depressive or anxiety disorders for the clinical group) were excluded. BN symptom severity and prior histories of Anorexia Nervosa (AN) were assessed with the Eating Disorders Examination (Cooper & Fairburn, 1987). Adolescents in the BN group (n = 28) met DSM5 criteria for BN, engaging in an average of one objective bulimic episode (OBE) and one compensatory behavior per week over the past 3 months. Adolescents in the SBN group (n = 16) were included if they engaged in an average of one LOC eating episode (i.e., objectively or subjectively large) and one compensatory behavior per week within the past 3 months (Fitzsimmons‐Craft et al., 2014). HC participants had no lifetime Axis I disorders. Diagnoses of BN, SBN (OSFED), and comorbid psychiatric disorders were made using the Structured Clinical Interview for DSM Disorders (First et al., 2002) for adolescents 18 years and older, and the Kiddie‐Sads‐Present and Lifetime Version (Kaufman et al., 1997) for those under 18 years. The DuPaul‐Barkley Attention‐Deficit Hyperactivity Disorder Rating Scale (DuPaul, 1991) quantified symptoms of inattention and hyperactivity. Full‐scale IQs were estimated using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1981). Data from 12 BN, 5 SBN, and 18 HC adolescents were included in a previous fMRI study (Marsh et al., 2011). Data from the remaining BN and HC participants were collected between 2011 and 2014. Participants provided informed consent or assent for a protocol approved by the Institutional Review Board of the New York State Psychiatric Institute. See Supporting Information Methods for a complete description of the participants.
Table 1.
Demographic and clinical characteristics of female adolescents with threshold and SBN, and age‐matched female healthy comparison participants
| BN (n = 28) | SBN (n = 16) | HC (n = 40) | Analysis | ||
|---|---|---|---|---|---|
| Characteristic | Mean (SD) | Mean (SD) | Mean (SD) | F(df) or χ2(df, n) | p |
| Age (years) | 18.0 (1.6) | 16.3 (2.2) | 17.0 (2.1) | F(2,81)=4.56 | .013a |
| Body mass index (kg/m2) | 21.6 (2.6) | 22.1 (2.0) | 21.7 (2.9) | F(2,81)=0.23 | .797 |
| Duration of illness (months) | 33.4 (22.1) | 18.9 (16.4) | F(1,42)=5.24 | .027 | |
| WAIS IQ score (Full) | 109.3 (11.3) | 105.9 (12.5) | 110.9 (15.0) | F(1,80)=0.74 | .481 |
| Eating Disorders Examination ratings | |||||
| OBEs (past 28 days) | 30.4 (24.0) | 3.9 (54.6) | F(2,42)=18.76 | <.001 | |
| Subjective bulimic episodes (past 28 days) | 21.8 (25.7) | 15.3 (14.9) | F(2,42)=0.86 | .358 | |
| Vomiting episodes (past 28 days) | 51.9 (45.6) | 19.1 (29.5) | F(2,42)=6.64 | .014 | |
| LOC (past 28 days) | 52.2 (36.1) | 19.1 (13.4) | F(2,42)=12.34 | .001 | |
| Prior AN (n/%) | 6/21.4 | 2/12.5 | χ2(1,44)=0.55 | .460 | |
| Comorbid MDD (n/%) | 8/28.6 | 4/25.0 | χ2(1,44)=0.07 | .798 | |
| Comorbid Anxiety (n/%) | 2/7.1 | 5/31.2 | χ2(1,44)=4.42 | .035b | |
| SSRIs use (n/%) | 5/17.8 | 5/31.2 | χ2(1,44)=1.04 | .308 | |
| Treatment | |||||
| Inpatient (n/%) | 10/35.7 | 2/12.5 | χ2(1,44)=2.77 | .096 | |
| Outpatient (n/%) | 5/17.9 | 6/37.5 | χ2(1,44)=2.10 | .148 | |
Post‐hoc analyses revealed that a significant difference in age between BN and SBN (p < .05) but not between HC and any of the clinical samples (ps > .05).
More SBN than BN adolescents had comorbid anxiety diagnoses, likely due to our relaxed inclusion criteria given the difficulty recruiting younger, less severely ill adolescents with eating disorders.
Abbreviations: AN = Anorexia Nervosa; BN = Bulimia Nervosa; HC = healthy control; MDD = major depressive disorder; SBN = subthreshold Bulimia Nervosa; SSRIs = selective serotonin reuptake inhibitors; WAIS = Wechsler Adult Intelligence Scale.
2.2. fMRI paradigm
All participants completed the Simon Spatial Compatibility task, as previously described in Marsh et al. (2011). Briefly, in each trial, participants were presented with a leftward or rightward pointing arrow that was either congruent or incongruent with their position (left or right) on the screen. Participants were instructed to respond as quickly and accurately as possible to the direction in which the arrow was pointing by pressing a button on a response box using the index finger for left and the middle finger for right. Stimulus duration was 1,300 ms, with jittered intervals ranging from 4,160 to 6,960 ms (M = 5,350, SD = 1,159.98) between each trial. Each of 3 runs contained 55 stimuli, with 11 blank stimuli, 22 congruent stimuli, and 22 incongruent stimuli. Across the three runs, a total of 30 congruent and 34 incongruent stimuli were preceded by a congruent stimulus (i.e., post‐congruent Congruent [cC] and post‐congruent Incongruent [cI]). The E‐prime software (Psychology Software Tools, Inc., Sharpsburg, PA) was used to program and run the experiment, and to record participants' responses and response times (RTs).
2.3. Behavioral analyses
RTs on correct trials were entered as dependent variables in a repeated measures, mixed‐model analysis of variance (ANOVA) in IBM SPSS Statistics 22.0 (Armonk, NY: IBM Corp.) with Stimulus (post‐congruent Incongruent [cI] vs. post‐congruent Congruent [cC]) as the within‐subject variable, and Diagnostic Sample (BN, SBN, HC) as the between‐subject variable.
2.4. Image acquisition and preprocessing
All participants data were collected using the same scanner, equipment, sequence and protocol. Images were collected using a GE Signa 3 Tesla LC scanner (Milwaukee, WI). Functional images were acquired using a T2* sensitive, gradient‐recalled, single shot, echo‐planar pulse sequence (repetition time = 2,200 milliseconds, echo time = 30 milliseconds, 90 degree flip angle, single excitation per image, 24*24 cm field of view, 64*64 matrix, 34 slices 3.5‐mm thick, no gap, covering the entire brain). We collected 140 echo‐planar imaging volumes for each run. All images were preprocessed using the SPM12 (Wellcome Department of Imaging Neuroscience, London [http://www.fil.ion.ucl.ac.uk/spm/]) standard procedure. Functional images were corrected for differences in slice timing using sinc‐interpolation, and head movement was corrected using a least‐squares approach and a six‐parameter rigid body spatial transformation. Structural data were coregistered to the functional data and segmented into tissues probability maps, bias corrects and spatially normalized to the Montreal Neurological Institute (MNI) space of 1 × 1 × 1 mm3 voxels. Using the deformation fields of these segmented images, the functional images were subsequently spatially normalized to MNI space of and 3 × 3 × 3 mm3 voxels. An 8‐mm full‐width/half‐maximum isotropic Gaussian smoothing kernel was applied to all normalized functional images. All analyses included a temporal high‐pass filter (128 s), correction for temporal autocorrelation using an autoregressive AR(1) model, and each image was scaled to have a global mean intensity of 100.
2.5. First level analyses
First‐level parametric analyses were performed for each participant using the general linear model (GLM) provided by SPM12. For each participant, preprocessed time series data from all three Simon task runs (420 volumes) were modeled using a GLM with six conditions: (a) Incongruent correct trails preceded by congruent trials (cI), (b) Congruent correct trials preceded by incongruent trials (iC), (c) Incongruent correct trials preceded by incongruent trials (iI), (d) Congruent correct trials preceded by congruent trials (cC), (e) fixation trials, and (f) incorrect trials (incongruent or congruent), including trials with reaction times below the minimal RT of 200ms for stimulus detection and processing. These events were convolved with the canonical HRF and then least‐squares regression was used to estimate parameters for each independent variable for each participant. Only correct trials were included, given the limited number of incorrect trials. Runs in which a participant had >30% error rate (ER) on the task or more than a voxel of total displacement in any of the six standard motion parameters were excluded from our analyses.
Because activation of fronto‐striatal regions is greatest when level of conflict is maximal (i.e., when incongruent stimuli are preceded by congruent stimuli) (Horga et al., 2011), we focused our analyses on these post‐congruent trials. Parameter estimates averaged across the three runs were used to produce a post‐congruent Incongruent versus post‐congruent Congruent (cI–cC) contrast for each participant to access brain activation associated with the engagement of selfregulatory control and resolution of cognitive conflict, when conflict is maximal. Given that the samples included herein are not independent from those included in our previous study of adolescents with BN (Marsh et al., 2011), replication using univariate GLM‐based analyses are not the focus of this paper. Nevertheless, group level t‐maps showing activation associated with the task in the whole sample (Supporting Information Figure S1) and with post‐congruent conflict (cI–cC) within and between groups (Supporting Information Figure S2) are presented in the supplementary materials.
2.6. Multi‐voxel pattern analyses
We conducted MVPA in Matlab using functions from the Princeton MVPA Toolbox (https://github.com/princetonuniversity/princeton-mvpa-toolbox; Polyn, Natu, Cohen, & Norman, 2005) with a linear support vector machine (SVM) (Cortes & Vapnik, 1995) to classify participants as ill or healthy (BN vs. HC or SBN vs. HC) based on distributed patterns of activation during response to post‐congurent conflict (cI–cC). These classification analyses focused on fronto‐striatal regions, specifically the IFG pars opercularis, orbitalis, and triangularis, the ACC and the putamen, given their involvement in the resolution of cognitive conflict (Liu, Banich, Jacobson, & Tanabe, 2004; Peterson et al., 2002; Rubia et al., 2006) and our previous findings from adults (Marsh et al., 2009) and adolescents (Marsh et al., 2011) with BN performing this task. Because the inclusion of a large number of uninformative features (i.e., in this case, activation voxels) usually results in poor classification performance, we applied an a priori mask comprised of these fronto‐striatal regions, as defined by the Automated Anatomical Labeling atlas, to select a subset of putatively relevant activation features for use in the classification models.
Three sets of classification analyses were conducted. In the first set, contrast maps from the 28 BN and 28 randomly selected HC participants were divided into training and test datasets by leaving out the map from one participant per group (i.e., BN and HC) at a time in a leave‐one‐subject‐per‐group‐out cross‐validation scheme. Similarly, in the second set of analyses, the SVM classifier was trained and cross‐validated on the maps from the 16 SBN and 16 randomly selected HC participants. To ensure that the training of the classifier was unbiased within each sample, the pairs of subjects entered into these classification analyses were randomized over 50 permutations. In the third set of classification analyses, the SVM classifier was trained on the maps from participants in the first set of classification analyses (28 BN and 28 HC participants), and then tested (i.e., validated) on the maps from 12 SBN participants and the 12 HC participants whose maps were left out of the training phase. The number of SBN participants selected for testing the classifier was down‐sampled from 16 to 12 to balance the numbers of SBN and HC participants. The HC maps selected for training and testing and the SBN maps selected for testing were randomized over 50 permutations, thereby guaranteeing unbiased training and testing of the classifier.
To further reduce dimensionality and facilitate the classifier's performance, an ANOVA (p < .05) revealed voxels (i.e., features) that did not vary significantly between groups within each cross‐validation training set and were therefore unselected as features for classification. Note that this ANOVA was conducted iteratively on the training data, strictly independent from the test data, to ensure that feature selection did not spuriously/illegitimately improve classification of the test data in any way. Statistical significance for each classification analysis was determined by permutation testing with 1,000 iterations. Metrics of accuracy, specificity (i.e., HC classification accuracy), sensitivity (i.e., BN or SBN classification accuracy) and significance were averaged across the 50 permutations for each classification model.
To ensure that findings from the a priori classification analyses within fronto‐striatal circuits were not driven by the effects of medication or comorbid illnesses, additional post‐hoc classification analyses were performed in after excluding BN and SBN participants (a) taking SSRIs, (b) with lifetime AN, 9c) comorbid MDD, or (d) comorbid anxiety. Exploratory classification analyses using BN versus HC as a training sample and SBN vs. HC as a validation sample were also conducted whole‐brain, as well as within each anatomical region comprised in the a priori fronto‐striatal mask.
3. RESULTS
3.1. Participants
Twenty‐eight female adolescents meeting DSM‐5 criteria for BN (mean age: 18.0 years; age range: 15–21 years), 16 female adolescents with SBN (mean age: 16.3 years; age range: 13–21 years) and 40 age‐matched healthy female adolescents were included in this study (mean age: 17.0 years; age range: 13–21 years). An additional two participants (one HC and one SBN) were tested but excluded from analyses because their overall ER on the task exceeded 30%. When compared with the BN adolescents, those with SBN were younger (p = .004, Table 1), consistent with the demographic characterization of these clinical presentations in adolescents (Eddy et al., 2008). The SBN adolescents were also less advanced in their illness duration (p=.027), and less symptomatic, engaging in less frequent LOC eating episodes (defined as the sum of objective and subjective binge‐eating episodes within the past 28 days; p = .001), OBEs (p < .001), and self‐induced vomiting episodes (p = .014).
3.2. Behavioral performance
Supporting information Table S1 presents descriptive statistics and group comparisons on task performance (RT and ER) for each condition (cC, cI, iC, and iI). On average, participants responded correctly to 95% (SD = 7%) of the trials. As a result, only 5% of the trials were excluded from our image analyses. The repeated measures ANOVA revealed a significant main effect of stimulus indicated that all participants responded faster to congruent compared with incongruent stimuli (F[1,80] = 150.84, p < .001). No significant Group effect or Diagnostic Sample‐by‐Stimulus interaction was detected (ps > .1), suggesting that performance did not differ across BN, SBN, and HC adolescents (Figure 1).
Figure 1.
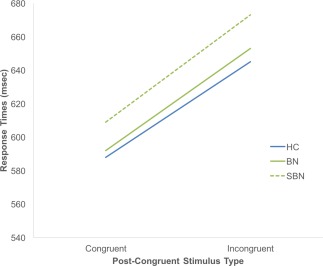
RTs in response to post‐congruent stimuli on the Simon task in the BN, SBN and HC groups. Reaction times are depicted as a function of stimulus type across all trials. Abbreviations: BN, Bulimia Nervosa; HC, healthy control; SBN, subthreshold Bulimia Nervosa [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Multi‐voxel pattern analyses
3.3.1. A priori classification analyses
The SVM was able to correctly classify BN vs. HC participants and SBN vs. HC participants based on conflict‐related activation patterns within fronto‐striatal regions (BN vs. HC: 58.4%, p < .001; SBN vs. HC: 64.3%, p < .001). Using SBN vs. HC as validation samples, classifiers trained on activation patterns in BN versus HC also led to accurate classification of participants as ill versus healthy (60%, p < .001). Figure 2 shows the relative contribution of each voxel within these fronto‐striatal regions to group classification based on classifiers trained on contrast maps from BN versus HC (left panel) and from SBN versus HC (right panel). Accuracy, sensitivity, specificity, and significance metrics are presented in Table 2.
Figure 2.
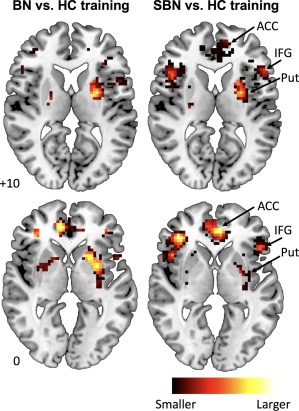
Relative contribution of each voxel within the a priori fronto‐striatal mask to group classification following training on conflict‐related activation patterns from BN versus HC (left panel) and from SBN versus HC (right panel). Abbreviations: ACC, anterior cingulate cortex; BN, Bulimia Nervosa; HC, healthy control; IFG, inferior frontal gyrus; Put, putamen; SBN, subthreshold Bulimia Nervosa
Table 2.
Performance metrics of classification of BN versus HC and of SBN versus HC using a cross‐validation scheme, and of classification of SBN versus HC based on training in BN versus HC
| Analysis | Acc (%) | Spec (%) | Sens (%) | p |
|---|---|---|---|---|
| BN vs. HC CV | 58.4 | 57.7 | 59.7 | <.001 |
| SBN vs. HC CV | 64.3 | 66.8 | 62.6 | <.001 |
| Train BN vs. HC, Test SBN vs. HC | 65.9 | 66.0 | 66.9 | <.001 |
Abbreviations: Acc = accuracy; BN = Bulimia Nervosa; CV = cross‐validation; HC = rol; SBN = subthreshold Bulimia Nervosa; Sens = sensitivity; Spec = specificity.
3.3.2. Exploratory classification analyses
Classification findings within the fronto‐striatal mask remained significant after excluding BN and SBN participants who were either taking SSRIs, had comorbid diagnoses of MDD, or a lifetime history of AN (Table 3). Classification findings also remained significant after excluding participants with a comorbid anxiety disorder when cross‐validating within BN versus HC and within SBN vs. HC, but not when validating in SBN versus HC the classifier trained on patterns in BN versus HC (Table 3). Supporting Information Figure S3 shows the relative contribution of each voxel within these fronto‐striatal regions to group classification based on classifiers trained on contrast maps from BN versus HC (Supporting Information Figure S3A) and from SBN versus HC (Supporting Information Figure S3B).
Table 3.
Performance metrics of post‐hoc classification analyses conducted within the fronto‐striatal mask after excluding BN and SBN participants (a) with a history of AN, (b) with comorbid anxiety, (c) with comorbid depression, and (d) taking SSRIs
| BN vs. HC CV | SBN vs. HC CV | Train BN vs. HC, test SBN vs. HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Acc (%) | Spec (%) | Sens (%) | p | Acc (%) | Spec (%) | Sens (%) | p | Acc (%) | Spec (%) | Sens (%) | p |
| No AN | 62.3 | 61.0 | 64.3 | <.001 | 64.4 | 65.7 | 63.6 | <.001 | 57.1 | 55.9 | 59.7 | <0.01 |
| No Anx | 55.5 | 55.2 | 55.8 | <.05 | 61.1 | 61.2 | 61.3 | <.001 | 53.2 | 52.5 | 55.2 | ns |
| No MDD | 60.3 | 59.4 | 61.7 | <0.01 | 64.0 | 65.0 | 63.4 | <.001 | 56.7 | 56.3 | 58.1 | <0.05 |
| No SSRIs | 59.7 | 59.8 | 59.8 | <0.01 | 59.6 | 60.4 | 59.2 | <.01 | 57.4 | 55.9 | 60.4 | <0.01 |
Abbreviations: AN = anorexia nervosa; Anx = anxiety; BN = Bulimia Nervosa; CV = cross‐validation; HC = healthy control; MDD = major depressive disorder; SBN = subthreshold Bulimia Nervosa; Sens = sensitivity; Spec = specificity; SSRIs = selective serotonin reuptake inhibitors.
Whole‐brain and regional activation patterns from BN versus HC led to accurate classification of SBN and HC participants as ill vs. healthy with maximal accuracy in right putamen (69%, p < .001) and left ACC (65%). Accuracy, sensitivity, specificity, and significance metrics for these models are also reported in Table 4.
Table 4.
Performance metrics of exploratory classification models
| Train BN vs. HC, test SBN vs. HC | |||||
|---|---|---|---|---|---|
| Region | Side | Acc (%) | Spec (%) | Sens (%) | p |
| Whole‐brain | Bilateral | 61.3 | 62.2 | 61.2 | <.001 |
| ACC | Left | 64.5 | 67.5 | 62.7 | <.001 |
| Right | 51.3 | 61.3 | 50.7 | ns | |
| IFG oper | Left | 50.3 | 50.2 | 50.9 | ns |
| Right | 55.0 | 54.3 | 57.7 | <.05 | |
| IFG orb | Left | 59.7 | 57.7 | 65.6 | <.001 |
| Right | 48.7 | 48.0 | 48.8 | ns | |
| IFG tri | Left | 58.3 | 66.0 | 57.5 | <.001 |
| Right | 52.0 | 53.3 | 52.4 | ns | |
| Putamen | Left | 51.4 | 58.3 | 51.1 | ns |
| Right | 69.1 | 63.6 | 83.6 | <.001 | |
Abbreviations: Acc = accuracy; ACC = anterior cingulate cortex; Bulimia Nervosa; CV = cross‐validation; HC = healthy control; oper = opercularis; orb = orbitalis; IFG = inferior frontal gyrus; SBN = subthreshold Bulimia Nervosa; Sens = sensitivity; Spec = specificity; tri = triangularis.
4. DISCUSSION
Findings from this study show, for the first time, that specific patterns of functional disturbances in fronto‐striatal regions can characterize adolescents with BN spectrum disorders early in the course of the illness. MVPA based on machine learning revealed patterns of conflict‐related activation within IFG, ACC and putamen that discriminate adolescents with BN spectrum disorders from healthy adolescents, regardless of the severity of their clinical presentation. Crucially, the patterns detected in the adolescents with the more severely ill presentation (BN) compared with healthy adolescents accurately discriminated the adolescents with less severely ill presentation (SBN) from healthy adolescents. Sample differences in age and illness duration did not affect these classification findings. Thus, we were able to classify adolescents as BN spectrum versus healthy based on patterns of activation within fronto‐striatal regions despite the different clinical presentations as well as the different ages and illness durations across clinical samples. These findings suggest that deficient functioning of these circuits may indeed be a neurobiological marker or a risk factor for the illness.
These findings have novel research and clinical implications. In particular, these circuits may constitute promising targets for understanding the developmental trajectory of BN in future longitudinal studies, as well as for treatment selection, the prediction of treatment response, and the development of novel treatments (or additions to evidence‐based treatments). For instance, our recent longitudinal findings suggest increasing engagement of fronto‐striatal circuits over time in adolescents who are most resilient to persistent BN, perhaps pointing to a compensatory mechanism that permits the regulation of eating behaviors over development (Cyr et al., in press). As such, noninvasive brain‐based technologies, such as real‐time fMRI neurofeedback, repetitive transcranial magnetic stimulation, and transcranial direct‐current stimulation may be promising tools to target fronto‐striatal circuits and enhance self‐regulatory control over conflicting drives to binge‐eat and remain thin (Dunlop et al., 2015; Lapenta, Sierve, de Macedo, Fregni, & Boggio 2014; Ochsner, Silvers, & Buhle, 2012). Future studies should also examine whether fronto‐striatal abnormalities can predict response to empirically supported therapeutic approaches such as cognitive or dialectic behavioral therapy, and how the integrity of fronto‐striatal circuits may mediate the effects of therapy on specific BN symptoms.
Despite these research and clinical implications of our findings, it is worth noting that the classification accuracy was only moderate (no >73.2%). Thus, the classifier's performance could be further improved through the integration of multiple modalities (Hahn et al., 2011), or the use of more advanced algorithms and methods that have been applied with machine learning (Kim, Calhoun, Shim, & Lee, 2016; Peng, Lin, Zhang, & Wang, 2013; Watanabe, Kessler, Scott, Angstadt, & Sripada, 2014). Future studies should therefore expand our findings by integrating other features into a single highly selective and specific prediction or classification model of BN. Future longitudinal studies should examine prospectively whether these fronto‐striatal deficits are present prior to the onset of BN symptoms, conferring vulnerability for BN spectrum disorders, or whether they arise with the onset of BN symptoms. Given that a substantial portion (over 30%) of adolescents with SBN progress to BN (Stice et al., 2013), fronto‐striatal alterations may constitute early risk markers for BN, potentially preceding the onset of BN symptoms. Alternatively, these alterations may mark specific BN symptoms rather than the disorder per se.
Pattern recognition techniques involving machine learning have already been applied to identifying biomarkers and diagnostically classifying several neurological and psychiatric disorders (Fu & Costafreda, 2013; Haubold, Peterson, & Bansal, 2012; Kloppel et al., 2012; Orru, Pettersson‐Yeo, Marquand, Sartori, & Mechelli, 2012). Recent findings showed accurate discrimination between individuals with eating disorders (AN or BN) and healthy participants based on anatomical brain features (Cerasa et al., 2015). Other data suggest that activation patterns associated with food cues in reward related brain areas can differentially diagnose individuals with BN and binge‐eating disorder (Weygandt, Schaefer, Schienle, & Haynes, 2012). These neuroimaging‐based computational approaches for the identification of biomarkers offer great hope for clinical applications, from diagnosis to treatment selection and the prediction of treatment outcomes (Huys, Maia, & Frank, 2016). However, most prior studies that have used machine learning to identify markers of psychiatric illnesses have iteratively trained and cross‐validated their classifiers on data within a single sample, an approach that likely overestimates the classifier's performance (Hastie, Tibshirani, & Friedman, 2009) and limits generalizability to new data. Therefore, our approach of training and validating in independent samples permits generalizability of our findings to a younger, less symptomatic sample. Nevertheless, these findings should be replicated in a larger sample consisting of males and females, as well as adolescents with other psychiatric disorders or symptoms.
Additional limitations are worth noting. First, our study samples were cross‐sectional and modest in size, thereby warranting replication of our findings in larger, longitudinal samples. Given our sample size, we limited our analyses to specific fronto‐striatal regions, thereby precluding the inclusion of potentially informative features that would lead to more accurate performance of the classifier. Second, the presence of comorbid anxiety may have influenced our findings since exclusion of BN and SBN adolescents with comorbid anxiety did not reveal accurate classification of SBN versus HC following training in BN vs. HC. Thus, the presence of comorbid anxiety may have contributed to our initial findings or, alternatively, excluding these cases may have reduced our statistical power. Similarly, the pattern of voxel contribution to the classification may have been influenced, in part, by the presence of comorbid MDD, but excluding BN and SBN adolescents with this comorbidity still revealed accurate classification. Nevertheless, the heterogeneity of our study samples reflects the general population of adolescents with BN, in which the presence of comorbid anxiety and depressive disorders is common (Swanson, Crow, Le Grange, Swendsen, & Merikangas, 2011). Third, we did not control for hunger, which can affect attentional and executive processes (Green & Rogers, 1998; Kemps, Tiggemann, & Marshall, 2005; Shaw & Tiggemann, 2004) and therefore confound our findings. Thus, future studies should control for satiety. Fourth, we did not account for menstrual status, which might impact neural functioning in women (Dreher et al., 2007). However, it is unlikely that menstrual status differed systematically across adolescents in the clinical and healthy samples to confound our results. Finally, it is worth noting that no significant group differences in task performance were detected, which may render our fMRI findings difficult to interpret (Wilkinson & Halligan, 2004). However, the absence of behavioral group differences may also facilitate the interpretation of our fMRI classification findings. Indeed, when group performances are matched, differences in fMRI activation cannot be attributed to artifacts of differential compliance with or capacity to perform the behavioral task (Callicott et al., 2003).
Despite these potential limitations, our study has important implications for understanding the developmental trajectory of brain functioning in BN. We showed that abnormal activation patterns associated with conflict resolution in BN spectrum disorders can be identified early in the course of the illness, in adolescents with less severe clinical presentations. Our findings also provide new support for the hypothesis that fronto‐striatal disturbances constitute a useful biomarker or risk factor for BN. Future longitudinal studies are required to determine whether disturbances in these circuits precede BN, in the form of a vulnerable self‐regulatory system, or instead arise with the onset of disordered eating behaviors. Such longitudinal studies are also required to understand how fronto‐striatal disturbances might contribute to the persistence of BN symptoms over adolescence and adulthood. Finally, the application of machine learning‐based data‐driven analysis methods to psychiatric research is still in its infancy, with new and advanced techniques developing rapidly. Such techniques should be incorporated into future research aimed at replicating and extending these marker findings in BN spectrum disorders.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
Cyr M, Yang X, Horga G, Marsh R. Abnormal fronto‐striatal activation as a marker of threshold and subthreshold Bulimia Nervosa. Hum Brain Mapp. 2018;39:1796–1804. 10.1002/hbm.23955
Funding information National Institute of Mental Health, Grant/Award Number: R01MH090062 and K23MH101637.
REFERENCES
- Callicott, J. H. , Mattay, V. S. , Verchinski, B. A. , Marenco, S. , Egan, M. F. , & Weinberger, D. R. (2003). Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. The American Journal of Psychiatry, 160, 2209–2215. [DOI] [PubMed] [Google Scholar]
- Casey, B. J. , Somerville, L. H. , Gotlib, I. H. , Ayduk, O. , Franklin, N. T. , Askren, M. K. , … Shoda, Y. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences of the United States of America, 108, 14998–15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa, A. , Castiglioni, I. , Salvatore, C. , Funaro, A. , Martino, I. , Alfano, S. , … Quattrone, A. (2015). Biomarkers of eating disorders using support vector machine analysis of structural neuroimaging data: Preliminary results. Behavioral Neurology, 2015, 924814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, Z. , & Fairburn, C. (1987). The eating disorder examination: A semi‐structured interview for the assessment of the specific psychopathology of eating disorders. International Journal of Eating Disorders, 6, 1–8. [Google Scholar]
- Cortes, C. , & Vapnik, V. (1995). Support‐vector networks. Machine Learning, 20, 273–297. [Google Scholar]
- Cyr, M. , Fontaine, M. , Stefan, M. , Terranova, K. , Kopala‐Sibley, D. C. , Attia, E. , & Marsh, R. (in press) A longitudinal functional magnetic resonance imaging study of task control circuits and bulimic symptoms over adolescence. Journal of Child Psychology and Psychiatry, xx:xx–xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr, M. , Kopala‐Sibley, D. C. , Lee, S. , Chen, C. , Stefan, M. , Fontaine, M. , … Marsh, R. (2017). Reduced inferior and orbital frontal thickness in adolescent Bulimia Nervosa persists over two‐year follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 866–874 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr, M. , Wang, Z. , Tau, G. Z. , Zhao, G. , Friedl, E. , Stefan, M. , … Marsh, R. (2016). Reward‐based spatial learning in teens with bulimia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 962–971 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A. (1988). Abilities and neural mechanisms underlying AB performance. Child Development, 59, 523–527. [PubMed] [Google Scholar]
- Dreher, J. C. , Schmidt, P. J. , Kohn, P. , Furman, D. , Rubinow, D. , & Berman, K. F. (2007). Menstrual cycle phase modulates reward‐related neural function in women. Proceedings of the National Academy of Sciences of the United States of America, 104, 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop, K. , Woodside, B. , Lam, E. , Olmsted, M. , Colton, P. , Giacobbe, P. , & Downar, J. (2015). Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. NeuroImage Clinical, 8, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul, G. J. (1991). Parent and teacher ratings of ADHD symptoms: Psychometric properties in a community‐based sample. Journal of Clinical Child and Adolescent Psychology, 20, 245–253. [Google Scholar]
- Eddy, K. T. , Celio‐Doyle, A. , Hoste, R. R. , Herzog, D. B. , & le Grange, D. (2008). Eating disorder not otherwise specified in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 156–164. [DOI] [PubMed] [Google Scholar]
- Emond, V. , Joyal, C. , & Poissant, H. (2009). [Structural and functional neuroanatomy of attention‐deficit hyperactivity disorder (ADHD)]. L'Encephale, 35, 107–114. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002) Biometrics research In Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. SCID‐I/P. New York State Psychiatric Institute, New York: [Google Scholar]
- Fitzsimmons‐Craft, E. E. , Ciao, A. C. , Accurso, E. C. , Pisetsky, E. M. , Peterson, C. B. , Byrne, C. E. , & Le Grange, D. (2014). Subjective and objective binge eating in relation to eating disorder symptomatology, depressive symptoms, and self‐esteem among treatment‐seeking adolescents with bulimia nervosa. European Eating Disorders Review, 22, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C. H. , & Costafreda, S. G. (2013). Neuroimaging‐based biomarkers in psychiatry: Clinical opportunities of a paradigm shift. The Canadian Journal of Psychiatry, 58, 499–508. [DOI] [PubMed] [Google Scholar]
- Green, M. W. , & Rogers, P. J. (1998). Impairments in working memory associated with spontaneous dieting behaviour. Psychological Medicine, 28, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Hahn, T. , Marquand, A. F. , Ehlis, A. C. , Dresler, T. , Kittel‐Schneider, S. , Jarczok, T. A. , … Fallgatter, A. J. (2011). Integrating neurobiological markers of depression. Archives of General Psychiatry, 68, 361–368. [DOI] [PubMed] [Google Scholar]
- Hastie, T. , Tibshirani, R. , & Friedman, J. (2009). The elements of statistical learning: Data mining, inference, and prediction. New York: Springer. [Google Scholar]
- Haubold, A. , Peterson, B. S. , & Bansal, R. (2012). Annual research review: Progress in using brain morphometry as a clinical tool for diagnosing psychiatric disorders. Journal of Child Psychology and Psychiatry, 53, 519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Stefan, M. , Terranova, K. , Steinglass, J. , & Marsh, R. (2015). Altered white matter microstructure in adolescents and adults with Bulimia Nervosa. Neuropsychopharmacology, 41, 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga, G. , Maia, T. V. , Wang, P. , Wang, Z. , Marsh, R. , & Peterson, B. S. (2011). Adaptation to conflict via context‐driven anticipatory signals in the dorsomedial prefrontal cortex. Journal of Neuroscience, 31, 16208–16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys, Q. J. , Maia, T. V. , & Frank, M. J. (2016). Computational psychiatry as a bridge from neuroscience to clinical applications. Nature of Neuroscience, 19, 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, J. , Birmaher, B. , Brent, D. , Rao, U. M. A. , Flynn, C. , Moreci, P. , … Ryan, N. (1997). Schedule for affective disorders and schizophrenia for school‐age children‐present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kaye, W. , Strober, M. , & Jimerson, D. C. (2004). The neurobiology of eating disorders In Charney D., Nestler E.J. (Eds.), The neurobiology of mental illness (pp 1112–28). New York: Oxford Press. [Google Scholar]
- Kemps, E. , Tiggemann, M. , & Marshall, K. (2005). Relationship between dieting to lose weight and the functioning of the central executive. Appetite, 45, 287–294. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Calhoun, V. D. , Shim, E. , & Lee, J. H. (2016). Deep neural network with weight sparsity control and pre‐training extracts hierarchical features and enhances classification performance: Evidence from whole‐brain resting‐state functional connectivity patterns of schizophrenia. Neuroimage, 124, 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, D. A. , & Walsh, B. T. (2003). Eating disorders. International Review of Psychiatry (Abingdon, England), 15, 205–216. [DOI] [PubMed] [Google Scholar]
- Kloppel, S. , Abdulkadir, A. , Jack, C. R., Jr. , Koutsouleris, N. , Mourao‐Miranda, J. , & Vemuri, P. (2012). Diagnostic neuroimaging across diseases. Neuroimage, 61, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenta, O. M. , Sierve, K. D. , de Macedo, E. C. , Fregni, F. , & Boggio, P. S. (2014). Transcranial direct current stimulation modulates ERP‐indexed inhibitory control and reduces food consumption. Appetite, 83, 42–48. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Banich, M. T. , Jacobson, B. L. , & Tanabe, J. L. (2004). Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event‐related fMRI. Neuroimage, 22, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Lock, J. , Garrett, A. , Beenhakker, J. , & Reiss, A. L. (2011). Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. American Journal of Psychiatry, 168, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi, A. , Takerkart, S. , Regragui, F. , Boussaoud, D. , & Brovelli, A. (2012). Multivoxel pattern analysis for FMRI data: A review. Computational and Mathematical Methods in Medicine, 2012, 961257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. , Horga, G. , Wang, Z. , Wang, P. , Klahr, K. W. , Berner, L. A. , … Peterson, B. S. (2011). An FMRI study of self‐regulatory control and conflict resolution in adolescents with bulimia nervosa. American Journal of Psychiatry, 168, 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. , Stefan, M. , Bansal, R. , Hao, X. , Walsh, B. T. , & Peterson, B. S. (2015). Anatomical characteristics of the cerebral surface in bulimia nervosa. Biological Psychiatry, 77, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. , Steinglass, J. E. , Gerber, A. J. , Graziano O'leary, K. , Wang, Z. , Murphy, D. , … Peterson, B. S. (2009). Deficient activity in the neural systems that mediate self‐regulatory control in bulimia nervosa. Archives of General Psychiatry, 66, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. , Zhu, H. , Wang, Z. , Skudlarski, P. , & Peterson, B. S. (2007). A Developmental fMRI Study of Self‐Regulatory Control in Tourette's Syndrome. The American Journal of Psychiatry, 164, 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel, W. , Shoda, Y. , & Rodriguez, M. I. (1989). Delay of gratification in children. Science (New York, N.Y.), 244, 933–938. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru, G. , Pettersson‐Yeo, W. , Marquand, A. F. , Sartori, G. , & Mechelli, A. (2012). Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neuroscience & Biobehavoural Reviews, 36, 1140–1152. [DOI] [PubMed] [Google Scholar]
- Peng, X. , Lin, P. , Zhang, T. , & Wang, J. (2013). Extreme learning machine‐based classification of ADHD using brain structural MRI data. PLoS One, 8, e79476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, B. S. , Kane, M. J. , Alexander, G. M. , Lacadie, C. , Skudlarski, P. , Leung, H. C. , … Gore, J. C. (2002). An event‐related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Research. Cognitive Brain Research, 13, 427–440. [DOI] [PubMed] [Google Scholar]
- Polyn, S. M. , Natu, V. S. , Cohen, J. D. , & Norman, K. A. (2005). Category‐specific cortical activity precedes retrieval during memory search. Science, 310, 1963–1966. [DOI] [PubMed] [Google Scholar]
- Raz, A. , Zhu, H. , Yu, S. , Bansal, R. , Wang, Z. , Alexander, G. M. , … Peterson, B. S. (2009). Neural substrates of self‐regulatory control in children and adults with Tourette syndrome. The Canadian Journal of Psychiatry, 54, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Smith, A. B. , Woolley, J. , Nosarti, C. , Heyman, I. , Taylor, E. , & Brammer, M. (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event‐related tasks of cognitive control. Human Brain Mapping, 27, 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, J. , & Tiggemann, M. (2004). Dieting and working memory: Preoccupying cognitions and the role of the articulatory control process. British Journal of Health Psychology, 9, 175–185. [DOI] [PubMed] [Google Scholar]
- Simon, J. R. (1969). Reactions toward the source of stimulation. Journal of Experimental Psychology, 81, 174–176. [DOI] [PubMed] [Google Scholar]
- Skunde, M. , Walther, S. , Simon, J. J. , Wu, M. , Bendszus, M. , Herzog, W. , & Friederich, H. C. (2016). Neural signature of behavioural inhibition in women with bulimia nervosa. Journal of Psychiatry & Neuroscience, 41, E69–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice, E. , Marti, C. N. , & Rohde, P. (2013). Prevalence, incidence, impairment, and course of the proposed DSM‐5 eating disorder diagnoses in an 8‐year prospective community study of young women. Journal of Abnormal Psychology, 122, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice, E. , Marti, C. N. , Spoor, S. , Presnell, K. , & Shaw, H. (2008). Dissonance and healthy weight eating disorder prevention programs: Long‐term effects from a randomized efficacy trial. Journal of Consulting and Clinical Psychology, 76, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, S. A. , Crow, S. J. , Le Grange, D. , Swendsen, J. , & Merikangas, K. R. (2011). Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Archives of General Psychiatry, 68, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T. , Kessler, D. , Scott, C. , Angstadt, M. , & Sripada, C. (2014). Disease prediction based on functional connectomes using a scalable and spatially‐informed support vector machine. Neuroimage, 96, 183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1981). WAIS‐R manual: Wechsler adult intelligence scale‐revised. New York: Psychological Corporation. [Google Scholar]
- Weygandt, M. , Schaefer, A. , Schienle, A. , & Haynes, J. D. (2012). Diagnosing different binge‐eating disorders based on reward‐related brain activation patterns. Human Brain Mapping, 33, 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D. , & Halligan, P. (2004). The relevance of behavioural measures for functional‐imaging studies of cognition. Nature Reviews Neuroscience, 5, 67–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


