Abstract
Background
Connective tissue progenitors (CTPs) embody the heterogeneous stem and progenitor cell populations present in native tissue. CTPs are essential to the formation and remodeling of connective tissue, and represent key targets for tissue-engineering and cell-based therapies. To better understand and characterize CTPs we aimed to compare the: 1) concentration and prevalence, 2) early in vitro biological behavior, and 3) expression of surface-markers and transcription factors among cells derived from marrow space (MS), trabecular surface (TS), and adipose tissues (AT).
Methods
Cancellous-bone and subcutaneous-adipose tissues were collected from 8 patients. Cells were isolated and cultured. Colony formation was assayed using Colonyze™ software based on ASTM-standards. Cell concentration ([Cell]), CTP concentration ([CTP]), and CTP prevalence (PCTP) were determined. Attributes of culture-expanded cells were compared based on (a) effective-proliferation rate; and (b) expression of surface-markers CD73, CD90, CD105, SSEA-4, SSEA-3, SSEA-1/CD15, Cripto-1, E-Cadherin/CD324, Ep-CAM/CD326, CD146, hyaluronan, and transcription factors Oct3/4, Sox-2, Nanog, using flow cytometry.
Results
Mean [Cell], [CTP], and PCTP were significantly different between MS and TS samples (p=0.03, p=0.008, and p=0.0003), respectively. AT-derived cells generated the highest mean total-cell yield at day 6 of culture; 4-fold greater than TS and over 40-fold greater than MS per million cells plated. TS-colonies grew with higher mean density than MS colonies (290 ± 11 vs. 150 ± 11 cell per mm2) (p=0.0002). Expression of classical-mesenchymal stromal cell (MSC) markers was consistently recorded (>95%) from all tissue sources, whereas all the other markers were highly variable.
Conclusion
The prevalence and biological potential of CTPs are different between patients and tissue sources, while having lack of variation in classical-MSC markers. Other markers are more likely to discriminate differences between cell populations in biological performance. Understanding the underlying reasons for variation in the concentration, prevalence, marker expression, and biological potential of CTPs between patients and source tissues, and means of managing this variation, will contribute to the rational development of cell-based clinical diagnostics and targeted cell-based therapies.
Keywords: Stem cell, Connective Tissue Progenitor, Mesenchymal Stromal Cell, Adipose Tissue, Bone, Trabecular Bone, Colony Forming Unit Assay, Flow Cytometry, Surface Markers, Transcription Factor
Introduction
Stem and progenitor cells in native tissues are essential for the formation and remodeling of new tissues. They constitute a target cell population for a broad range of tissue engineering applications [1]. They also represent a therapeutically-useful starting material for the generation of culture-expanded progeny for cellular therapies, including cartilage, bone, and soft tissue regeneration [2].
The term connective tissue progenitors (CTP) has been used to define the heterogeneous populations of stem and progenitor cells present in native connective tissues that are able to proliferate and differentiate into one or more connective tissue phenotype [3,4]. Colony founding CTPs are found in virtually every connective tissue in adults [3–7].
Stem and progenitor cells can be assayed from any tissue in order to characterize their concentration, prevalence, and range of biological phenotypes using colony forming unit (CFU) assays; based on the assumption that each colony is formed by the progeny of one stem cell or progenitor [8]. Tissue-resident stem cells and progenitors are by nature heterogeneous in biological state and potential [9]. Differences between the colonies formed under standardized-conditions reflect the heterogeneity in biological potential among the colony-founding cells [10–14].
CFU assays are traditionally performed by a skilled operator by performing a manual colony count. However, evidence suggests that manual counting provides poor repeatability and reproducibility [15]. Moreover, subjective manual methods are unable to capture biologically important metrics of colony formation and differentiation systematically. This information, which is not being collected by manual-colony counting, if obtained, could be used for better biological characterization of these colonies and cells, which potentially could identify clinically relevant differences between colony-founding cells and their clonal progeny [15,16].
The ASTM International (previously American Society for Testing and Materials), as a globally recognized leader in the development and delivery of consensus standards, has provided a Standard Test Method for Automated Colony-Forming Unit Assays that address the limitations of manual methods (F2944 –12 Standard) [15]. The main benefit of the use of automated methods is that it reduces the variation in measurement that results from subjective differences between observers. The improved repeatability and reproducibility of automated-CFU assays enhances the utility of these assays for a variety of applications, including: a) comparison and selection of optimal anatomic sites and methods for stem and progenitor cell harvest, b) assessment of the effect of in vitro processing on CFU concentration, prevalence, and biological performance, c) exploring the relationship between CTP concentration, prevalence, and biological performance to local tissue health or the progression of disease, d) predicting cell quality or potency and the likelihood of clinical-efficacy if used for treatment, or e) enabling systematic-rational discrimination and selection among CFU-subtypes to enhance control over cell-source quality and outcomes.
Several studies have described significant differences between tissue sources with respect to biological potential for the harvest of stem and progenitor cells [17–19]. Variation has been reported between patients, related to gender, age [20,21], surgical site, and harvesting techniques [6,22–24]. Even among cultured-expanded populations, there have been reports of variations between tissues and among separate cells isolated from the same tissue [10–13]. Heterogeneity has even been reported within an apparent clone [14].
Bone marrow [7,17,23], trabecular bone [17,20,25,26], and adipose tissue [17,27,28] are the most common sources of CTPs for both research and clinical applications. However, these sources have been reported to vary significantly in cell concentration, prevalence and biological attributes. Nancarrow-Lei R., et. al. [17] provided a systematic review of cell source options. The data reported in the field are not sufficiently homogeneous in methods of analysis or reporting to allow a meta-analysis to systematically quantify the magnitude and extent of variation between sources. Therefore, this paper could only provide qualitative comparisons and consensus statements. Bone marrow aspirates are considered to be the reference standard against which all other tissue sources are compared. Adipose tissue is accepted to provide the highest prevalence of CFUs among tissue resident cells, but adipose-derived cells tend to lag in differentiation potential towards bone and cartilage phenotypes compared to marrow-derived cells. Even within a given donor or tissue, heterogeneity within and between donors is large [29,30]. Rational clinical development demands further investigation and direct comparison of these cell sources with respect to their concentration, prevalence, and the biological performance.
When culture expanded in vitro, cells from each of these sources can be used to generate populations of culture-expanded cells that can be categorized as “mesenchymal stromal cells” or MSCs. It has been shown that culture-expanded MSCs may be indistinguishable from fibroblasts, based on conventional markers [31,32]. However, the International Society for Cellular Therapy (ISCT) has defined standardized terminology and minimal criteria for classification of culture-expanded cells as MSCs based on the presence of surface markers CD73, CD90, and CD105 and the absence of hematopoietic markers CD34, CD45, CD14, CD19, HLA-DR [33]. The MSC Committee of the ISCT has also proposed the need to add functional analysis, including the immunological modulatory effects, to enable standardization of clinical cell-based therapies [34]. There is evidence that in vitro expansion induces or selects for the expression of MSC surface markers in a diversity of starting cells [35]. However, human MSCs isolated from different sources often differ in their expression of surface markers and exhibit distinct differentiation patterns [17]. Plating density and cell isolation may influence the expression of markers of differentiation [36]. Moreover, recent data demonstrate that expression of this MSC-marker profile is not predictive of biological behavior with respect to bone, cartilage and fat differentiation in vitro [37–39]. This suggests that expression of traditional-MSC surface markers may not be sufficient as attributes that predict biological potential [40]. Other surface markers must also be considered [41,42].
Various other markers have been reported to be associated with biological potential, specifically the property of long term in vitro expansion and multi-lineage differentiation potential. The transcription factors Oct3/4, Sox-2, and Nanog [43] and stage-specific embryonic antigen-4 (SSEA-4) [44] are examples. Cripto-1, a member of the epidermal growth factor family involved in determination of cell fate during embryonic development [45], has been considered as a “stemness marker” [46]. Others include: Epithelial cell adhesion molecule (Ep-CAM/CD326), a trans-membrane glycoprotein with roles in cell-cell adhesion [47]; Ep-CAM/CD326 and E-Cadherin/CD324 have been linked to activation of Nanog and Oct3/4 and considered indicators of successfully-reprogramed inducedpluripotent stem cells (iPSCs) [48]; CD146, a cell surface glycoprotein with roles in proliferation, cell-cell interactions, migration, and angiogenesis [49]; and hyaluronan (HA), a glycosaminoglycan component of the extracellular matrix of most mammalian tissues [50] that is often found in stem cell niches [50,51]. HA is involved in regulation of stem cell self-renewal by interaction with Oct3/4, Sox-2, and Nanog through CD44v3 [52]. HA has also been reported as a surface marker on an osteogenic subset of bone marrow-derived CTPs [53].
To advance the state of knowledge regarding options for cell source selection, our study examined differences between three clinically-relevant tissue sources for human stem and progenitor cells, specifically cells from bone marrow space (MS), trabecular surface (TS), and adipose tissue (AT). The outcome of in vitro expansion of these cell sources to generate MSCs was also examined using cell surface markers and pluripotency-associated transcription factors. There were three aims to: 1) define and compare the concentration and prevalence of human stem and progenitor cells (CTPs) among MS-, TS-, and AT-derived cells, 2) compare the early in vitro biological behavior of the progeny of CTPs based on day 6 colony metrics, and 3) compare the expression of surface markers and transcription factors that have been associated with in vitro biological performance among culture-expanded cells.
Materials and Methods
Human Tissue Samples
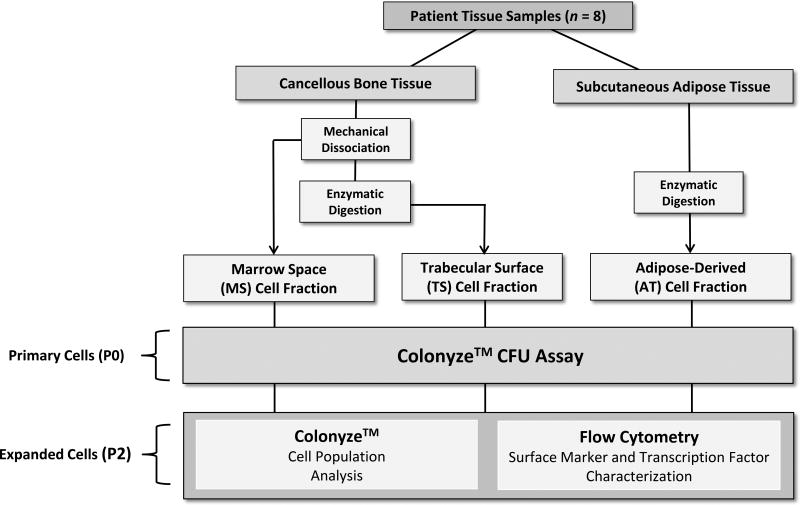
Bone and adipose tissues were obtained from 8 patients, 6 males and 2 females (mean age, 55 ± 5 years; range, 27–78 years), undergoing elective total-hip arthroplasty (THA) in an Institutional Review Board approved-protocol and with appropriate informed consent. All patients had advanced arthritis of the hip. The underlying cause of hip disease was osteoarthritis (5 patients), adult rheumatoid arthritis, juvenile rheumatoid arthritis, and osteonecrosis. Relevant patient characteristics are presented in Table S1. Using a posterior approach to the hip, adipose tissue was collected from the subcutaneous fat (~2–3 gm) 1–2 cm below the skin in the region overlying the greater trochanter. Bone tissue (~2–3 cm3) was obtained using a core biopsy tool, from the proximal femoral metaphysis, following transection of the femoral neck. Overall study design is presented (Figure 1).
Figure 1.
Study Design. Sample harvest, processing and analysis pathway.
Bone and Adipose Tissue Processing
Cancellous-bone samples were weighed and then minced into 1–2 mm3 pieces with a sharp osteotome. Bone-derived cells were collected in two fractions as previously described [54]. The loosely adherent cells released by mincing and mechanical agitation were collected and defined as the MS fraction. Cells that remained adherent to the trabecular surface were then dissociated by enzymatic digestion with 111U/mL collagenase type-I and 24U/mL dispase (Worthington Biochemical, NJ) for 90 minutes at 37 °C. This fraction of cells was defined as the TS fraction. AT-samples were minced with a surgical scalpel and cells were released by enzymatic digestion with 111U/mL collagenase type-I and 24U/mL dispase for 90 minutes at 37 °C. Cells from each source were filtered through a 70 µm nylon cell strainer (Corning, NY) to separate tissue debris and to prepare suspensions of mostly single cells. Cells were centrifuged at 300×g for 5 minutes. The pellet was re-suspended in medium containing: alpha-minimum essential medium (αMEM), fetal-bovine serum (FBS) 16.5% (Atlanta Biologicals, GA), L-glutamine 2–4 mM, 1U/mL penicillin, and 0.1 mg/mL streptomycin (Sigma, MO). Cell number was determined using a hemocytometer. Cell concentration ([Cell]) (cells per gram of tissue) from MS, TS, and AT were calculated.
Primary Cell Culture (P0)
Cells from MS, TS, and AT were cultured on 2-well Lab-Tek™ chamber-slides (Thermofisher Scientific, NY). The following plating densities were used per each cell source: MS, 5 × 105; TS, 2.5 × 105; and AT, 1 × 105 nucleated cells per chamber (4.2 cm2). Plating density was selected based on prior reports of the prevalence of CTPs in these tissues in an attempt to observe the development of separated and distinct CTP-derived CFUs. Cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Non-adherent cells were removed 48-hours after initial plating. Adherent cells were grown for 6 days with medium exchange every 2 days.
Culture Expansion (P2)
MS, TS, and AT populations were also cultured in 162 cm2 flasks under the same conditions. Cells were expanded following an established protocol [55]. Briefly, adherent passage zero (P0) cells were harvested using 0.5% trypsin/1mM EDTA (Sigma-Aldrich, St. Louis, MO) for 3 minutes at 37 °C when they reached 60% – 80% confluence. These P0 cells were re-plated at 100 cells/cm2 and expanded again to 60% – 80% confluence. These passage 1 (P1) cells were re-plated at 100 cells/cm2, expanded again to 60% – 80% confluence, and harvested as passage 2 (P2) cells for analysis.
Characterization of Bone and Adipose Tissue Derived Cells
(a) Proliferation and Morphology Assessment
(a-i) Assessment of Primary Cultures (P0)
Primary cultures on chamber-slides from MS, TS, and AT were fixed at day 6 of initial culture with acetone-methanol (1:1). DAPI (4', 6-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA) staining of nuclei allowed discrete localization of cells and colonies. Fluorescent images of the entire culture area were captured and processed using quantitative large field of view (LFOV) image analysis as described by Powell et al. [16] using excitation at 360 nm and emission at 460 nm. Automated-quantitative CFU assay was performed using Colonyze™ image analysis software [15,16]. A colony at day 6 was defined as a group of 8 or more cells, based on proximity of less than or equal to 79.65 microns between nuclei centroids, suggesting origin from a single founding cell (i.e. CTP). CTP prevalence (PCTP) was calculated as the number of colonies observed per million nucleated cells plated [56]. CTP concentration (CTPs per gram of tissue) from MS, TS, and AT was also calculated as the product of [Cell] and PCTP.
The biological performance of the progeny of each founding CTP was described based on two morphometric colony measurements: a) cells per colony (a metric of proliferation) and b) cell density (cells per mm2) (a function of cell-cell adherence and cell migration following cell division) [15,16]. The total cell yield per million nucleated cells plated was also used as a metric that is relevant for those interested in bulk manufacturing, including cells within colonies and cells not included within colonies (“free cells”).
(a-ii) Assessment of the Proliferation Rate of Culture-expanded (P2) Cells
Culture-expanded MS, TS, and AT cell populations were harvested at P2, when they reached 60% – 80% confluence, using 0.5% trypsin/1mM EDTA. Cells were re-plated on 2-well Lab-Tek™ chamber-slides at 100 cell/cm2 plating density and incubated for 6 days. Colonyze™ was used to measure total cells. Effective Proliferation Rate (EPR) of the culture-expanded (P2) cells was evaluated by calculating the proliferation rate in cell divisions per day, as indicated in the following equation: [EPR = (Log2 final cell count – Log2 initial cell count)/ Duration (6 days)] [15].
(b) Phenotypic Characterization of Culture-expanded (P2) Cells
Culture-expanded MS, TS, and AT cells were harvested at P2 when they reached 60% – 80% confluence using non-enzymatic dissociation by 0.5 mM EDTA (Fisher Scientific, MA) for 5 minutes at room temperature to preserve cell surface markers [57]. Cells were washed with PBS and suspended in staining buffer.
Anti-human fluorescent-conjugated antibodies were titrated based on manufacturer recommended concentrations and appropriate protocols [58] and used to label cell surface and nuclear antigens (Table S2).
For surface marker staining, cells were incubated with appropriate antibodies and blocking solution at 4 °C for 30 minutes. Transcription factors were labeled using an established protocol [59]. Cells and their nuclei were permeabilized and stained following manufacture’s protocol using an optimized staining buffer set (eBioscience, CA). For HA labeling, cells were incubated with biotinylated hyaluronic acid binding-protein (bHABP) (EMD Millipore, MA). Labeled HA was detected with fluorescent-labeled streptavidin. Cell viability was confirmed using a dead-cell staining kit (Molecular Probes, OR). Dead cells were excluded from the analysis. Labeled cells were re-suspended in 250 µL of 1× PBS. Appropriate isotype-matched fluorochrome-conjugated antibodies and unstained-cell preparations were used as negative controls. Characterized human BM-MSCs (purchased from Texas A&M Institute for Regenerative Medicine) were used as positive control for traditional-MSC markers. Human teratocarcinoma cells (CRL-2073) (ATCC®, VA) were cultured in the Malcuit’s lab at Kent State University and used as a positive control for Oct3/4, Sox2, Nanog, SSEA-3, and SSEA-4. Data were acquired using an LSR-Fortessa flow cytometer (Becton Dickinson). Percentage of cells with positive expression for each marker was reported. Analysis was performed using FlowJo Software (Ashland, OR).
Statistical Analysis
Data outcomes from the CFU-assay and flow cytometry analysis were presented as mean ± standard error. Two-tailed paired t-tests were used to compare cell sources for statistical significant differences. Differences were considered significant at p ≤ 0.05.
Results
Tissue Source Variation in CTP Concentration ([CTP]), CTP Prevalence (PCTP), and colony morphology
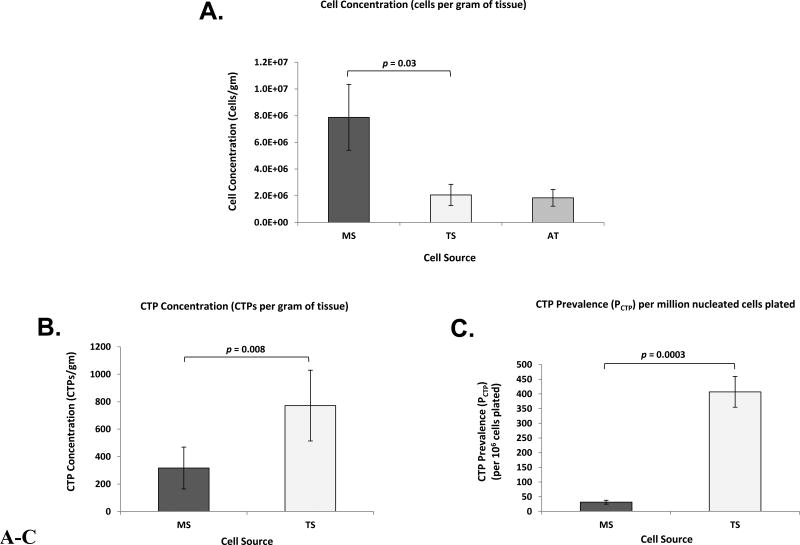
Subjects and tissue sources demonstrated significant variation in cell concentration [Cell], CTP concentration [CTP], and CTP prevalence (PCTP) (Table I-A and I-B). Mean [Cell] for MS and TS samples were 7.9 ± 2.5 × 106 and 2.1 ± 0.8 × 106 cells/gram of tissue, respectively (p = 0.03), while mean [Cell] for AT samples was 1.8 ± 0.6 ×106 cells/gram of tissue (Figure 2A). Mean [CTP] for MS and TS samples were 320 ± 150 and 770 ± 260 CTPs/gram of tissue, respectively (p = 0.008) (Figure 2B). Mean PCTP for MS and TS samples were 30 ± 7 and 410 ± 50 CTPs/million nucleated cells, respectively (p = 0.0003) (Figure 2C). Individual patient-based comparison of [Cell], [CTP], and PCTP per each cell source is presented in Figures S1–S3.
Table I.
Cell Source Comparison and Colony-Based Metrics. (I-A) Concentration; cell concentration [Cell] (for MS, TS, and AT) and CTP concentration [CTP] (for MS and TS). (I-B) Colonyze™ CFU Metrics; including: PCTP, adherent cell counts (total cells, cells within colony, and free cells) per million cells plated, and colony metrics (cells per colony, cell density, and colony area). (I-C) Effective proliferation rates (EPRs) of MS, TS, and AT-derived culture-expanded (P2) cells.
| I-A. Cell and CTP Concentration | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | MS | TS | AT | p-value |
| Cell Concentration [Cell] (Cells per gram of tissue) | 7.9 ×106 ± 2.5 ×106 (a) | 2.1 × 106 ± 0.8 × 106 (b) | 1.8 × 106 ± 0.6 × 106 (c) | 0.03(a,b) |
| 0.06(a,c) | ||||
| 0.85(b,c) | ||||
|
| ||||
| CTP Concentration [CTP] (CTPs per gram of tissue) | 320 ± 150 | 770 ± 260 | - | 0.008 |
| I-B. Colonyze™ Colony Forming Unit Assay Data of Primary Cells | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | MS | TS | AT | p-value |
| CTP Prevalence (PCTP) (CTP per Million Cells Plated) | 30 ± 7 | 410 ± 50 | - | 0.0003 |
|
| ||||
| Total Adherent Cells per Million Cells Plated | 3040 ± 670(a) | 35400 ± 5870(b) | 148000 ± 41300(c) | 0.0008(a,b) |
| 0.013(a,c) | ||||
| 0.053(b,c) | ||||
|
| ||||
| Cells within Colonies per Million Cells Plated | 1690 ± 610 | 25530 ± 5840 | - | 0.004 |
|
| ||||
| Free Cells per Million Cells Plated | 1350 ± 240 | 9880 ± 1170 | - | 0.0004 |
|
| ||||
| Cells per Colony | 80 ± 37 | 100 ± 29 | - | 0.29 |
|
| ||||
| Cell Density (Cells per mm2) | 150 ± 11 | 290 ± 11 | - | 0.0002 |
|
| ||||
| Colony Area (mm2) | 0.65 ± 0.20 | 0.48 ± 0.15 | - | 0.30 |
| I-C. Colonyze™ Cell Population Analysis of Culture Expanded (P2) Cells | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | MS | TS | AT | p-value |
| Effective Proliferation Rate (Cell Division per Day) | 0.54 ± 0.08(a) | 0.40 ± 0.05(b) | 0.47 ± 0.10(c) | 0.18(a,b) |
| 0.56(a,c) | ||||
| 0.39(b,c) | ||||
Data are presented as Mean ± Standard Error, n = 8. MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
Figure 2.
A–C. Bone and Adipose Tissue Derived Primary Cells - Cell Concentration, CTP Concentration, and CTP Prevalence. Bar graphs represent: (A) cell concentrations (cells per gram of tissue) of MS, TS, and AT; (B) CTP concentrations (CTPs per gram of tissue) of MS and TS; and (C) CTP prevalence (PCTP) per million nucleated cells plated of MS and TS. Data are presented as Mean ± Standard Error, (n = 8). CTP: Connective Tissue Progenitor, MS: Marrow Space, TS: Trabecular Surface, AT: Adipose Tissue.
Cultures of AT-derived cells grew out as distributed single cells that could not be defined as colonies using the criteria established for Colonyze™. Therefore, PCTP and [CTP] could not be estimated in the AT samples. Of note, the possible reason that discrete colonies could not be defined for AT-derived CTPs may be due to the fact that the progeny of these cells migrated sufficiently after each cell division to obscure their grouping into colonies having distinct boundaries thus blending cells from adjacent clones with one another. This behavioral attribute represented a fundamental difference in in vitro intercellular interactions and migration among AT-derived cells as compared to MS- or TS-derived cells.
Yield of Adherent Cells
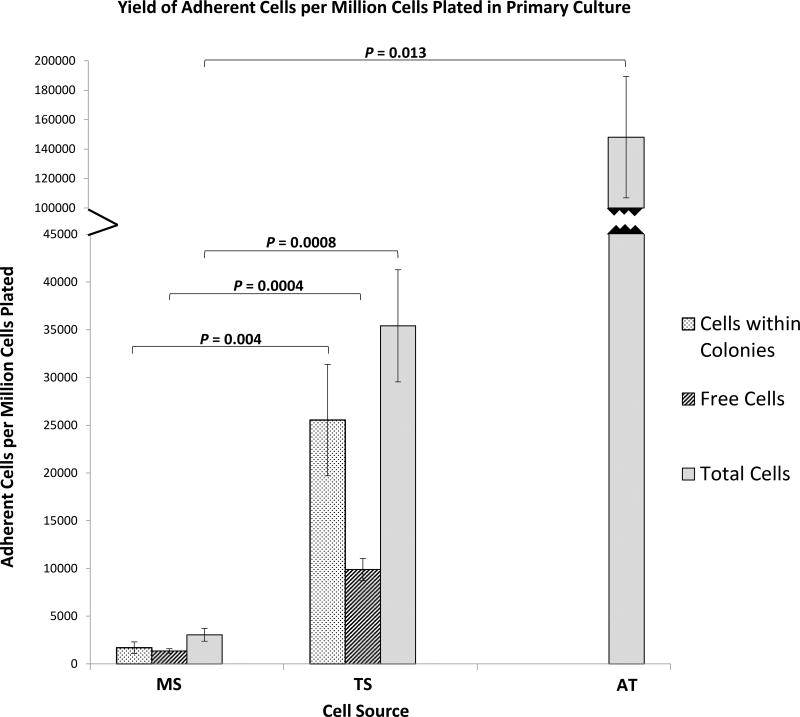
The yield of adherent cells per million cells plated at day 6 is shown in Table I-B. The yield from TS was significantly greater than MS (p = 0.0008). The yield from AT was also significantly higher compared with MS (p = 0.013), but not statistically greater than TS-derived cells (p = 0.053) (Figure 3). In five of the eight patients, AT-derived cells generated larger number of adherent cells at day 6 than TS-derived cells (Figure S4).
Figure 3.
Bone and Adipose Tissue Derived Primary Cells - Yield of Adherent Cells. Bar graph represents total adherent cell counts per million cells plated at day 6 of culture of MS, TS, and AT. Cells within colonies and free cells per million cells plated at day 6 of culture are presented for MS and TS. Data are presented as Mean ± Standard Error, (n = 8). CTP: Connective Tissue Progenitor, MS: Marrow Space, TS: Trabecular Surface, AT: Adipose Tissue.
In the MS- and TS-derived primary cultures, adherent cells that were within defined colonies could be discriminated from adherent cells that were not (“free cells”). MS- and TS-derived adherent cells at P0 differed significantly with respect to the mean number of cells within colonies per million cell plated (p = 0.004) and the mean number of cells not part of a colony “free cells” (p = 0.0004) (Figure 3), reflecting primarily the higher prevalence of CTPs in the TS-fraction (Table I-B). TS-derived cells yield was greater for cells within colonies and “free cells” in every patient (Figures S5 and S6).
Comparison of Colony Metrics
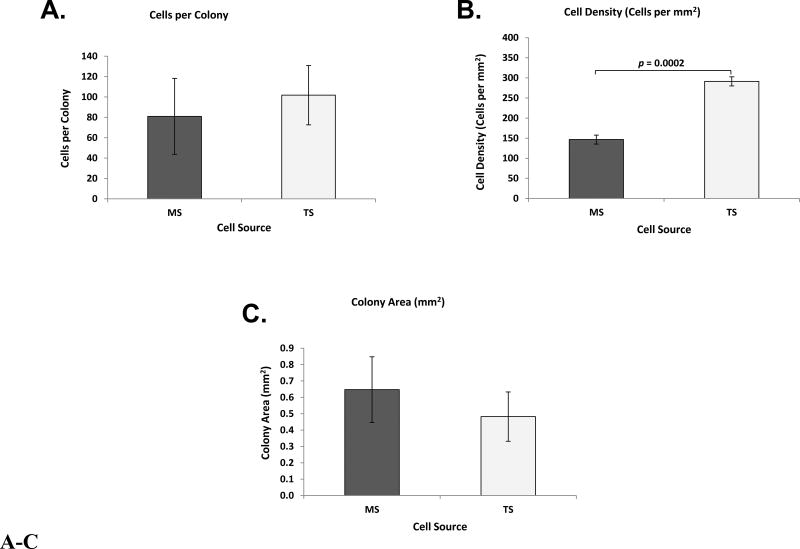
MS and TS CTP-derived colonies were analyzed with respect to colony morphology at day 6. Mean cells per colony for MS- and TS-derived colonies were not significantly different (Figure 4A). The mean number of cells in TS-derived colonies was greater than MS in 4 of the 8 patients (Figure S7).
Figure 4.
A–C. Bone Derived Primary Cells, Colony-Based Metrics. Bar graphs represent: (A) Cells per colony; (B) cell densities (cells per mm 2); and (C) colony areas (mm 2) of MS and TS. Data are presented as Mean ± Standard Error, (n = 8). CTP: Connective Tissue Progenitor, MS: Marrow Space, TS: Trabecular Surface.
Mean cell density in TS was consistently greater than MS-derived colonies (p = 0.0002) (Figure 4B). The mean density of TS-derived colonies was greater than MS in every patient (Figure S8). Mean colony areas for MS and TS were not significantly different (Figure 4C), (Table I-B).
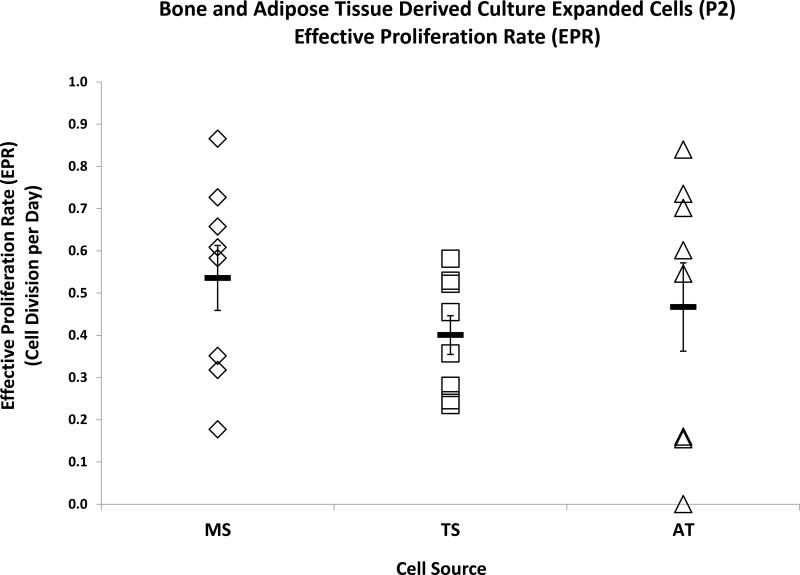
Effective Proliferation Rate of Culture-Expanded (P2) Cells
Colonyze™ was used to assess the EPR of culture-expanded cells based on total cell counts. EPRs were calculated in units of cell divisions per day (Table I-C). The mean EPR was comparable across the three cell sources during P2-expansion (Figure 5). Individual patient-based comparison of EPR per each cell source is presented in Figure S9.
Figure 5.
Bone and Adipose Tissue Derived Culture-Expanded (P2) Cells - Effective Proliferation Rates. Dot plots represent effective proliferation rates (EPRs) as determined by cell division per day of MS, TS, and AT. Data are presented as Mean ± Standard Error, (n = 8).
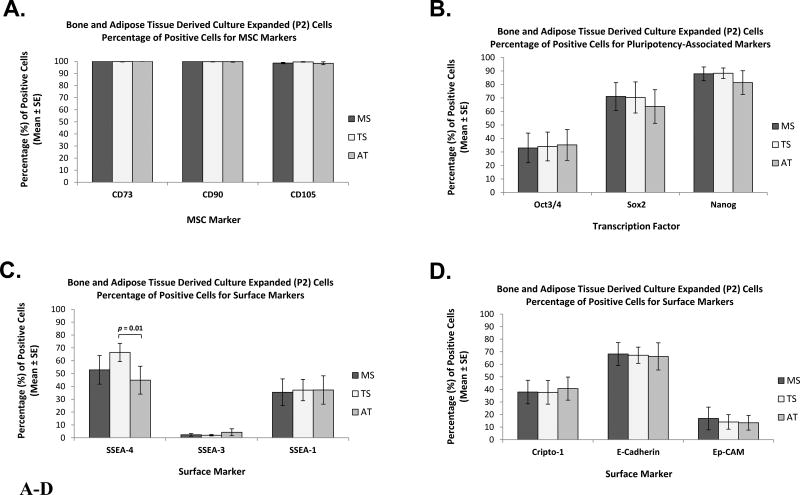
Marker Characterization in Culture-expanded (P2) Cells - Comparison between Tissue Sources
The mean percent values for cells staining positive for each of the selected cell-surface markers and pluripotency-associated transcription factors are shown in Table II and Figures 6 and 7. Representative flow cytometry histograms are presented in Figure S10.
Table II.
Marker Expression Metrics. Mean percent positive expression (± Standard Error) of each marker in MS, TS, and AT derived culture-expanded (P2) cells.
| Marker | Percentage of Positive Cells (%) | |||
|---|---|---|---|---|
| MS | TS | AT | ||
| Classical MSC Surface Markers | CD73 | 100 ± 0.01 | 99.8 ± 0.2 | 99.9 ± 0.05 |
| CD90 | 100 ± 0.01 | 99.7 ± 0.3 | 99.7 ± 0.3 | |
| CD105 | 98.6 ± 0.6 | 99.5 ± 0.2 | 98.4 ± 1.2 | |
| Pluripotency-Associated Transcription Factors | Oct3/4 | 32.9 ± 12.3 | 34.0 ± 11.6 | 35.2 ± 12.4 |
| Sox2 | 71.1 ± 11.4 | 70.4 ± 12.9 | 63.6 ± 12.2 | |
| Nanog | 87.9 ± 5.5 | 88.3 ± 4.2 | 81.4 ± 9.9 | |
| Pluripotency-Associated Surface Markers | SSEA-4 | 52.9 ± 11.1 | 66.5 ± 7.1 (*) | 44.8 ± 10.9 |
| SSEA-3 | 2.3 ± 1.0 | 2.0 ± 0.6 | 4.3 ± 2.7 | |
| SSEA-1/CD15 | 35.4 ± 10.4 | 37.1 ± 8.3 | 37.2 ± 11.0 | |
| Other Surface Markers | Cripto-1 | 37.9 ± 9.4 | 37.7 ± 9.4 | 40.7 ± 9.2 |
| E-Cadherin/CD324 | 68.2 ± 9.2 | 67.2 ± 6.5 | 66.3 ± 10.8 | |
| Ep-CAM/CD326 | 16.9 ± 9.0 | 14.1 ± 5.8 | 13.5 ± 5.9 | |
| HA | 26.6 ± 8.3 | 20.4 ± 8.2 | 28.5 ± 14.1 | |
| CD146 | 50.1 ± 9.0 | 49.0 ± 9.6 | 34.9 ± 10.6 (#,*) | |
MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
indicates TS significantly different compared with AT (p = 0.01); and
indicates AT significantly different compared with MS (p = 0.02).
Figure 6.
A–D. Marker Characterization of Bone and Adipose Tissue Derived Culture-Expanded (P2) Cells by Flow Cytometry - Comparison between Tissue Sources. Bar graphs represent percentage of positive cells for: (A) Classical MSC surface markers CD73, CD90, and CD105; (B) pluripotency-associated transcription factors Oct3/4, Sox2, and Nanog; (C) surface markers SSEA-4, SSEA-3, and SSEA-1; and (D) surface markers Cripto-1, E-Cadherin, and Ep-CAM. Data are presented as Mean ± Standard Error, (n = 8). MSC: Mesenchymal Stromal Cell; MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
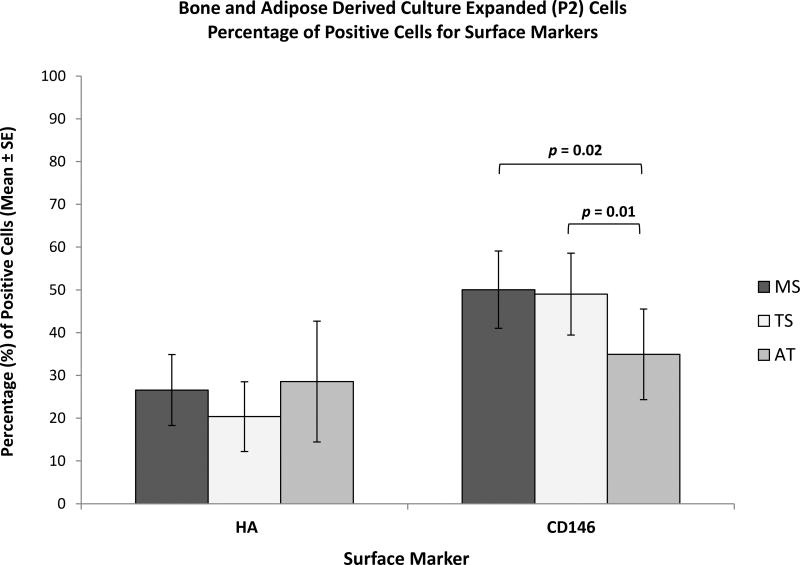
Figure 7.
Marker Characterization of Bone and Adipose Tissue Derived Culture-Expanded (P2) Cells by Flow Cytometry - Comparison between Tissue Sources. Bar graphs represent percentage of positive cells for surface markers HA and CD146. Data are presented as Mean ± Standard Error, (n = 8). MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
Not surprisingly, the classical MSC markers, CD73, CD90, and CD105, were consistently over 95% present among the CTP-derived progeny from all three tissue sources (Figure 6A). All tissue sources showed similar mean percentages of cells expressing Oct3/4, Sox-2, Nanog, SSEA-3, SSEA-1, Crypto-1, E-Cadherin, Ep-CAM, and HA, as illustrated in Figures 6B–D and 7.
However, large variations were seen between patients and between tissue sources within each patient (Table III and IV). Mean expression of two markers SSEA-4 and CD146 differed between AT-derived cells and the other tissues. The mean expression of SSEA-4 in TS-derived cells was significantly greater than AT-derived cells (p = 0.01) (Figure 6C). Mean CD146 expression in both MS- and TS-derived cells was significantly greater than AT-derived cells (p = 0.02 and p = 0.01, respectively) (Figure 7).
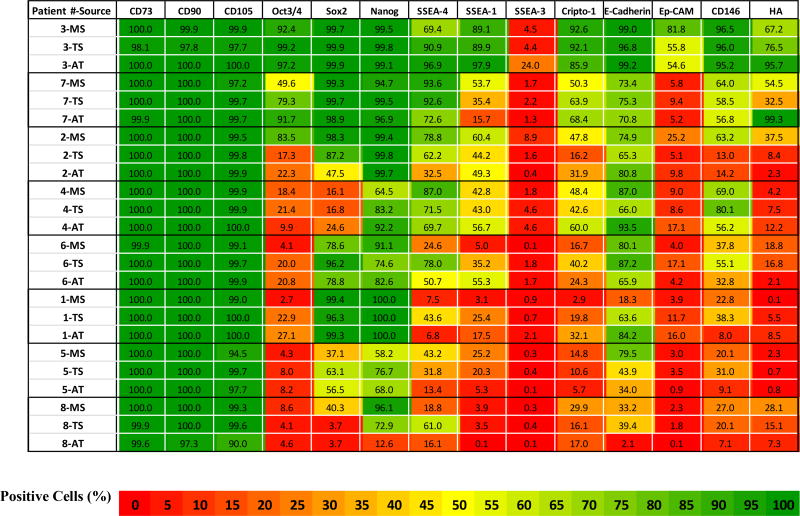
Table III.
Marker Expression Heat-Map Data. Percent expression for each marker in MS, TS, and AT derived culture-expanded (P2) cells per each patient sample. Flow cytometry data are presented as heat-map table using 3 color-scale (Red: Lowest (0%); Yellow: Midpoint (50%); and Green: Highest (100%)). Patients have been ranked roughly from top to bottom based on overall sum of expression levels to better illustrate the range of variation. All patients had advanced arthritis of the hip. Patients with a diagnosis of adult rheumatoid arthritis, juvenile rheumatoid arthritis, and osteonecrosis are patients 2, 7, and 4 respectively.
MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
Table IV.
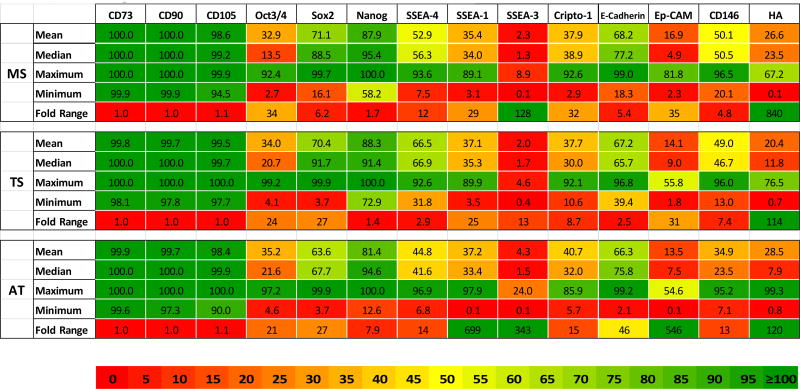
Marker Expression Range and Fold Variation Heat-Map. Mean, Median, Maximum, Minimum, and Fold Range (Max/Min) is presented for each marker and each tissue source.
Data are presented from culture-expanded (P2) cells. MS: Marrow Space; TS: Trabecular Surface; AT: Adipose Tissue.
Marker Variation between Patients and Tissue Samples in Culture-expanded (P2) Cells
The percent positive marker expression for each sample in each patient is presented in Table III. Data are grouped from top to bottom based on the sum percent for all markers. In general, high expression in one tissue was associated with higher expression in other tissues from the same patient. Samples from patient #3 had the highest levels and patient #8 had the lowest levels of expression of non-traditional markers across all three tissue sources.
In almost every patient, individual tissue sources presented different patterns of expression, manifest by 2–20 fold differences in individual markers. Overall, no one tissue source stood out particularly with respect to expression of non-traditional markers. However, AT-derived cells were distinct in having statistically lower expression of SSEA-4 and CD146 than TS-derived cells. In addition, AT-samples represented both the highest and the lowest scoring samples with respect to overall expression, and therefore the widest range of variation. In fact, AT-samples demonstrated over a 100-fold range with respect to 4 of the 11 non-traditional markers (SSEA-1, SSEA-3, Ep-CAM, and HA). In contrast TS-derived cells demonstrated the least variation, less than 25-fold for all makers except HA (Table IV).
Discussion
This study had three aims to: 1) define and compare the concentration and prevalence of human CTPs among MS-, TS-, and AT-derived cells, 2) define and compare the early in vitro biological behavior of the progeny of CTPs, and 3) compare the expression of surface markers and transcription factors that have been associated with in vitro biological performance of culture-expanded cells from these tissue sources.
The first two of these aims have been addressed in other studies with varying degrees of precision. However, to the best of our knowledge this is the first study to compare cell sources using quantitative LFOV image analysis, as outlined in the recently adopted ASTM Standard Method for Automated Colony Forming Unit Assays F2944-12 [15]. Aim three seeks to answer the important question, “How reproducible are the MSC populations that are derived from different patients and different tissue sources from the perspective of surface markers and transcription factors that might be predictive of differences in future biological performance?”
The concentration and prevalence of human colony founding cells in bone marrow aspirates (BMA) and fat-derived cells from liposuction aspirates have been compared in several studies [60,61]. However, the novelty of the present study is that the comparison between bone and fat was done at a tissue level, eliminating the potential artifacts in measurement associated with variation in dilution with peripheral blood and the exposure of both fat and marrow to serum and platelet degranulation products which is inevitable with BMA and liposuction techniques. We have recently published direct evidence for the large variation in efficiency of BMA as a means for harvest of bone and marrow-derived cells and the large dilution with peripheral blood that is associated with BMA [62]. By harvesting tissue during an open surgical procedure, the harvest site and composition of the harvested tissue is controlled and more reproducible than by BMA or liposuction. Moreover, these methods allow the separation of cells that can be mechanically released (MS-fraction) from cells that are recoverable only through enzymatic digestion of trabecular bone tissue surfaces (TS-fraction). This provides the most accurate estimation of the concentration and prevalence of cell populations in bone since they may contain different progenitor populations and stem cell niches.
Overall, our findings are consistent with prior studies [17,18,22,26], however, we found distinct findings with respect to variations between patients and tissue sources in early biological performance and non-traditional marker expression. The MS-compartment was far more cellular (cells per gram of tissue) than either the TS or AT. The prevalence of CTPs in the TS-compartment was over 10-times greater than the MS, roughly one CTP in 2500 cells.
Combining both the MS and TS compartments, the mean concentration of CTPs in the proximal femur was about 1100 CTPs per gram of tissue, and a mean of roughly 70% of all colony founding CTPs in bone resided in the TS compartment. These findings are comparable to data analyzing the cancellous bone collected from transcortical biopsies from the anterior iliac crest in a similar cohort of patients [62]. This suggests similarities in bone quality, bone health, and CTP populations in the iliac crest and proximal femur metaphysis in this elderly adult population. This approach of using tissues harvested without the diluting and contaminating effects of peripheral blood is particularly relevant for the assessments of variation between individuals and anatomic sites from the perspective of tissue health, donor site selection, and process optimization.
In Aim 2, this study provides in depth analysis of the biological variation of the cells in vitro performance by applying principles of ASTM standardized-automated image capture and analysis for CFU assay [15]. These methods allow quantitative characterization and comparison of the performance of the progeny of MS- and TS-derived CTPs using reproducible operator-independent methods. Despite the large variation between individual patients and individual colonies, TS-derived colonies exhibited not only a higher prevalence of colony founding CTPs, but also that their progeny tended to exhibit different morphology than MS-derived colonies with almost twice the mean cell density (in cells per mm2). AT-derived colonies also demonstrated a difference in cell behavior, growing at lower seeding density and possibly even higher proliferation, since by day 6 individual colonies had grown into each other, and isolated colonies could not be identified. The combination of high-adherent cell prevalence and population growth at low density among the progeny of AT-derived cells indicates that, in future studies, CFU assay of AT-derived CTPs from surgically collected fat will require an even lower plating density than that used in the current study. The early differences in biological performance among the progeny of CTPs from MS, TS, and AT sources may or may not provide information that can be used to predict future performance at the level of an individual tissue source or CTP-derived clone. However, knowledge of these quantifiable differences will enable future studies to refine our understanding of the origin and the implications of differences between individuals, tissues, and CTP-derived clones.
From a perspective of cell manufacturing, if the goal were to select a cell source based on the maximum yield of adherent cells (per million cells plated), assuming all adherent cells have equal quality, then AT-derived cells would appear to be the optimal choice, generating a mean of 148,000 adherent cells at 6 days (per million cells plated). This was almost 5-times greater than the yield from TS-derived cells, and 50-times greater than MS-derived cells cultured under the same “MSC expansion” conditions. When converted to yield cells per gram of tissue, the comparison of the yield for AT-derived cells remains 5-times greater than TS at 6 days, but 11-times greater than MS-derived cells. The data in Table I and Figures 2–5 further illustrate the advantages that advanced LFOV quantitative analysis now provides rigorous and reproducible analysis of CTP concentration, prevalence, and early biological performance. These methods can be applied to examine quantitative differences between individuals, tissue sources, harvest and processing techniques, and the effects of media conditions and surface modification for any CFU assay.
While we found the quantitative imaging used in Aim 1 and 2 to be a remarkably valuable tool, we consider Aim 3 to be the most important aspect of this work. In Aim 3, the attributes of culture-expanded populations from each of these individuals and tissue sources were evaluated, based on the expression of surface markers and transcription factors that have been associated with the biological performance of iPSC and embryonic stem cells (ESC). The motivation of this work was a series of publications reporting that the MSC-criteria, defined by the ISCT (greater than 95% expression of CD73, CD90 and CD105, and the absence of hematopoietic lineage markers) [33], do not necessarily predict differences in biological performance [11,35,63–66]. Indeed, from our experience, it is rare for a culture-expanded population of cells from BMA, MS, TS, or AT sources that does not meet these ISCT-criteria. Therefore, we contend that the existing ISCT criteria may present limited value as discriminators of the relative biological quality of one sample, tissue source or lot over another [37,40]. We speculated even further, that since the expression of this marker profile is almost always present it may have lulled the field into a false sense of security in assessing the reproducibility of generating MSCs with consistent biological properties.
The data presented in Table III and IV provide strong evidence that as expected, using established cell processing and culture-expansion methods, variation in expression of CD73, CD90, and CD105 was very low; however, variation in other markers was large. This finding, from the classical-MSC markers perspective, demonstrates a success in the consistency of these methods and protocols. However, with respect to other markers, differences of over two orders of magnitude were not uncommon between patients and tissues.
If we assume for the sake of discussion that higher levels of any one or all of these markers are desirable, these data provide evidence that some patients would seem to be better CTP-donors than others. However, no patient or sample lacked these markers entirely. In addition, the tissue sources within every patient varied in biological performance. Almost every patient demonstrated differences between tissue sources, manifest by 2–20 fold differences in the expression of individual markers.
While patients could be ranked based on overall expression levels, as shown in Table III, no one tissue source stood out in terms of consistent-expression of these additional markers. However, AT-derived cells had the distinction of having statistically lower expression of SSEA-4 and CD146 than TS-derived cells. MS-derived cells also had higher levels of CD146 than AT-derived cells. AT-samples were also distinct in having a larger range of variation. AT-samples demonstrated over a 100-fold range of variation with respect to 4 of the 11 non-traditional markers (SSEA-1, SSEA-3, Ep-CAM, and HA). In contrast TS-derived cells demonstrated the least variation, less than 25-fold for all makers except HA (Table IV). The presence of SSEA-1 positive cells could indicate committed progenies among the cell populations from all tissue sources.
The significant variation revealed in the expression levels of these other stem and progenitor cell markers raises several questions, and reveals important gaps in our understanding of the variation in phenotype and biological potential that may exist in the cell populations that have traditionally been referred to as homogenous “MSCs”. Few papers in the recent literature have systematically characterized culture-expanded MSCs based on any but the traditional set of ISCT-MSC markers [31,67–69]. Few papers examined the effect of variation in cell sources [17,18,24,70,71], or in culture-expansion protocols [10,72–76]. Of course, the findings of this study are limited to the one expansion protocol used, and alternative protocols may well have provided a different result.
The ISCT-criteria for MSCs also indicates that the cell population should have tri-lineage differentiation potential (i.e. that under stimulated conditions the cells should express either osteogenic, chondrogenic, or adipocytic markers). We did not attempt to test for tri-lineage potential in this initial assessment. The ISCT-standard does not define these metrics with a high level of precision, and most papers that took the time to test for differentiation potential simply evaluated subjective histochemical-staining for mineral deposition, negatively-charged extracellular matrix, or lipid aggregates. Yet staining using these markers is highly variable and interpretation can be highly subjective. Moreover, we are not aware of any papers that have clearly linked performance in assays for tri-lineage potential with other metrics of in vitro or in vivo performance [13]. Since recent papers have already shown the lack of correlation between traditional-marker expression and differentiation performance [17,37,40], we chose not to include an assessment of differentiation potential in the current study. It was most important first to determine the extent of variation of other accessible markers, which we have now done.
In order to move the field forward, effective cell therapy products will need defined critical quality attributes (CQAs) that are as closely linked and predictive of clinical outcome as possible. It is, therefore, essential that we as a community contribute to development of methods and standards for quantitative assessment and reporting of clinically relevant MSC functional attributes, as well as surface markers. The MSC Committee of the ISCT has contributed again to this discussion of functional metrics in a report by Krampera M., et. al. [34]. Functional metrics may include dynamic measures of in vitro cell performance. These metrics may include: proliferation rate; proliferative capacity (doubling potential); migration; or evidence of differentiation based on mRNA expression, secretome/proteome analysis, matrix synthesis, metabolic activity. The biological response of cells to defined surfaces, matrices, or soluble factors may be considered. Most markers of cell performance could be assayed using image analysis [15]. Standardized metrics for assessment of cell-cell interactions mediated through contact or paracrine effects may be particularly important for prediction of clinical immunomodulatory activity. Again, in most cases these metrics of cell-cell interaction (aggregation, death, motility, and proliferation) can be quantified using image analysis.
While an association between the non-traditional markers and pluripotency is established for iPS and ES cells, one cannot assume that expression of these additional markers will correlate directly with biological performance or differentiation outcomes of MSCs. However, now that this variation is known, there is a clear opportunity to test the hypothesis that one or more of these alternative-markers may be more predictive of MSC biological and differentiation performance. The correlation between the expression of any of these markers, alone or in combination, will need to be defined in future studies using standardized in vitro and in vivo models that measure specific biological attributes of performance (e.g. proliferation, migration, survival, doubling potential, engraftment, contribution to new tissue formation). Standardized methods for assay of immunomodulatory effects must not be forgotten [77], as immunomodulation is the presumed mechanism for most of the therapeutic effects attributed to culture-expanded MSC populations. If an association between these markers and biological performance is established, then this would represent only a first step forward. The larger challenge from the clinical, biological, and manufacturing perspective is to understand the underlying reason behind the wide variation in marker expression that has been demonstrated. Part of the variation between individuals is likely related to clinical factors of age, gender, co-morbid conditions, genetic background, and the health of tissue at the site of tissue harvests. In this small cohort of eight individuals, we are unable to contribute much insight into the impact of these clinical variables. Examination of hundreds of individuals would likely be necessary to build out models that incorporate the impact of clinical variables on the attributes and performance of culture-expanded cells at the level of an individual or a specific tissue type or location.
This initial report does not yet examine questions of repeatability (Would another aliquot of the same sample perform the same way?), or reproducibility (Would the same methods performed in another lab yield the same results?). These important aspects of measurement will need to be addressed if we are to move toward greater understanding of native CTP-populations [3,4] and their relationship to tissue health. Repeatability and reproducibility are also essential if we are to establish safe, efficient and effective methods to generate biologically-potent culture-expanded populations with defined CQAs with high reliability. The repeatability of outcome between labs and between individuals is essential, if we are to develop safe and predictably effective cell-therapies based on culture-expanded cell populations.
Clinical differences between patients, tissue samples, and tissue health may not explain all of the variation observed. It is likely that some, and potentially even most, of the variation that we found between individuals and tissues could be due to stochastic variation in the specific CTPs, subtypes, or mix of subtypes that happened to be sampled in the initial aliquot that was used for culture-expansion. It is well known that the colonies formed by these initial-founding cells vary greatly with respect to biological performance [78]. Some colonies proliferate rapidly, but do not sustain long term proliferation; others proliferate slowly. Slow growing colonies may be overwhelmed by more rapidly growing clones, particularly in early phases of expansion. Ultimately, only those cells with long term proliferative capacity will be represented, and those cells present in later phases of expansion may be derived from only a small subset of the CTPs that were plated initially. If the hierarchy of CTPs in MS, TS, or AT populations in native tissues contains a plurality of subtypes and those colonies with the greatest likelihood of generating progeny with high expression of these non-traditional makers are relatively low in number, then variation in the representation of these high performing colonies in the initial sample (particularly their absence) could play a large role in determining the end result. Similarly, if some clones have the attribute of secreting factors that down regulate the expression of one or more of these markers, then stochastic variation in the number and the proliferation rate of such clones could also have a profound effect. A direct assessment of the impact of individual clones on the outcome of culture-expanded populations is possible. This will require systematic methods for early and controlled isolation of clonal populations.
We have begun to develop and apply such tools [79], and intend to address these questions in future work. While clonal selection cannot be assumed to minimize variation, it seems reasonable that some of the variation that is present would be better controlled if colony selection based on key attributes of early colonies that predict future performance could be defined (e.g. colony morphology, size-cell number, or early expression of key markers).
This study had three principle limitations. First, the data set is limited to only 24 culture-expanded populations from three-tissue sources from each of eight patients. While this represents a substantial amount of work, and far greater numbers of culture-expanded populations than what are usually reported in studies of MSC, the volume of the data available does not enable robust “principle component analysis” to demonstrate the strength of associations and co-variation between the markers that we have added to the armamentarium of MSC analysis. Second, colony data in the AT-derived cell population is unavailable. This is partly due to the fact that colonies from fat proliferate with much lower density than colonies from MS and TS sources. Future studies seeking to measure CTP prevalence should consider lowering the plating density for AT-cell sources than what was used in the current study. Third, the nature of the founding-CTPs and colonies of the populations of culture-expanded “MSCs” is uncertain. This is a limitation that is common to virtually all studies using MSC-populations. We are unable to determine the early attributes of the CTP-derived clones that contributed to the ultimate population in each sample. Defining the relationship between the attributes of the population-founding CTPs and the attributes of a clonal culture-expanded population is a key unanswered question left for future work.
Conclusions
In summary, these findings further illustrate the large differences that exist between individuals and cell sources. These data also illustrate the advantage that is provided by quantitative imaging to enhance the rigor objectivity and reproducibility of CTP early-performance assays.
Expressions of traditional-MSC markers are highly predictable among culture-expanded human cell populations and may offer little objective value in determining potency or reproducibility. This deficiency may be augmented through the use of an expanded-array of markers.
Understanding the underlying reasons for variation in the concentration, prevalence, marker expression, and biological potential of CTPs between patients and source tissues, and means of managing this variation, will contribute to the rational development of cell-based clinical diagnostics and targeted cell-based therapies.
Supplementary Material
Acknowledgments
We acknowledge the funding source that support this work, the National Institutes of Health (R21 AR067357).
We acknowledge the assistance of Bunny Cotleur (Scientific Consultant of Flow Cytometry), Kewal Asosingh, (Scientific Director of Flow Cytometry), Cathy Shemo, Sage O'Bryant, and Patrick Barrett in the Flow Cytometry Core in the Lerner Research Institute of the Cleveland Clinic Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:1–24. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Silos V, Camacho-Morales A, Fuentes-Mera L. Mesenchymal Stem Cells Subpopulations: Application for Orthopedic Regenerative Medicine. Stem Cells Int. 2016;2016:1–9. doi: 10.1155/2016/3187491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002:66–80. doi: 10.1097/00003086-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86–A:1541–58. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 5.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–61. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyer CF, Berlet GC, Bussewitz BW, Hankins T, Ziegler HL, Philbin TM. Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am. 2013;95:1312–6. doi: 10.2106/JBJS.L.01529. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–36. [PubMed] [Google Scholar]
- 10.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 11.Harrington J, Sloan AJ, Waddington RJ. Quantification of clonal heterogeneity of mesenchymal progenitor cells in dental pulp and bone marrow. Connect Tissue Res. 2014;55(Suppl 1):62–7. doi: 10.3109/03008207.2014.923859. [DOI] [PubMed] [Google Scholar]
- 12.Aizman I, Holland WS, Yang C, Bates D. αSMA Expression in Large Colonies of Colony-Forming Units-Fibroblast as an Early Predictor of Bone Marrow MSC Expandability. Cell Med. 2016;8:79–85. doi: 10.3727/215517916X693357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell KC, Lacey MR, Gilliam JK, Tucker HA, Phinney DG, O’Connor KC. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol Bioeng. 2011;108:2716–26. doi: 10.1002/bit.23193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennerfeldt DA, Van Vliet KJ. Concise Review: When Colonies Are Not Clones: Evidence and Implications of Intracolony Heterogeneity in Mesenchymal Stem Cells. Stem Cells. 2016;34:1135–41. doi: 10.1002/stem.2296. [DOI] [PubMed] [Google Scholar]
- 15.ASTM. Standard Test Method for Automated Colony Forming Unit (CFU) Assays — Image Acquisition and Analysis Method for Enumerating and Characterizing Cells and Colonies in Culture 1. ASTM Int. 2012:1–11. doi: 10.1520/F2944. [DOI] [Google Scholar]
- 16.Powell KA, Nakamoto C, Villarruel S, Boehm C, Muschler G. Quantitative image analysis of connective tissue progenitors. Anal Quant Cytol Histol. 2007;29:112–21. [PubMed] [Google Scholar]
- 17.Nancarrow-Lei R, Mafi P, Mafi R, Khan W. A Systemic Review of the Sources of Adult Mesenchymal Stem Cells and their Suitability in Musculoskeletal Applications. Curr Stem Cell Res Ther. 2017;12 doi: 10.2174/1574888X12666170608124303. [DOI] [PubMed] [Google Scholar]
- 18.Kellner J, Sivajothi S, McNiece I. Differential properties of human stromal cells from bone marrow, adipose, liver and cardiac tissues. Cytotherapy. 2015;17:1514–23. doi: 10.1016/j.jcyt.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–46. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 20.Coipeau P, Rosset P, Langonne A, Gaillard J, Delorme B, Rico A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11:584–94. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 21.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 22.Toosi S, Naderi-Meshkin H, Kalalinia F, Peivandi MT, Hossein Khani H, Bahrami AR, et al. Comparative characteristics of mesenchymal stem cells derived from reamer-irrigator-aspirator, iliac crest bone marrow, and adipose tissue. Cell Mol Biol (Noisy-Le-Grand) 2016;62:68–74. [PubMed] [Google Scholar]
- 23.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–57. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 24.Trivanović D, Jauković A, Popović B, Krstić J, Mojsilović S, Okić-Djordjević I, et al. Mesenchymal stem cells of different origin: Comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci. 2015;141:61–73. doi: 10.1016/j.lfs.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Guijo FM, Blanco JF, Cruz G, Muntion S, Gomez M, Carrancio S, et al. Multiparametric comparison of mesenchymal stromal cells obtained from trabecular bone by using a novel isolation method with those obtained by iliac crest aspiration from the same subjects. Cell Tissue Res. 2009;336:501–7. doi: 10.1007/s00441-009-0778-x. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104:2728–35. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemotherapy. 2016;43:268–74. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012 doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phinney DG. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J Cell Biochem. 2012;113:2806–12. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 31.Denu RA, Nemcek S, Bloom DD, Goodrich AD, Kim J, Mosher DF, et al. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016;136:85–97. doi: 10.1159/000445096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14:516–21. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 34.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells-The international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–61. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713–23. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 36.Shoshani O, Ravid O, Massalha H, Aharonov A, Ovadya Y, Pevsner-Fischer M, et al. Cell isolation induces fate changes of bone marrow mesenchymal cells leading to loss or alternatively to acquisition of new differentiation potentials. Stem Cells. 2014;32:2008–20. doi: 10.1002/stem.1719. [DOI] [PubMed] [Google Scholar]
- 37.Lo Surdo JL, Millis BA, Bauer SR. Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells. Cytotherapy. 2013;15:1527–40. doi: 10.1016/j.jcyt.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein P, Sperling I, Corbeil D, Hempel U, Fickert S. Progenitor cells from cartilage-No osteoarthritis-grade-specific differences in stem cell marker expression. Biotechnol Prog. 2013;29:206–12. doi: 10.1002/btpr.1668. [DOI] [PubMed] [Google Scholar]
- 39.Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013;9:32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–5. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Camilleri ET, Gustafson MP, Dudakovic A, Riester SM, Garces CG, Paradise CR, et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther. 2016;7:107. doi: 10.1186/s13287-016-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv F-J, Tuan RS, Cheung KMC, Leung VYL. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 43.Ng H-H, Surani MA. The transcriptional and signalling networks of pluripotency. Nat Cell Biol. 2011;13:490–6. doi: 10.1038/ncb0511-490. [DOI] [PubMed] [Google Scholar]
- 44.Pacini S, Carnicelli V, Trombi L, Montali M, Fazzi R, Lazzarini E, et al. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs) PLoS One. 2010;5:e9861. doi: 10.1371/journal.pone.0009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parisi S, D’Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–14. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandt R, Normanno N, Gullick WJ, Lin JH, Harkins R, Schneider D, et al. Identification and biological characterization of an epidermal growth factor-related protein: cripto-1. J Biol Chem. 1994;269:17320–8. [PubMed] [Google Scholar]
- 47.Huang H-P, Chen P-H, Yu C-Y, Chuang C-Y, Stone L, Hsiao W-C, et al. Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. J Biol Chem. 2011;286:33520–32. doi: 10.1074/jbc.M111.256164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H-F, Chuang C-Y, Lee W-C, Huang H-P, Wu H-C, Ho H-N, et al. Surface marker epithelial cell adhesion molecule and E-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev. 2011;7:722–35. doi: 10.1007/s12015-011-9233-y. [DOI] [PubMed] [Google Scholar]
- 49.Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795:130–6. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Astachov L, Vago R, Aviv M, Nevo Z. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Biosci (Landmark Ed. 2011;16:261–76. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 51.Haylock DN, Nilsson SK. The role of hyaluronic acid in hemopoietic stem cell biology. Regen Med. 2006;1:437–45. doi: 10.2217/17460751.1.4.437. [DOI] [PubMed] [Google Scholar]
- 52.Bourguignon LYW, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–24. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caralla T, Boehm C, Hascall V, Muschler G. Hyaluronan as a novel marker for rapid selection of connective tissue progenitors. Ann Biomed Eng. 2012;40:2559–67. doi: 10.1007/s10439-012-0608-2. [DOI] [PubMed] [Google Scholar]
- 54.Jonsson KB, Frost A, Nilsson O, Ljunghall S, Ljunggren O. Three isolation techniques for primary culture of human osteoblast-like cells: a comparison. Acta Orthop Scand. 1999;70:365–73. doi: 10.3109/17453679908997826. [DOI] [PubMed] [Google Scholar]
- 55.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Tabatabaei M, Mosaffa N, Nikoo S, Bozorgmehr M, Ghods R, Kazemnejad S, et al. Isolation and partial characterization of human amniotic epithelial cells: the effect of trypsin. Avicenna J Med Biotechnol. 2014;6:10–20. [PMC free article] [PubMed] [Google Scholar]
- 58.Hulspas R. Titration of fluorochrome-conjugated antibodies for labeling cell surface markers on live cells. Curr Protoc Cytom. 2010 doi: 10.1002/0471142956.cy0629s54. Chapter 6: Unit 6.29. [DOI] [PubMed] [Google Scholar]
- 59.Festuccia N, Chambers I. Quantification of pluripotency transcription factor levels in embryonic stem cells by flow cytometry. Curr Protoc Stem Cell Biol. 2011 doi: 10.1002/9780470151808.sc01b09s19. Chapter 1: Unit 1B.9. [DOI] [PubMed] [Google Scholar]
- 60.Henrich D, Nau C, Kraft SB, Zollfrank M, Kontradowitz K, Oppermann E, et al. Effect of the harvest procedure and tissue site on the osteogenic function of and gene expression in human mesenchymal stem cells. Int J Mol Med. 2016;37:976–88. doi: 10.3892/ijmm.2016.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnanasegaran N, Govindasamy V, Musa S, Kasim NHA. Different isolation methods alter the gene expression profiling of adipose derived stem cells. Int J Med Sci. 2014;11:391–403. doi: 10.7150/ijms.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patterson T, Boehm C, Nakamoto C, Rozic R, Walker E, Piuzzi N, et al. The Efficacy of Bone Marrow Aspiration for Harvest of Connective Tissue Progenitors from the Human Iliac Creast. J Bone Jt Surg. 2017 doi: 10.2106/JBJS.17.00094. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 64.Rasini V, Dominici M, Kluba T, Siegel G, Lusenti G, Northoff H, et al. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy. 2013;15:292–306. doi: 10.1016/j.jcyt.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–91. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanavde V, Vaz C, Rao MS, Vemuri MC, Pochampally RR. Research using Mesenchymal Stem/Stromal Cells: quality metric towards developing a reference material. Cytotherapy. 2015;17:1169–77. doi: 10.1016/j.jcyt.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon Y-J, Kim J, Cho JH, Chung H-M, Chae J-I. Comparative Analysis of Human Mesenchymal Stem Cells Derived From Bone Marrow, Placenta, and Adipose Tissue as Sources of Cell Therapy. J Cell Biochem. 2016;117:1112–25. doi: 10.1002/jcb.25395. [DOI] [PubMed] [Google Scholar]
- 69.Denu RA. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/5841716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. No Identical Mesenchymal Stem Cells at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Breunig MJ, Escalante LE, Bhatia N, Denu RA, Dollar BA, et al. Biologic and immunomodulatory properties of mesenchymal stromal cells derived from human pancreatic islets. Cytotherapy. 2012;14:925–35. doi: 10.3109/14653249.2012.684376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffmann A, Floerkemeier T, Melzer C, Hass R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2153. [DOI] [PubMed] [Google Scholar]
- 73.Baustian C, Hanley S, Ceredig R. Isolation, selection and culture methods to enhance clonogenicity of mouse bone marrow derived mesenchymal stromal cell precursors. Stem Cell Res Ther. 2015;6:151. doi: 10.1186/s13287-015-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balint R, Richardson SM, Cartmell SH. Low-density subculture: a technical note on the importance of avoiding cell-to-cell contact during mesenchymal stromal cell expansion. J Tissue Eng Regen Med. 2015;9:1200–3. doi: 10.1002/term.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res Int. 2014;2014:951512. doi: 10.1155/2014/951512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adamzyk C, Emonds T, Falkenstein J, Tolba R, Jahnen-Dechent W, Lethaus B, et al. Different Culture Media Affect Proliferation, Surface Epitope Expression, and Differentiation of Ovine MSC. Stem Cells Int. 2013;2013:387324. doi: 10.1155/2013/387324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bloom DD, Centanni JM, Bhatia N, Emler CA, Drier D, Leverson GE, et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy. 2015;17:140–51. doi: 10.1016/j.jcyt.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gronthos S, Zannettino ACW, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 79.Kwee E, Herderick EE, Adams T, Dunn J, Germanowski R, Krakosh F, et al. Integrated Colony Imaging, Analysis, and Selection Device for Regenerative Medicine. SLAS Technol. 2017;22:217–23. doi: 10.1177/2211068216676587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.