Abstract
Several cancer immunotherapy approaches have been recently introduced into the clinics and they have shown remarkable therapeutic potentials. The groundbreaking cancer immunotherapeutic agents function as a stimulant or modulator of the body immune system to fight against or kill cancers. Although targeted immunotherapies such as immune check point inhibitors (CTLA-4 or PD-1/PD-L1), DNA vaccination and CAR-T therapy are revolutionizing cancer treatment, the delivery efficacy can be further improved while their off-target toxicity can be mitigated through nanotechnology approaches. Recent research has demonstrated that nanotechnology has multifaceted role for (i) reeducating tumor associated macrophages (TAM) to function as tumor suppressor agent, (ii) serving as an efficient alternative for Chimeric Antigen Receptor (CAR)-T cell generation and transduction, and (iii) selective knockdown of Kras oncogene addiction by nano-Crisper-Cas9 delivery system. The function of host immune stimulatory signals and tumor immunotherapies can further be improved by repurposing of nanomedicine platform. This review summarizes the role of multifunctional polymeric, lipid, metallic and cell based nanoparticles for improving current immunotherapy.
Keywords: Cancer, immunotherapy, nanomedicine, dendritic cell vaccine, CAR-T, CRISPR-Cas9, tumor associated macrophages, PD-1/PDL-1 targeting, CTLA-4 targeting, cytokine storm
Graphical abstract
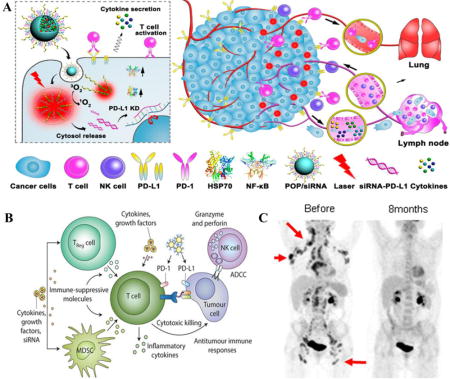
A) Photoactivatable nanomicelles complex with PDL-1 siRNA for combination of cancer immunotherapy and photodynamic therapy. (B) Molecular mechanism of tumor immune cell inhibition and activation of T-cell. (C) PET/CT scan of patient indicates the superior tumor growth inhibition after 8 months treatment of immunotherapy. Image (A) [35], B [17], (C)[89] are adopted and reproduced with permissions.
1. Introduction
Nanomedicine and immunotherapy are two widely discussed themes of this decade for achieving better outcomes in cancer treatment. The role linking cancer and immune surveillance system is a complicated biological network process. It is expected that body immune system should spontaneously reject the formation of cancer as a ‘foreign’ cell due to their unique and aberrant mutational properties. Our immune system has two arms, namely (i) the innate immunity, consisting of neutrophils and macrophages that defends against invasion of pathogens; and (ii) the adaptive immunity comprising of CD8+ cytotoxic T cells, B cells, T-regulatory (Treg) cells and natural killer (NK) cells that recognize and destroy infected cells, or memorize the antigens for fighting against them in the future[1]. In adaptive immunity such as vaccination, the B cells produce antibodies against the specific antigens and neutralize the ability of pathogens to attack host cells[2]. Due to the antigen-specific response of B and T-lymphocytes, they are called as adaptive immunity[3]. Another fundamental group of immune cells is the antigen-presenting cells (APCs). APCs, such as Dendritic Cells (DCs) reside in peripheral tissues and collect antigens from lymphatic fluids to trigger the activation of T cells mediated immune response[4]. Among the many innate immune receptors that are expressed in DC, macrophages and NK-cells, toll-like receptors (TLRs) play a prominent role in functioning against pathogens[5]. Tumor environment has unique characteristics to manipulate and evade immune surveillance by generating immunosuppressive cytokines through activation of immune checkpoint molecules [6]. Immune checkpoint proteins downplay the antitumor immunity. The best and successful anticancer strategy is to block the checkpoint molecules to rejuvenate active immune system, resulting in the suppression of tumor growth. The checkpoint molecules are classified into two categories, namely, (i) stimulatory checkpoint proteins (such as tumor necrosis factor family CD40, OX40 and CD27) and (ii) inhibitory checkpoint proteins (such as PD1, CTLA-4, and LAG3). The programmed cell death-1 (PD1) protein, expressed in T-cells and B-cells interacts with its ligand (PDL-1) of tumor cells to evade a T-cell mediated immune attack on cancer cells [7]. Similarly, another checkpoint molecule, CTLA-4 has been widely studied for cancer therapy. Besides the excellent progression-free survival rate compared to the conventional chemotherapy, the antitumor effects of PD-1, CTLA-4 antibody inhibitors are uncertain, and that is attributed to transient expression of checkpoint molecules, drug resistance and the inability of drugs to penetate the tumor stromal barriers.
Besides the significant research and clinical success, cancer nanomedicines are currently facing challenges in clinical translation. However, a deeper understanding of the tumor-associated environment such as heterogeneity, stromal barrier and tumor immune system opens a new paradigm for nanotechnology in cancer immune therapy. In this regard, a rational design of nanomedicines have shown promising potentials for tumor immune therapy and diagnosis. To set the stage for nanomedicine in cancer immune therapy, in this review we will briefly discuss the prospect of nanotechnology in the ongoing revolution of immunology and active immunotherapy.
2. Mechanism of tumor immune evasion
2.1. Role of immune system in cancer progression
The human body immune system is a dominant factor for homeostatic functions, defense against foreign pathogens, tissue repair, and clearance of dead cells. Our immune cells continuously screen every cell for normal function and if they recognize any aberrant gene mutation or cancerous cell formation, they immediately eliminate the malfunctioning cell (Figure 1). Macrophages mainly perform this task. Neutrophils and natural killer (NK) cells act as the first line of protection and second line defense are medicated with CD8+ cytotoxic T-cells. The activation of T-cell is associated with macrophages and DCs which represent tumor-associated antigens (TAAs) to T cells so that T-cell get activated and induce a potent adaptive immune response[8]. Despite these powerful immune defense mechanism, tumor microenvironment reprograms themselves to evade the immune system on several aspects, such as changing the expression of checkpoint markers to become invisible to immune cells, polarizing tumoricidal macrophages to tumorigenic macrophages and neutralizing cytotoxic CD8+T cell function. All these tumorigenic immune responsive factors create the immunosuppressive tumor environment through the secretion of chemokines, metabolic mediators, and cytokines as well as through cell signaling mechanisms. Thus, understanding the role of tumor-associated immune cells and inhibiting their contribution to tumor progression has become the central focus of cancer research [6, 73–76]. Inhibitors of immune-suppressing molecules have been widely used in the clinic for treating various types of solid and hematological cancers.
Figure 1.
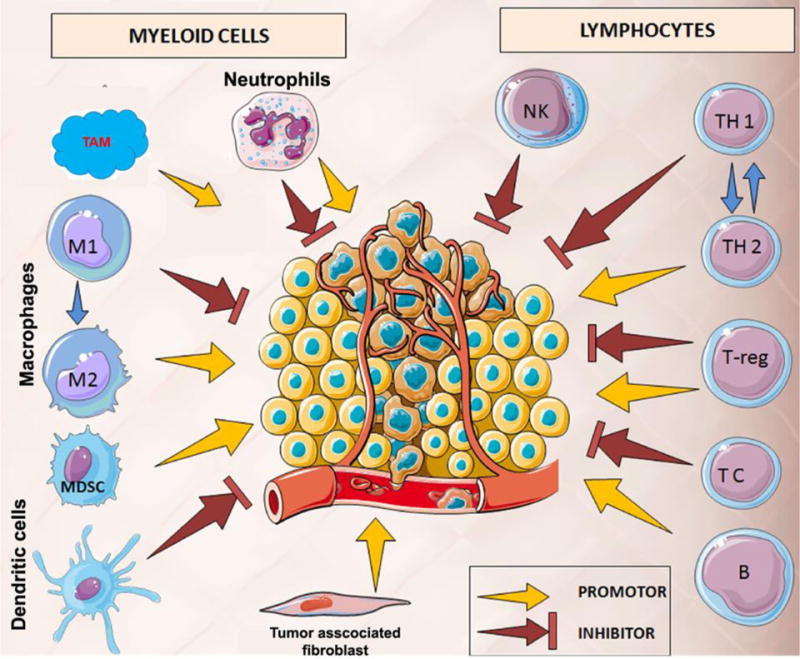
Dual tumoricidal and tumorigenic role of tumor-associated immune cells. Thus, selective up-modulation of tumoricidal macrophages is the prime goal of cancer immune resurrection and immunotherapy. This figure is adopted and modified from ref. [8] with permissions. TAM: tumor-associated macrophages, TC: T cells, NK: natural killer cells, MDSC: myeloid-derived suppressor cells.
2.2 Polarization of macrophages
The polarization of macrophage depends on tumor environmental stimuli such as cytokines, chemokine and growth factors that trigger specific biomarkers for tumor immune responses (Figure 1). In a simplified point-of-view, macrophages have been described as a “double-edged sword,” and they can transform both tumors promoting and suppressing factor in tumor milieu[9]. For examples, polarized M1-macrophages are considered as a promoter of tumoricidal immune functions by secreting pro-inflammatory cytokines, such as IL-12, IFNγ, IL-1, and IL-23, iNOS[10]. Thus, activated M1 macrophages reeducate the DC, and CD4+ T cells for destroying tumor cells, and present tumor antigens for cytotoxic CD8+ T medicated killing. On the other hand, pro-oncogenic M-2 and tumor-associated macrophages (TAM) represents as a major anti-inflammatory element of the tumor stroma. Accumulated research indicates that TAM and M2-macrophages have a significant role in the formation of tumor-associated fibroblast, angiogenesis, and oncogenic addiction that lead to suppression of adaptive immunity[11].
Thus, use of nanoparticle for specific modulation of macrophage subtypes is a smart approach in modern cancer immunotherapy[12]. It is reported that a polymeric-liposomal gel system co-combined with TGF-β inhibitor can able enhance natural killer (NK) cell activity against several cancer types with a significant reduction of tumor growth in vivo and an increased immune response[13]. Saeid et al. demonstrated that FDA-approved iron supplement ferumoxytol nano-micelles can induce reactive oxygen species (ROS) mediated pro-apoptotic protein upregulation[14]. This iron nanoparticle was capable of tumor growth suppression by inducing pro-inflammatory macrophage polarization in tumor tissues.
2.3. CTLA-4 PD-1, PD-L1 mechanistic pathway
Ipilimumab is the first FDA approved checkpoint inhibitor for targeting CTLA4 in metastatic melanoma. CTLA4, majorly expressing on T cells, regulates the early stages of T cell activation and co-stimulatory T cell receptor (CD28) neutralization [15].
Thus, the immunosuppressive role of CTLA4 appears through CD4+ T cells mediated signaling, such as downregulation of helper T cell and upregulation of regulatory T (Treg) cells. Therefore, inhibition of CTLA4 results in enhancement of immune-stimulatory responses through activation of CD4+ T cells and down-modulation of Treg cells [17]. Another checkpoint receptor, PD-1 is a promising tumor target with a diverse role in potential immune modulations that are capable of manipulating anti-tumor immune responses. In healthy cells, PD-1 protein counteracts the cytotoxic effects of peripheral T cells in response to inflammation and autoimmunity, but in the tumor environment, this immune regulation gets converted into a major immune resistance [18] [19]. PD-1 up-modulation is induced through activation of T cells, that results in binding with one of its ligands, such as PD-L1 (also known as B7-H1) or PDL-2 (also known as B7-DC). The engagement of PD-1 with PD-L1/2 in the tumor is a master step for blocking multiple anti-tumor immune responses, such as suppression of T cell-antibody presenting cell (APC) interaction, exhaustion or depletion of CD8+ T cells functions, and increasing Treg cells’ infiltration in the tumor [20]. Just as PD-1 is expressed in the majority of the tumor infiltrating lymphocytes (TILs), PD-1 ligand, PDL-1 is frequently overexpressed in cancer cells, myeloid cells, tumor-associated macrophages, and TILs. Recent reports in renal cell carcinoma suggest that the PDL-1 expression in tumor microenvironment predicted a poor prognosis than the PDL-1 negative counterpart tumor types [21]. In some tumors such as glioblastomas, lymphoma and lung cancer, it has been found that PDL-1 expression is regulated by oncogenic signaling kinases, including PI3K-AKT, anaplastic lymphoma kinase (ALK), and signal transducer and activator of transcription 3 (STAT3) pathway [22] (Figure 2).
Figure 2.
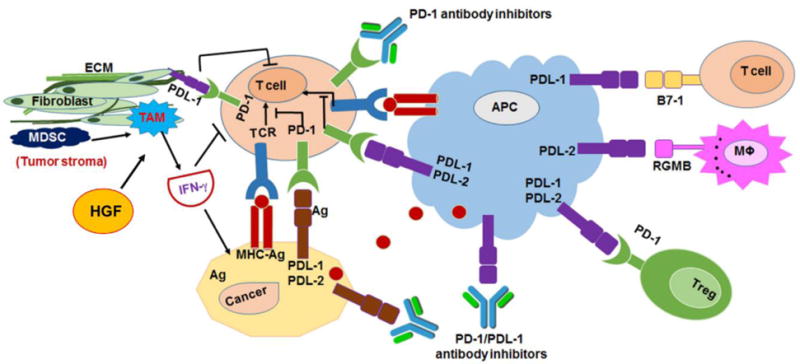
Mechanism of PD-1 and PDL-1 pathway in tumor environment and the complex crosstalk between cancer, tumor, T-cell, APC and macrophage and tumor stroma. The image was modified and reproduced from [16]. ECM: extracellular matrix, HGF: Hepatocyte growth factor, Ag: antigen.
3. Evolution of immune check-point inhibitor therapy
3.1 Anti-PD-1/PDL-1 and CTLA-4 therapy
3.1.1. Success of checkpoint blockers
With regard immunotherapy, thus far five checkpoint antibody inhibitors, including Ipilimumab (for targeting CTLA4), Nivolumab/Pembrolizumab (for targeting PD-1) and Atezolimuab/Durvalumab (for targeting PD-L1) have been commercialized for treating different types of primary, metastatic and unresectable tumor. There are close to a dozen human immunotherapy based trials that have been completed with more than 50 clinical trials under investigation [23]. The current market size of immune checkpoint blockers in the United States alone is ~US$7 billion, and it is predicted to reach US$15 billion by 2024 [23].
One of the successes for targeting and inhibiting PD1/PD-L1 interaction was after discovering nivolumab (Opdivo; Bristol Myers Squibb, Princeton, NJ). Nivolumab is the first humanized anti PD-1 monoclonal antibody that has been approved in treating melanoma. This discovery opens a new avenue for cancer treatment [24]. Nivolumab currently approved to treat multiple cancer such as melanoma, lung cancer, colon cancer and renal cancer [25,26]. Pidilizumab is another humanized anti-PD-1 antibody that has been found to be effective on multiple hematological malignancies such as acute myeloid leukemia, chronic lymphocyte leukemia and Hodgkin’s and non-Hodgkin’s lymphoma [27]. Atezolizumab and Durvalumab showed antitumor activity; therefore, these agents have been approved recently to treat multiple solid cancers[28].
4.1.2. siRNA for PD-1 and PD-L1 knockdown
RNAi is one of the technologies that is used to interfere with the expression of specific genes in cancer cells which consequently leads to tumor inhibition[29]. Short half live, degradation in the presence of nucleases and poor stability are obstacles that complicate using naked siRNA. Therefore, encapsulation of siRNA in nanoparticle will improve the overall stability and targetability of siRNA[30]. RNAi can work either by inhibiting immune suppression or by enhancing the immune response[31]. The best strategy of using RNAi is to inhibit immune repression and induce the immune response to eliminate most of the tumors [32]. Achievement of the best treatment using siRNA depends on many factors such as success either conjugation or encapsulation of siRNA with/in nanoparticles, route of administration, the stability of the formulation, and targetability to minimize the adverse effects[33]. Studies reported the ability of nanoparticles in targeting the immune cells and induce the innate immune activation via toll-like receptor (TLR) mediated pathway. Heo et al. demonstrated the significant knockdown of PLGA nanoparticles co-encapsulated with a STAT3-specific siRNA and TLR-7 agonist[34]. This unique system works via internalization of dendritic cells (DC) and activation TLR7. Activated TRL7 has a crucial role in suppression of immunosuppressive gene which leads to inhibit tumor growth [34]. Cubillos-Ruiz et al. demonstrated linear polyethyleneimine-based (PEI-based) nano-micelles encapsulating siRNA were significantly engulfed by regulatory DCs expressing CD11c and PDL-1 at the ovarian tumor in mice. The selective uptake of PEI-siRNA transformed the DC from immunosuppressive cells to activated antigen-presenting cells, resulting in activation of T-lymphocytes. Activation of TLR5, TLR7 supports that PEI is functioning as an agonist of TLR mediated DC activation[35]. This observation supports the use of nanotechnology platform as an opportunity for regulating immunosuppression activity of the cancer cells.
4. Vaccination of tumor
4.1. Dendritic cell (DC)
DC is one of the major antigen presenting cells, which have a vital role in communication between innate and adaptive immunity [36]. DC is able to induce either immune tolerance or immune enhancement [37] depending on the type of antigens [38].
DC has the ability to stimulate the two central components of the adaptive immune system including activation T-cell and differentiation of B-cells [40]. The DC is originated from the bone marrow, and they are classified into myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) [41][37]. DC communicate with T-cell through binding with major histocompatibility molecules (MHC) protein [41]. The selective T-cell response depends on the types of an antigen presenting molecule of the DC surface (Figure 3). It is known that the DC expresses both major histocompatibility type I, II (MHC-I & MHC II) [41]. If the antigen is presented by MHC-I, then it activates CD8+T-cytotoxic, whereas if the antigen is presented by MHC-II, then it attracts CD4+T-helper cell [42]. CD8+ T-cell immunity is pathogen specific, and its responsibility is to encounter the infection and to create memory T-cell which is able to survive in the absence of foreign antigen to provide sustained protection against recurrent infection [43]. CD4+ T-cell immunity has different pathways depending on its differentiation either into Th1, Th2, Th17, follicular helper T-cell and to Treg [44].
Figure 3.
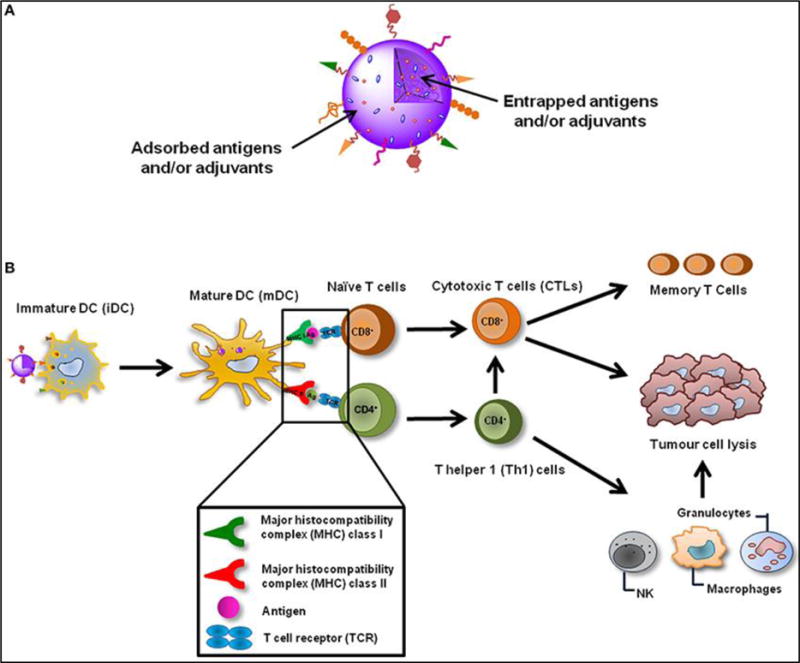
Outline of tumor vaccination with nanoparticle: A. Antigen encapsulated nanoparticle to activate peripheral dendritic cells. B. This immune-modulating nanoparticle triggers the adaptive immune responses through reactivation of CD8+ cytotoxic T cells to function against the specific tumorigenic proteins. The figure was obtained and reproduced from ref.[39] with permissions.
4.2. Dendritic cell immunotherapy, DNA vaccination
Despite all the enormous scientific efforts, the key to cure and prevent most of the cancer types has not yet found. Cancer vaccine holds the hope even there are difficulties on its application. Recently, many types of cancer vaccine, such as cell-based, synthetic proteins, protein antigens, DNA vaccines, antibodies have been emerged [45]. Developing of therapeutic cancer vaccine is aimed for recognition and destruction of the cancerous cells. More specifically cancer vaccine triggers the immune defense mechanism against tumor-associated antigens. Then the awaked immune system will start looking for the antigens of interest and kill the tumor cells [45].
DNA vaccines are genetic information delivery vehicles that are transfected to target cells for producing antigen of interest. For cancer, DNA vaccine is developed against tumor-specific antigens that are encoded into plasmid DNA under the control of a mammalian promoter (i.e., CMV-intA, CMV immediate/early promoter, and its adjacent intron A sequence) and they can be easily produced in the bacteria. Many attempts have been carried out to develop and translate the DNA vaccination in the clinic for inhibition tumor proliferation[41]. The first cell-based based cancer vaccine, Sipuleucel-T/APC8015 (Provenge®) was approved in 2010 against metastatic castration-resistant prostate cancer[45]. This vaccine stimulates the immune activation against the common prostate cancer antigen, Prostatic Acid Phosphate (PAP). This vaccine is patient specific as it is prepared by isolating patient DCs from the blood by leukapheresis process, then cultured in ex-vivo with a recombinant fusion protein (PAP) fused with granulocyte–macrophage colony-stimulating factor (PAP-GM-CSF). Then the engineered APCs are re-injected to the patient to stimulate the patients’ T-cells for recognizing and killing PAP overexpressed prostatic cancer cells. [46]. The mechanism of action of DC in the immune system is simply illustrated in Figure 4.
Figure 4.
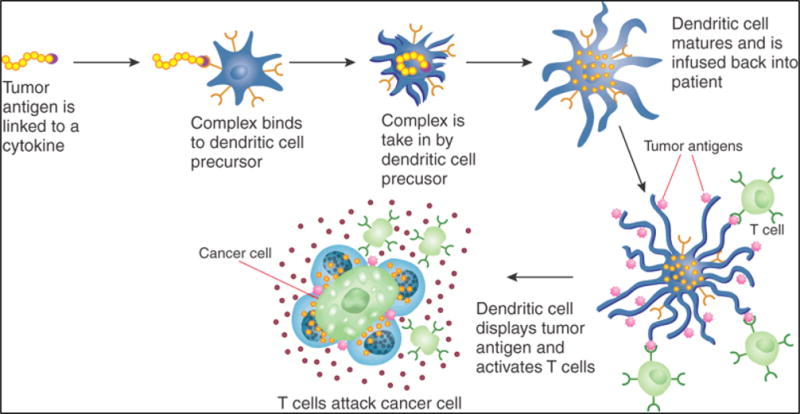
Off-the-shelf engineering of patient’s dendritic cell vaccine specific to the tumor antigen. This DC infused to patients so that it can express the tumor antigen and guide the T-cell to kill tumor cells. The figure is adopted form ref. [45] with permissions.
5. Application of nanotechnology to tumor immunotherapy
5.1. Nanomedicine for tumor vaccine
One of the targeted vaccination strategies is the use of nanotechnology-based DC vaccine. It has been shown that nanotechnology can enhance the stimulation of immune system against infectious and malignant diseases[47]. Nanotechnology provides wide range applications, such as improving drug and gene delivery, delivering of theranostic agents [48][16,49–51], improving the bioavailability of water-insoluble agents[52–54][55]. The advantages of nanoparticle-vaccine are that it increases antigen delivery to DC, non-immunogenic in nature and sustain antigen releasing ability [39]. Alongside, Nano-engineered DC-vaccine also utilized to eliminate the post-surgical residual malignancy thus preventing tumor relapse [56]. The liposome-based DC vaccine, DepoVax™ (DPX-0907) is currently studying in phase I clinical trials for breast, ovarian, prostate cancer. It consists of novel mixture of seven tumor-specific epitopes (TAAs), such as TNF-α-converting enzyme (TACE/ADAM17), B-cell receptor-specific protein 31 (CDM protein), topoisomerase II α, Abelson homolog 2 (Abl2), epithelial discoidin domain receptor 1 (EDDR1), γ catenin (Junction plakoglobin)[57]. This blend of peptide epitopes and adjuvants can be manipulated for the need for specific tumor phenotypes and activation cytotoxic and helper T-cells. Clinical data showed that efficient trapping of the DPX-0907 liposome to DC at the site of injection and effective activation of cytotoxic T-cells[58]. Another study unveils the useful application of theranostic nanoparticles in DC-based vaccination field [59]. The selected nanoparticle was an iron oxide and zinc oxide core-shell nanoparticle which was able to deliver both genes of interest and imaging agent to DC at the same time. The result shows successful gene translation and specific accumulation in circulatory DC[60]. Another promising example of theranostic Up Conversion Nano-Particle (UPNP) coated polymeric NP. The ovalbumin model antigen (OVA) was loaded to UPNP using electrostatic force to form nanoparticle/antigen complex[61]. This complex has the unique optical feature for tracking of mature migratory DCs in vivo data indicated antigen/nanoparticle complex increases the CD8+ cytotoxic T-cell propagation [62].
5.2 Nanoparticle for targeting circulating tumor immune cells
Current approaches of targeted immunotherapy are directed towards reduction of heterogeneity among malignant cells and preparing the body’s own defense to combat circulating tumor cells. While much has been documented about the Enhanced Permeability and Retention (EPR) effect, and its performance in vivo based on the morphology of nanoparticles and the tumor type, little has been known about the role of circulating monocytes in nanoparticle uptake that gives a contrast to the functioning of the EPR effect. In a study conducted by Bryan et al., has been shown that a certain subset of circulating monocytes can preferentially take up single-walled carbon nanotubes (SWNT), rather than depending on the EPR effect that more often fails to operate because of heterogeneity amongst tumor types[63]. Since these monocytes further differentiate into tumor-associated macrophages (TAMs) and can penetrate through hypoxic and necrotic tumor regions which are more often inaccessible to conventional targeted nanoparticles. SWNT uptake opens the possibility of drug delivery in hypoxic regions. Additionally, these nanoparticles are viable for as long as the carrier immune cells (or monocytes) are in circulation throughout the body [63]. Interesting findings on the role of immune cells in cancer therapy have made a consensus shift in research, and scientists are developing nanocarriers armed with immune cell targeting as a viable approach in anticancer therapy. The goal of cancer immunotherapy is to enhance the natural ability of the immune system to identify, scavenge and eliminate cancer cells.
5.3 Liposomes in cancer immunotherapy
Various approaches to deliver the drug by liposomes have been studied with different targeting approaches utilized to procure the best outcome. For delivery of advanced medicine such as gene or immunotherapy, localizing the delivery of the cargo to the intracellular compartment is crucial for maximizing therapeutic efficacy[64][65]. Toward this dextran modified liposome encapsulated with ovalbumin showed tumor pH-sensitive ovalbumin delivery to the cytosol of dendritic cells, triggering antigen-specific cellular immunity via MHC-I pathway [66]. The activity of immunotherapy agent is enhanced manifold when delivered with an adjuvant that sensitizes the dendritic cells to express co-stimulatory molecules (like CD80 and CD 86). With that perspective, cationic lipids have been studied to activate dendritic cells to enhance the effect of cellular immunity. Another study reported that liposomes containing antigenic protein to trigger cellular immunity. The liposomes were surface-modified with pH-sensitive polymer to specifically fuse with and release cargo in the mildly acidic tumor environment. Moreover, these liposomes were engineered to carry cationic lipid that served the purpose of adjuvant [67]. Cyclic di-GMP has been reported to trigger the innate immunity system, to bind with DDX41 in the cytosol, and to form a complex with interferon stimulating protein (STING) that sends a message to produce interferon type-1. However, the major issue with cyclic di-GMP is the high polarity that does not allow the passage through phospholipid membrane. Hence, cyclic di-GMP has been formulated into liposomes made of lipid with high fusogenic character and ability to release in mildly acidic tumor environment [68]. With an aim to minimize the biodistribution of immunotherapy agents and to avoid infiltration to distal organs in a bid to concentrate localized therapy, PEGylated liposomes functionalized with anti-CD40 and CpG oligonucleotides were developed. CpG oligonucleotides are ligands for toll-like receptors (TLR) and potent immunostimulatory agents, whereas CD40-antiCD40 ligand triggers a signaling mechanism to promote anti-tumor T-cell response [69].
5.4 Polymer-based nanocarriers in cancer immunotherapy
Polymeric micelles which are self-assembled structures have been in cancer research for quite some time owing to high drug loading and ability to modulate surface characteristics by simple chemistry[70]. Polymeric micelles loaded with 6-thioguanine have been studied to suppress the effect of Myeloid-suppressor cells (MDSCs) and to enhance anti-tumor T-cell response in a murine model [71]. MDSCs are responsible for downregulating the efficacy of antitumor immunity and are supposed to be a major hindrance to therapy. The cationic polymer, such as polyethyleneimine (PEI), has property to induce necrosis through recruiting inflammatory cytokines at the site of the tumor. To overcome necrotic effect, PEI-polymeric micelles was composed cationic charge masking hyaluronic acid (HA). Thus, once HA-PEI micelle internalized into the cell, it sheds off the HA layer, revealing the cationic complex in the cytosol of the cell that leads to selective production of cytokines like monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor α (TNF-α) further inhibiting cell growth [72]. Another study, self-assembled block copolymer composed of poly(ethylene oxide)-block-poly(α-carboxylate-ε-caprolactone) was conjugated with a STAT3 inhibitor able to reverse the immunosuppressive effect in a class of tumor cells, and the effect was enhanced in the presence of adjuvants [73]. Although the literature on polymeric micelles in cancer immunotherapy is not well explored, current trends showing more research outcome in polymeric micelles for tumor immune modulation.
5.5 Magnetic nanoparticles in cancer immunotherapy
Magnetic nanoparticles have been widely studied in the theranostic domain because of their Magnetic Resonance Imaging (MRI) property. Literature increasingly suggests the popularity of magnetic nanoparticles, predominantly Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as the imaging component in multifunctional theranostic nanoparticles. Magnetic nanoparticles are often coated with a biocompatible material to reduce aggregation. In one study, magnetic nanoparticles coated with dextran and further functionalized with MHC-Ig dimer and anti-CD28 have been shown to enhance T-cell activation by localizing the nanoparticles by external magnetic field [74]. The powerful helper T cell subset, Th1 response is mediated by cytokines such as IFN-γ. Magnetic nanoparticles coated with dimercaptosuccinic acid and IFN-γ as the anti-tumor agent were localized at the site of action using the external magnetic field in tumor mouse model, leading to enhanced immune response and subsequent reduction in tumor site [75]. Similar cytokine was delivered by adsorbing on magnetic nanoparticles surface functionalized with carboxylic acid [76]. Nanoparticles composed of iron oxide core and zinc oxide shell, and engineered with suitable antigen for uptake by dendritic cells have been developed, and they show the promise of cell uptake without the use of toxic transfection agents [60].
5.6 Other inorganic nanoparticles in cancer immunotherapy
Beside the lipid and polymer nanoparticles, inorganic nanoparticles such as gold, CuS have also been studied for cancer immunotherapy. These nanoparticles present a large surface area, small size, and ability to manipulate the surface while maintaining the core functionality. Moreover, they have also been explored for combination therapy to enhance the efficacy of immunotherapy, making the tumor cells more susceptible to attack by the immune system. Likewise, photothermal ablation therapy was applied to CuS hollow nanoparticles coated with chitosan, and carrying the CpG oligonucleotide that specifically activates the toll-like receptor 9 (TLR9) signaling in plasmacytoid DCs. Interestingly, on laser irradiation, these hollow nanoparticles were broken down into small CuS nanocrystals that held the propensity to assemble into spontaneous polymeric nanoparticles, making a smooth uptake by DCs [77]. Gold nanoparticles have been widely explored in theranostics since they are non-toxic and provide good resolution by computed tomography (CT) imaging[55]. Gold nano-vaccines that were able to stimulate the immune system were developed with red fluorescent protein as the model antigen, and CpG oligonucleotide conjugated onto the surface by a series of chemical reactions [78][79].
5.7 Biomimetic nanoparticles in cancer immunotherapy
Materials that mimic the biological components are advantageous in a way that there is no unwanted systemic toxicity associated with them. Viral proteins naturally mount an immune response, but if these viral proteins are mimicked with functional antigen recognition without associated virulence, then this paves the way for a novel system that can be used for cancer immunotherapy. The non-viral E2 subunit of pyruvate dehydrogenase capable of activating dendritic cells and encapsulating CpG were formulated as nanocarriers [80]. Cholesterol-pullulan nanogel encapsulating IL-2 were developed and studied for tumor suppression in mice [81]. Polymer core nanoparticles surface decorated with tumor-associated antigens were developed which when injected into the body, would recruit professional antigen presenting cells to specifically scavenge, adding a novel dimension to cancer vaccine therapy [82]. Likewise, plenty of literature suggests a coating of core nanoparticles with biomimetic membranes suitable for tumor vaccines.
5.7 Rational design of nanoparticles with better immunotherapy and minimum side effect
Beside the excellent clinical outcome of immunotherapy, it has been found that the response rate of patients remain modest (<20%). The systemic immune activation by immunetherapeutics has often led to severe toxicity, including colitis, pneumonitis, cytokine storm that is due to undesired interatciton between immunetherapeutics and host cells[83][84]. To resolve these challanges, nanoparticle approaches have shown promising preclinical outcome. Schmid D. et. al., have developed FDA-approved polymers poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) nanoparticle surface finctionalized with CD8α fragmented antibody (Fab)[85]. The idea of this design is to target the CD8α receptor of endogenous T cell subpopulation in blood, secondary lymphoid organs, and tumors. These nanoparticles would augment T cell function better than systemic administration of free drug and the study showed specific binding in vitro and in vivo. In order to selectively target the PD-1 receptor of T-cell PLGA-PEG nanoparticle was further conjugated with PD-1 antibody (namely, PD-1-PLGA-PEG). This system was co-encapsulated with small molecule TGFβ inhibitors (SD-208), which showed sustained released of drug in systematic circulation. The mice harboring colorectal tumors when treated with PD-1-PLGA-PEG nanoparticle showed significant improvement in survival rate as compared to free drug. Similarly, co-delivery of Toll-like receptor (TLR7/8) agonist (R848) with PD-1-PLGA-PEG nanoparticle had recruited higher amount of payload in T-lymphocytes of tumors, providing a novel off-the-shelf approach for improving limitations of current cancer immunotherapy (Figure 5). SD-208 encapsulated PLGA-PEG nanoformulation is safer compared to free SD-208 as it release the SD-208 after binding with infiltrating T-cells, thus reduction of non-specific toxicity associated with autoimmune responses. Rui. K et. al., has developed high-density lipoprotein-mimicking nanodiscs coupled with antigen (Ag) peptides and adjuvants for personalized immunotherapy with patient-specific neoantigens for stimulating strong CD8α+ cytotoxic T-lymphocyte (CTL) responses in Melanona[86]. Strikingly, nanodiscs produced 47-fold greater neoantigen-specific CTLs activity than soluble vaccines and 31-fold greater activity than the clinically approved CpG adjuvant (namely Montanide™) (Figure 6). The combination of Nanodiscs vaccine and anti-PD-1 therapy has completely eradicated the existing tumor. These findings represent a new and powerful approach for cancer immunotherapy and suggest a general strategy for personalized nanomedicine. The reasons of the strong anti-tumor immune response of nanodics are attributed to (i) smaller size (~20 nm) that help faster access of lymphatic tissue uptake than free peptide, (ii) in vivo stability of antigen peptide in nanodics formulation compared to free peptide, and (iii) efficient expression of antigen in the surface of antigen presenting cells.
Figure 5.
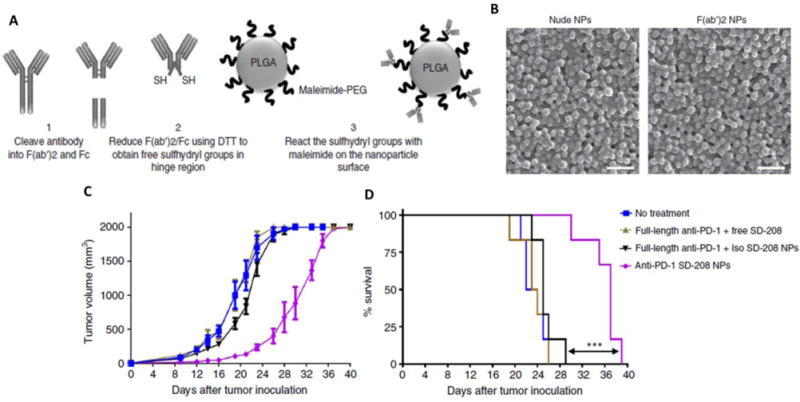
A. schemetic representation of F(ab′)2 antibody ligation to PLGA-PEG polymeric nanoparticles through thiol-maliamide chemistry. B Scheme of antibody fragment conjugation to the surface of pre-formulated maleimide-functionalized PEG-PLGA polymeric nanoparticles (NPs). B Scanning electron microscopy images showed the nanoparticulate nature of free and antibody conjugated nanoparticle. C. Tumor growth inhibiton of TLR7/8 agonist encapsulated anti-PD-1 PLGA-PEG nanoparticle than combination of individual components. D. Smilarlt, significant improvement survial rate in nanoformulation encapsulated with TGFβ inhibitor (SD-208). These data demonstrate the superiority of nanoformulation for improving current cancer immunothrapy with least side effect. Images were reproduced form ref. [85].
Figure 6.
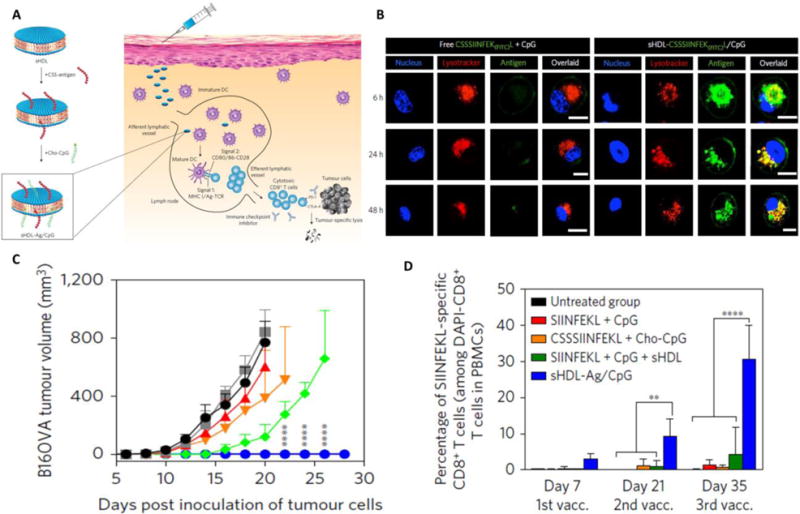
A. Development of ~20 nm nanodics and co-encapsulated with antigen and adjuvant and due to smaller size nanodisc has faster access to lyphatic vessel, thus generating stronger immue responses compared to conventional peptide antigen. B. Higher expression of antigen (right panel) in case of nanodisc treated dendritic cell as compared to free peptide form of antigen (left panel). C. Complete rejection of tumor in sHDL-Ag/CpG (nanodics) vaccinated mice injected with B16Ova cancer cells. D. The strong activation of T-cell in sHDL-Ag/CpG vaccinated mice is the main reason for anti-tumor immune responses. images were reproduced from ref. [86]
6. Engineering of CAR-T cell, and delivery to tumor immune resurrection
Chimeric antigen receptors (CAR) is one of the best strategies to reprogrammed patient own T-cell function for cancer immunethrapy[87]. It is consist of (i) bio-engineered fusion proteins of antigen recognition site, T-cell receptor signaling site, a costimulatory site that can be expressed in T cells and reprogram them to behave as cytotoxic T cells for killing tumor cells (Figure 7). Recently FDA approves CD19 targeting CAR T cells for B-cell malignancies with improved survival rate[88]. Another group from the Moffitt Cancer Center just announced promising results from the phase I clinical trial of the CD19 antigen-specific CAR-modified T cells, namely ZUMA-1 (KTE-C19) to treat B-cell lymphoma.[89][90]. Diffuse large B-cell lymphoma is an aggressive B-cell cancer that can spread quickly throughout the body—requiring immediate treatment, including drug therapy, radiation therapy, and possibly a stem cell transplant. The method of treatment is to isolate T cells from a patient’s blood and engineered them for targeting the protein called CD19, that is found in lymphoma cells[91]. The engineered T cells were then injected back into the same patient. KTE-C19 T cells could distinguish cancerous lymphoma cells that overexpress CD19 and target them for destruction. Also, the objective of one portion in the ZUMA-1 study was to determine the safety of KTE-C19, as evaluated by the frequency of dose-limiting toxicities in cancer patients with diffuse large B-cell lymphoma who were refractory to prior therapy which combined of anti-CD20 therapy and an anthracycline-containing regimen.
Figure 7.
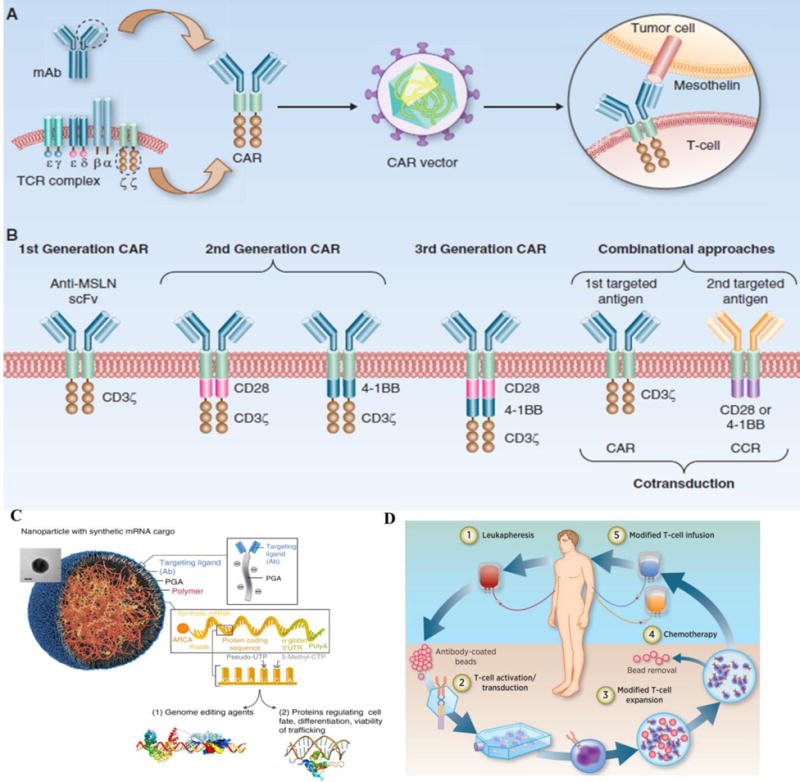
Outline of CAR T-cell design, advancement, nano-formulation and application. (A) structure of the CAR gene. The CAR gene contains (i) an intracellular signaling domain(CD3ζ), (ii) co-stimulatory domain (CD28 and/or 4-1BB), (iii) transmembrane domain, (iv) an scFv binding domain specific to tumor antigen (here methicillin, MSLN). This CAR gene is transduced to host cell along with viral packaging plasmid to form a pseudo-viral particle, and this particle is traduced to host cells. (B) Advancement of different generations of CAR vector. The main difference between first, second and third generation CAR is the introduction of more specific co-stimulatory domain to increase the activation strength of T cells. Combinational antigen recognition with balanced signaling has been described. (C) a non-viral nanoparticle for containing mRNA of interest to re-program therapeutic T-cells that can act like CAR-T cells. (D) Brief schematic explanation on how to isolate T-cells from patients, in vitro reprogrammed to express therapeutically relevant transgenes carried in CAR-gene, then further expanded to more number of T-cells and transfused back to the patient. Figures organized in such as for ease of understanding the CAR-T technology and application. Figure A, B was reproduced from [92], C obtained from [93] and D reproduced from[94] with permissions.
7. Challenges
Beside excellent clinical outcome of DC vaccine, PD-1/PDL-1 checkpoint inhibitors, CAR-T cell and CRISPR-Cas9 gene editing technology, many challenges are documented in recent years. For DC vaccine the most important issue is the faint immune response which is related to stability and reactivity of TAA on the MHC presenting molecule on the surface of DCs. In general, this type of vaccination is costly, time-consuming, and exhausting that is due to patient specificity. It requires well qualifications to extract DCs from the patient, incubate cells ex vivo, decide which type of DCs to be stimulated, the level of DCs maturation, selection of the antigen to be loaded and how it will be loaded and the required dose of this vaccine [39]. Many studies are on-going to overcome the limitation of ex vivo DC vaccine. One of these approaches is to develop in vivo DC vaccines which mean direct target of antigen to patient DCs receptors in vivo [95]. The transient expression checkpoint molecules and higher abundance of tumor stroma limit the therapeutic outcome in solid tumors such pancreatic cancer[16]. There are several challenges for CAR-T therapy, such as (i) isolation of T-cells from cancer patients, (ii) controlling the cytokine storm of the CAR-T treated patients that appear due to activation of other immune systems[96]. For the CRISPR-Cas9 gene editing, the investigation are very recent and are in the pre-clinical model. In this realm, one of the major challenges is the (i) use of a viral vector that showed immunogenic responses, and (ii) off-target effect of CRISPR guide RNA[97]. More data is warranted as the technology progressess to more mature stage.
8. Conclusion and prospects
The cancer immune therapy using checkpoint inhibitors, CAR-T and CRISPR-Cas9 gene editing system is very new approach, and few studies have reported their use in nanotechnology-based drug delivery. The future of cancer immunotherapy is predicted to be a game changer for modern cancer treatment. The poor responses of immune therapy to some solid tumor can be improved by using carncer immunotherapy in combination with chemotherapy and radiotherapy regimen. Importantly, nanotechnology provides great prospects for making immune therapy more efficient and a leading anticancer candidate. For example, developing non-viral gold nanoparticle-based CRISPR-Cas 9 gene editing system have shown excellent homology-directed repair to targeted gene in pre-clinical tumor model[98], providing great promise for future clinical applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The listed authors confirm being the sole contributors to this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DM, Simon JK, Baker JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 5.Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of Tumor Growth and Metastasis: The Role of Tumor Microenvironment. Cancer Growth Metastasis. 2014;7:CGM.S11285. doi: 10.4137/CGM.S11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 Tumour-Associated Macrophages (TAMs) and Th1/Th2 Cytokine Profiles in Patients with NSCLC. Cancer Microenviron. 2016;9 doi: 10.1007/s12307-015-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran TH, Rastogi R, Shelke J, Amiji MM. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci Rep. 2015;5 doi: 10.1038/srep16632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizrahy S, Hazan-Halevy I, Landesman-Milo D, Ng BD, Peer D. Advanced strategies in immune modulation of cancer using lipid-based nanoparticles. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:1–15. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura H. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science (80-) 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 19.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–41. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13:883–884. doi: 10.1038/nrd4476. [DOI] [PubMed] [Google Scholar]
- 24.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, Juergens RA, Laurie SA, Nathan FE, Shen Y, Harbison CT, Hellmann MD. Nivolumab Monotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J Clin Oncol. 2016;34:2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi N, Chaft J, Balmanoukian A, Goldberg SB, Sanborn RE, Steele KE, Rebelatto MC, Gu Y, Karakunnel JJ, Antonia S. Tumor response from durvalumab (MEDI4736)+ tremelimumab treatment in patients with advanced non-small cell lung cancer (NSCLC) is observed regardless of PD-L1 status. J Immunother Cancer. 2015;3:P193. [Google Scholar]
- 29.Tatiparti K, Sau S, Kashaw S, Iyer A. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials. 2017;7:77. doi: 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conde J, Arnold C, Tian F, Artzi N. RNAi nanomaterials targeting immune cells as an anti-tumor therapy: the missing link in cancer treatment? Mater Today. 2016 [Google Scholar]
- 31.Navarro G, Pan J, Torchilin VP. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol Pharm. 2015;12:301–313. doi: 10.1021/mp5007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldsmith M, Mizrahy S, Peer D. Grand challenges in modulating the immune response with RNAi nanomedicines. Nanomedicine. 2011 doi: 10.2217/nnm.11.162. [DOI] [PubMed] [Google Scholar]
- 33.Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo M, Lim Y. Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, Sentman CL, Kedl R, Conejo-Garcia JR. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–44. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vu Manh T-P, Dalod M. Characterization of dendritic cell subsets through gene expression analysis. 2016 doi: 10.1007/978-1-4939-3606-9_16. [DOI] [PubMed] [Google Scholar]
- 37.Vanloubbeeck Y, Hostetter J, Jones DE. The biology of dendritic cells and their potential use in veterinary medicine. Anim Health Res Rev. 2003;4:131–142. doi: 10.1079/ahr200354. [DOI] [PubMed] [Google Scholar]
- 38.Sozzani S, Del Prete A, Bosisio D. Dendritic cell recruitment and activation in autoimmunity. J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, Florindo HF, Barata TS. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2 doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klippstein R, Pozo D. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomedicine Nanotechnology, Biol Med. 2010;6:523–529. doi: 10.1016/j.nano.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Strioga M, Schijns V, Powell DJ, Pasukoniene V, Dobrovolskiene N, Michalek J. Dendritic cells and their role in tumor immunosurveillance. Innate Immun. 2013;19:98–111. doi: 10.1177/1753425912449549. [DOI] [PubMed] [Google Scholar]
- 42.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böttcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Höchst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, Flecken T, Giesen D, Engel D, Jung S, Busch DH, Protzer U, Thimme R, Mann M, Kurts C, Schultze JL, Kastenmüller W, Knolle PA. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat Commun. 2015;6:8306. doi: 10.1038/ncomms9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahic M, Yaqub S, Bryn T, Henjum K, Eide DM, Torgersen KM, Aandahl EM, Taskén K. Differentiation of naive CD4+ T cells into CD4+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. J Leukoc Biol. 2008;83:1111–7. doi: 10.1189/jlb.0507329. [DOI] [PubMed] [Google Scholar]
- 45.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27:129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 46.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 47.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol Pharm Bull. 2006;29:1790–1798. doi: 10.1248/bpb.29.1790. [DOI] [PubMed] [Google Scholar]
- 49.Sau S, Alsaab HO, Kashaw SK, Tatiparti K, Iyer AK. Advances in antibody-drug conjugates: A new era of targeted cancer therapy. Drug Discov Today. 2017;0 doi: 10.1016/j.drudis.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luong D, Kesharwani P, Alsaab HO, Sau S, Padhye S, Sarkar FH, Iyer AK. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surfaces B Biointerfaces. 2017 doi: 10.1016/j.colsurfb.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sau S, Alsaab HO, Kashaw SK, Tatiparti K, Iyer AK. Advances in antibody?drug conjugates: a new era of targeted cancer therapy. Drug Discov Today. 2017 doi: 10.1016/j.drudis.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahu P, Kashaw SK, Jain S, Sau S, Iyer AK. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J Control Release. 2017;253:122–136. doi: 10.1016/j.jconrel.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Sau S, Mondal SK, Kashaw SK, Iyer AK, Banerjee R. Combination of cationic dexamethasone derivative and STAT3 inhibitor (WP1066) for aggressive melanoma: a strategy for repurposing a phase I clinical trial drug. Mol Cell Biochem. 2017:1–18. doi: 10.1007/s11010-017-3084-z. [DOI] [PubMed] [Google Scholar]
- 54.Gawde KA, Kesharwani P, Sau S, Sarkar FH, Padhye S, Kashaw SK, Iyer AK. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J Colloid Interface Sci. 2017;496:290–299. doi: 10.1016/j.jcis.2017.01.092. [DOI] [PubMed] [Google Scholar]
- 55.Sau S, Agarwalla P, Mukherjee S, Bag I, Sreedhar B, Pal-Bhadra M, Patra CR, Banerjee R. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale. 2014;6:6745–54. doi: 10.1039/c4nr00974f. [DOI] [PubMed] [Google Scholar]
- 56.Bhargava A, Bunkar N, Khare NK, Mishra D, Mishra PK. Nanoengineered strategies to optimize dendritic cells for gastrointestinal tumor immunotherapy: from biology to translational medicine. Nanomedicine (Lond) 2014;9:2187–202. doi: 10.2217/nnm.14.115. [DOI] [PubMed] [Google Scholar]
- 57.Karkada M, Berinstein NL, Mansour M. Therapeutic vaccines and cancer: focus on DPX-0907. Biol Targets Ther. 2014;8:27. doi: 10.2147/BTT.S55196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsaab H, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer A. No TitlePD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017 doi: 10.3389/fphar.2017.00561. doi: https://doi.org/10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed]
- 59.Garu A, Moku G, Gulla SK, Chaudhuri A. Genetic immunization with in vivo dendritic cell-targeting liposomal DNA vaccine carrier induces long-lasting antitumor immune response. Mol Ther. 2016;24:385–397. doi: 10.1038/mt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 61.Basto AP, Badenes M, Almeida SCP, Martins C, Duarte A, Santos DM, Leitão A. Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein. Mol Immunol. 2015;64:36–45. doi: 10.1016/j.molimm.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J, Feng L, Cheng L, Peng R, Liu Z. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy. ACS Nano. 2015;9:6401–6411. doi: 10.1021/acsnano.5b02014. [DOI] [PubMed] [Google Scholar]
- 63.Smith BR, Ghosn EEB, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat Nanotechnol. 2014;9:481–7. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee S, Sau S, Madhuri D, Bollu VS, Madhusudana K, Sreedhar B, Banerjee R, Patra CR. Green synthesis and characterization of monodispersed gold nanoparticles: Toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J Biomed Nanotechnol. 2016;12:165–181. doi: 10.1166/jbn.2016.2141. [DOI] [PubMed] [Google Scholar]
- 65.Bhise K, Kashaw SK, Sau S, Iyer AK. Nanostructured Lipid Carriers Employing Polyphenols as Promising Anticancer Agents: Quality by Design (QbD) approach. Int J Pharm. 2017 doi: 10.1016/j.ijpharm.2017.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 2014;35:3091–3101. doi: 10.1016/j.biomaterials.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35:8186–8196. doi: 10.1016/j.biomaterials.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 68.Miyabe H, Hyodo M, Nakamura T, Sato Y, Hayakawa Y, Harashima H. A new adjuvant delivery system “cyclic di-GMP/YSK05 liposome” for cancer immunotherapy. J Control Release. 2014;184:20–27. doi: 10.1016/j.jconrel.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32:5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheriyan VT, Alsaab HO, Sekhar S, Stieber C, Iyer AK, Rishi AK. A CARP-1 functional mimetic loaded vitamin E-TPGS micellar nano-formulation for inhibition of renal cell carcinoma. 2017 doi: 10.18632/oncotarget.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeanbart L, Kourtis IC, van der Vlies AJ, Swartz MA, Hubbell JA. 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice. Cancer Immunol Immunother. 2015;64:1033–1046. doi: 10.1007/s00262-015-1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yim H, Park W, Kim D, Fahmy TM, Na K. A self-assembled polymeric micellar immunomodulator for cancer treatment based on cationic amphiphilic polymers. Biomaterials. 2014;35:9912–9919. doi: 10.1016/j.biomaterials.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 73.Garg SM, Vakili MR, Molavi O, Lavasanifar A. Self-Associating Poly(ethylene oxide)-block-poly(α-carboxyl-Îμ -caprolactone) Drug Conjugates for the Delivery of STAT3 Inhibitor JSI-124: Potential Application in Cancer Immunotherapy. Mol Pharm. 2017;14:2570–2584. doi: 10.1021/acs.molpharmaceut.6b01119. [DOI] [PubMed] [Google Scholar]
- 74.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced t cell receptor clustering by nanoparticles enhances t cell activation and stimulates antitumor activity. ACS Nano. 2014;8:2252–2260. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mejías R, Pérez-Yagüe S, Gutiérrez L, Cabrera LI, Spada R, Acedo P, Serna CJ, Lázaro FJ, Villanueva Á, Morales MdelP, Barber DF. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials. 2011;32:2938–2952. doi: 10.1016/j.biomaterials.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Mejías R, Costo R, Roca AG, Arias CF, Veintemillas-Verdaguer S, González-Carreño T, del Puerto Morales M, Serna CJ, Mañes S, Barber DF. Cytokine adsorption/release on uniform magnetic nanoparticles for localized drug delivery. J Control Release. 2008;130:168–174. doi: 10.1016/j.jconrel.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 77.Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, Yan B, Lu W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8:5670–5681. doi: 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S. Imageable Antigen-Presenting Gold Nanoparticle Vaccines for Effective Cancer Immunotherapy In Vivo. Angew Chemie. 2012;124:8930–8935. doi: 10.1002/ange.201203193. [DOI] [PubMed] [Google Scholar]
- 79.Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, Jaffar J, Hellstrom I, Liu J, Hellstrom KE. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J Am Chem Soc. 2010;132:6906–6907. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molino NM, Anderson AKL, Nelson EL, Wang SW. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7:9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, Akiyoshi K, Otsuji E, Mazda O. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367:330–335. doi: 10.1016/j.bbrc.2007.12.112. [DOI] [PubMed] [Google Scholar]
- 82.Fang RH, Hu CMJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 85.Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2016 doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther - Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: Interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, Jiang Y, Xue AX, Elias M, Aycock J, Wiezorek J, Go WY. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts ZJ, Better M, Bot A, Roberts MR, Ribas A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk Lymphoma. 2017:1–12. doi: 10.1080/10428194.2017.1387905. [DOI] [PubMed] [Google Scholar]
- 91.Neelapu SS. An interim analysis of the ZUMA-1 study of KTE-C19 in refractory, aggressive non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2017;15:117–120. [PubMed] [Google Scholar]
- 92.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: Driving T cells to solid Tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moffett HF, Coon ME, Radtke S, Stephan SB, McKnight L, Lambert A, Stoddard BL, Kiem HP, Stephan MT. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22:1875–1884. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 96.DeFrancesco L. CAR-T cell therapy seeks strategies to harness cytokine storm. Nat Biotechnol. 2014;32:604. doi: 10.1038/nbt0714-604. [DOI] [PubMed] [Google Scholar]
- 97.Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther - Nucleic Acids. 2015;4 doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, Shobha T, Mehdipour M, Liu H, Huang WC, Lan F, Bray NL, Li S, Corn JE, Kataoka K, Doudna JA, Conboy I, Murthy N. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 2017;1:889–901. doi: 10.1038/s41551-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


