Abstract
Purkinje neurons, one of the largest neurons in the brain, are critical for controlling body movements, and the dysfunction and degeneration of these cells cause ataxia. Purkinje neurons require a very efficient energy supply from mitochondria because of their large size and extensive dendritic arbors. We have previously shown that mitochondrial division mediated by dynamin-related protein 1 (Drp1) is critical for the development and survival of Purkinje neurons. Drp1 deficiency has been associated with one of the major types of ataxia: autosomal recessive spastic ataxia of Charlevoix Saguenay. Using post-mitotic Purkinje neuron-specific Drp1 knockout (KO) in mice, we investigated the molecular mechanisms that mediate the progressive degeneration of Drp1-KO Purkinje neurons in vivo. In these Purkinje neurons, p62/sequestosome-1, a multi-functional adaptor protein that balances apoptotic cell death and cell survival, was recruited to large mitochondria resulting from unopposed fusion in the absence of mitochondrial division. To test the role of p62 in Drp1-deficient neurodegeneration, we created mice lacking both Drp1 and p62 and found that the additional loss of p62 significantly extended the survival of Purkinje neurons lacking Drp1. These results provide insights into the neurodegenerative mechanisms of mitochondrial ataxia and a critical foundation for therapeutic interventions for this disease.
Keywords: Mitochondria, Organelle division, cell death, Purkinje neurons, Dynamin-related GTPase
1. Introduction
Although it has been over a century since the significance of the cerebellum for motor control was first identified, ataxia caused by cerebellar impairment remains an incurable disease (Bird, 1993; Fine et al., 2002; Schmahmann, 2004). Cerebellar impairment causes ataxia characterized by an impaired gait, difficulty coordinating one's extremities, disordered eye movement, poor articulation and tremor. The basic deficit common to the motor incapacity is the impairment of the rhythm, rate and force of contraction (Diener and Dichgans, 1992; Marsden and Harris, 2011). When the motor syndrome is fully manifest, it is a severely disabling condition. However, the mechanisms of pathogenesis and therapeutic strategies for ataxia remain elusive.
Cerebellar Purkinje neurons are one of the most important neurons for motor control, and their dysfunction leads to impaired motor coordination and locomotor learning (Kapfhammer, 2004; Tyrrell and Willshaw, 1992; Zhang et al., 2010). As the largest neurons in the central nervous system, Purkinje neurons require a very efficient energy supply through mitochondrial oxidative phosphorylation due to their highly arborized dendritic trees and their lengthy projection distances (Herndon, 1963; Phillips et al., 2016). Highly active mitochondrial oxidative phosphorylation in Purkinje neurons generates large amounts of toxic reactive oxygen species (ROS) as byproducts. One victim of ROS is the mitochondrion itself (Bhatti et al., 2016; Tonnies and Trushina, 2017); ROS impair mitochondrial components, such as mitochondrial membranes, proteins and DNA, rendering Purkinje neurons highly vulnerable to mitochondrial damage (Batlevi and La Spada, 2011; Federico et al., 2012; Itoh et al., 2013; Stadtman, 2006). In fact, studies have shown that mitochondrial health is significantly correlated with the pathogenesis of ataxia, which is termed mitochondrial ataxia (Batlevi and La Spada, 2011; Chrysostomou et al., 2016; Di Bella et al., 2010; Itoh et al., 2013; Lax et al., 2012; Mori et al., 2000; Vedanarayanan, 2003; Zeviani et al., 2012). Therefore, elucidating the mechanisms of Purkinje neuron death caused by mitochondrial damage will provide major insights into therapeutic strategies for ataxia.
Post-mitotic Purkinje neurons must maintain mitochondrial homeostasis throughout their lifespan. A key mechanism for the maintenance of mitochondria is the autophagic degradation of damaged mitochondria, which is termed mitophagy (Roy et al., 2015; Shirihai et al., 2015; Youle and van der Bliek, 2012). This degradation process depends on mitochondrial division, which makes mitochondria smaller and facilitates the efficient engulfment of mitochondria by autophagosomes, which eventually fuse with lysosomes for degradation.
Dynamin-related protein 1 (Drp1) mediates mitochondrial division as a critical mechano-chemical enzyme together with another GTPase, dynamin-2 (Bui and Shaw, 2013; Lee et al., 2016; Roy et al., 2015; Tamura et al., 2011). During mitochondrial division, Drp1 assembles as oligomeric complexes on the mitochondrial outer membrane. These complexes wrap around mitochondria and constrict to initiate the division of the mitochondria. Importantly, studies have suggested that Drp1-mediated mitochondrial division is compromised autosomal recessive spastic ataxia of Charlevoix Saguenay, one of the major forms of human mitochondrial ataxia that is associated with increased mitochondrial size, mitochondrial dysfunction and Purkinje neuron degeneration (Bouchard et al., 1978; Bradshaw et al., 2016; Girard et al., 2012).
By generating Drp1-knockout (KO) mice, we demonstrated that cerebellar Purkinje neurons, which strongly express Drp1, are highly sensitive to Drp1 loss (Kageyama et al., 2014; Kageyama et al., 2012; Wakabayashi et al., 2009; Yamada et al., 2016). The conditional KO of Drp1 in post-mitotic Purkinje neurons using the Cre recombinase under the control of the L7 promoter (Barski et al., 2000) caused progressive Purkinje neuron degeneration and ataxia with decreased motor coordination in mice (Kageyama et al., 2014; Kageyama et al., 2012). Mitochondria in Drp1-KO Purkinje neurons dramatically increased their size due to unopposed fusion in the absence of division, showed decreased mitophagy and became defective in oxidative phosphorylation (Kageyama et al., 2014; Kageyama et al., 2012; Yamada et al., 2016). These studies have demonstrated that Purkinje-neuron-specific Drp1-KO mice are an excellent model for studying mitochondrial ataxia.
We have recently shown that post-mitotic Drp1-KO Purkinje neurons degenerate through necroptotic cell death (Yamada et al., 2016). The KO of receptor-interacting protein kinase 3 (Rip3) that is involved in necroptosis significantly delays the death of Drp1-KO Purkinje neurons. However, Rip3 loss did not completely block the neurodegeneration. These data suggest that neurodegeneration caused by Drp1 deficiency is mediated by multiple cell death pathways.
In this study, we investigated the role of p62/sequestosome-1, a scaffold protein that controls cell death and survival (Katsuragi et al., 2015; Manley et al., 2013), in Drp1-deficient neurodegeneration because we observed the recruitment of p62 to mitochondria in cultured Purkinje neurons in the absence of Drp1. We knocked out p62 in Drp1-Purkinje neurons in mice and analyzed the survival of Purkinje neurons and the mitochondrial morphology. Our results revealed that p62 KO significantly slows the degeneration of Purkinje neurons. However, in contrast to Rip3 KO, p62 did not interfere with ROS-induced morphological transformation of mitochondria in response to the loss of Drp1-mediated mitochondrial division. These data suggest that p62 and Rip3 act at different times during the degeneration of Purkinje neurons caused by Drp1 deficiency.
2. Materials and Methods
2.1. Animals
All of the work with animals was conducted according to guidelines established by the Johns Hopkins University Committee on Animal Care and Use. Drp1flox/flox and p62-/- mice have been described previously (Komatsu et al., 2007; Wakabayashi et al., 2009). The L7-Cre mice were obtained from the Jackson Laboratory (Barski et al., 2000). By breeding p62+/-∷L7-Cre+/- ∷Drp1flox/flox mice and p62+/-∷Drp1flox/flox mice, we generated littermate control (Drp1flox/flox), L7-Drp1-KO (L7-Cre+/-∷Drp1flox/flox), p62-KO (p62-/-) and L7-Drp1p62-KO (L7-Cre+/-∷Drp1flox/flox∷ p62-/-) mice. The Drp1flox/flox mice were phenotypically wildtype (Kageyama et al., 2012).
2.2. Antibodies
We used the following primary antibodies: Car8 (Car8-Rb-Af330, Frontier Institute, Hokkaido, Japan), PDH (ab110333, Abcam, MA, USA) and p62 (GP-62C, Progen, Heidelberg, Germany). We purchased the following secondary antibodies from Invitrogen (CA, USA): Alexa 488 anti-Rabbit IgG (A21206), Alexa 568 anti-guinia pig IgG (A11073) and Alexa 647 anti-mouse IgG (A31571).
2.3. Confocal immunofluorescence microscopy
We performed immunofluorescence microscopy of cerebellar Purkinje neurons as previously described (Kageyama et al., 2012) with some modifications (Kageyama et al., 2014; Yamada et al., 2016). The mice were anesthetized by intraperitoneal injection of Avertin and fixed by cardiac perfusion of ice-cold 4% paraformaldehyde in PBS as previously described (Kageyama et al., 2014; Yamada et al., 2016). The brain of each mouse was dissected, fixed in 4% paraformaldehyde in PBS for 2 hours at 4°C, incubated in PBS containing 30% sucrose overnight and frozen in OCT compound (Tissue-Tek, Torrance, CA). The frozen sections were cut, washed in PBS, and blocked in 10% donkey or sheep serum. The sections were then incubated with primary antibodies, followed by fluorescently labeled secondary antibodies. We examined the samples using a Zeiss LSM800 laser scanning confocal microscope equipped with a 10× (0.4 NA) objective and a Zeiss LSM780 FCS laser scanning confocal microscope equipped with a 100× (1.3 NA) objective.
3. Results
3.1. p62 is recruited to mitochondria in Purkinje neurons in L7-Drp1-KO mice
We have previously shown that p62 is recruited to mitochondria in cultured Drp1-KO Purkinje neurons in vitro (Kageyama et al., 2012). To test whether this mitochondrial accumulation of p62 also occurs in vivo, we produced control mice (Drp1flox/flox), L7-Drp1-KO mice (L7-Drp1flox/flox), p62-KO mice (p62-/-) and L7-Drp1p62-KO mice (L7-Cre+/-∷Drp1flox/flox∷ p62-/-) by breeding. We fixed these mice at an age of 3 months by cardiac perfusion of 4% paraformaldehyde, dissected the brain and cut frozen sections around the median line. The brain sections were immunostained using primary antibodies against the Purkinje neuron marker carbonic anhydrase 8 (Car8), p62 and a mitochondrial protein, pyruvate dehydrogenase (PDH), and fluorescently labeled secondary antibodies. Using confocal microscopy, we found that mitochondria become large spheres in Drp1KO Purkinje neurons, consistent with our previous studies (Kageyama et al., 2014). Upon loss of Drp1, mitochondria first elongate due to ongoing fusion in the absence of Drp1-mediated mitochondrial division and subsequently these elongated tubules transform into large spheres due to oxidative damage (Kageyama et al., 2014). As shown in Fig. 1A, we found that p62 was recruited to large spherical mitochondria in L7-Drp1-KO mice but not short tubular mitochondria in control mice. We also found that the p62 signal was not observed in p62-KO and L7-Drp1p62-KO mice, demonstrating the specificity of anti-p62 antibodies we used (Fig. 1A).
Figure 1. p62 KO partially rescues degeneration of Drp1-KO Purkinje neurons.
(A) Control, L7-Drp1-KO, p62-KO and L7-Drp1p62-KO mice were fixed at an age of 3 months. Sagittal sections were cut around the median line of the cerebellum and processed for immunofluorescence microscopy using antibodies against a Purkinje neuron marker, Car8, and p62. The boxed regions are shown enlarged, and the scale bar corresponds to 10 μm. (B) Sagittal sections of the cerebellum from the indicated mice were immunostained using anti-Car8 antibodies. The scale bar corresponds to 1 mm. (C) Quantification of Purkinje neuron density. The number of soma of the Purkinje neurons was measured and normalized relative to the length of the Purkinje cell layer. The values represent the mean ± SEM (n = 6 animals for each genotype). We used the Student's t-test to statistically analyze the difference between the L7-Drp1-KO and L7-Drp1p62-KO mice. *p < 0.05, ***p < 0.001.
3.2. The loss of p62 slows the death of Drp1-KO Purkinje neurons
To determine the impact of the KO of p62, we quantified the number of Purkinje neurons in cerebellar sections stained with anti-Car8 antibodies in control, L7-Drp1KO, p62-KO and L7-Drp1p62-KO mice. Since the cell body of Purkinje neurons form a monolayer in the Purkinje cell layer of the cerebellum, we calculated the density of Purkinje neurons by dividing the total number of Purkinje neurons by the total length of the Purkinje cell layer. We found that approximately 60% of the Purkinje neurons were degenerated in the L7-Drp1-KO mice compared with the control mice (Fig. 1B and C), consistent with previous studies (Kageyama et al., 2014; Yamada et al., 2016). p62-KO mice exhibited a normal number of Purkinje neurons indistinguishable from that of the control mice (Fig. 1B and C). In L7-Drp1p62-KO mice, we observed a significant increase in the number of Purkinje neurons compared with the L7-Drp1-KO mice (Fig. 1B and C). These data suggest that a significant population of Drp1-KO Purkinje neurons die via a mechanism involving p62 by an age of 3 months.
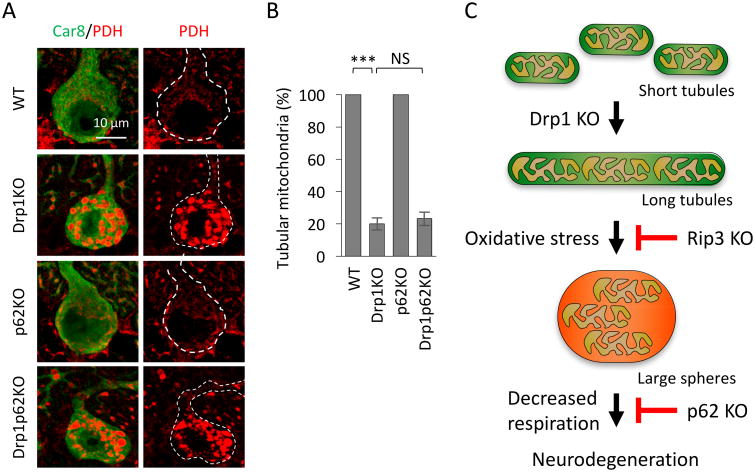
3.3. The loss of p62 does not affect mitochondrial morphology
We have previously shown that the loss of Rip3 that is involved in necroptosis slows the degeneration of Purkinje neurons caused by the loss of Drp1 (Yamada et al., 2016). The extent to which Rip3 loss rescues Drp1-deficient neurodegeneration in L7-Drp1Rip3-KO mice is similar to that observed in L7-Drp1p62-KO mice. In addition to increasing the viability of Purkinje neurons, Rip3 deletion significantly increased the percentage of Purkinje neurons that contained non-spherical mitochondria in L7-Drp1Rip3-KO mice (Yamada et al., 2016). Therefore, Rip3 drives the morphological transformation of mitochondria from elongated tubules into large spheres induced by oxidative stress when mitochondrial division is inhibited. We were accordingly interested in testing whether the loss of p62 also blocks the formation of large spherical mitochondria. We immunostained cerebellar sections with antibodies to the mitochondrial protein PDH and Car8 (Fig. 2A). Distinct from the loss of Rip3, the percentage of Purkinje neurons that contained non-spherical mitochondria did not change in L7-Drp1p62-KO mice compared with L7-Drp1-KO mice (Fig. 2B). Therefore, p62 and Rip3 likely functions at distinct steps and p62 promotes Purkinje neuron death downstream of the morphological transformation of mitochondria from elongated tubules to large spheres (Fig. 2C).
Figure 2. p62 KO does not affect the formation of large spherical mitochondria in Drp1-KO Purkinje neurons.
(A) Mitochondrial morphology. Sagittal sections of the cerebellum in the control, L7-Drp1-KO, p62-KO and L7-Drp1p62-KO 3-month-old mice were stained using antibodies against Car8 and the mitochondrial protein PDH. The scale bar corresponds to 10 μm. (B) The percentage of Purkinje neurons that contained tubular mitochondria was calculated by dividing the number of cells that contained tubular mitochondria by the number of surviving Purkinje neurons. Values represent the mean ± SEM (n = 6 animals for each genotype). We used the Student's t-test to statistically analyze the difference between the L7-Drp1-KO and L7-Drp1p62-KO mice. ***p < 0.001. (C) Model for the pathway that leads to degeneration of Purkinje neurons in the absence of Drp1.
4. Discussion
p62 functions as a critical signaling hub through interactions with components of oxidative stress response, starvation and autophagy, and it regulates cell death and survival (Katsuragi et al., 2015; Manley et al., 2013). Here, we have shown that the loss of p62 partially rescues the degeneration of Purkinje neurons in the absence of Drp1-mediated mitochondrial division. It appears that p62 acts after the accumulation of oxidative damage in mitochondria and that this step is distinct from the step in which Rip3 acts (Fig. 2C), as discussed below in more detail.
The loss of Drp1 leads to elongation of mitochondria that directly results from continuous fusion over decreased mitochondrial division. This elongation of mitochondria results in the accumulation of oxidative damage, as revealed by the accumulation of 4-hydroxynonenal in mitochondria of Drp1-KO Purkinje neurons likely due to decreased turnover of mitochondria, which is mediated by parkin-independent mitophagy (Kageyama et al., 2014). Oxidative damage induces the second morphological transformation into large spheres, although its exact molecular mechanisms currently remain unknown (Kageyama et al., 2012) (Fig. 2C). The formation of large spherical mitochondria is accelerated when the Parkinson-disease-associated ubiquitin E3 ligase parkin is simultaneously knocked out with Drp1, leading to increased neurodegeneration (Kageyama et al., 2014). Large spherical mitochondria exhibited decreased activity in the electron transport chains (Kageyama et al., 2012), and this decreased activity may further produce ROS in mitochondria. This oxidative damage and compromised mitochondrial function accompany the progressive degeneration of Purkinje neurons (Kageyama et al., 2012).
The death of Drp1-deficient Purkinje neurons can be delayed by either antioxidant treatments by feeding L7-Drp1-KO mice the coenzyme Q10 or by genetic removal of the Rip3-mediated necroptosis pathway (Fig. 2C) (Kageyama et al., 2012; Yamada et al., 2016). Both manipulations result in similar neuronal survival in Drp1-KO Purkinje neurons (Kageyama et al., 2012; Yamada et al., 2016). Since Rip3-mediated necroptosis is induced by oxidative stress (Moriwaki and Chan, 2013; Zhou and Yuan, 2014), increases in ROS levels in the absence of Drp1 may cause Rip3-mediated necroptosis (Fig. 2C). Antioxidants and Rip3-KO also inhibit the secondary change in mitochondrial morphology caused by the accumulation of oxidative damage (Kageyama et al., 2012).
We have demonstrated that p62 KO partially improves the survival of Drp1-KO Purkinje neurons, similar to antioxidant treatments and Rip3-KO. However, we found that p62 KO does not suppress secondary morphological changes. This result was surprising because we observed a tight correlation between the formation of large spherical mitochondria—as described above for antioxidant treatments and Rip3-KO. We therefore propose that p62 works in cell death resulting from Drp1 deficiency after the second transformation of mitochondrial shape (Fig. 2C). We speculate that ROS-accumulated spherical mitochondria may lead to apoptotic cell death, which involves p62 but is independent of Rip3.
Since p62 and Rip3 appear to function at distinct steps (Fig. 2C), we are interested in testing the effect of simultaneous depletion of these two proteins on the survival of Drp1-KO Purkinje neurons in future studies. We hope that we can ultimately prevent the degeneration of Purkinje neurons by inhibiting multiple cell death mechanisms in mouse models and provide a critical foundation for therapeutic interventions for human mitochondrial ataxia.
Highlights.
-p62 accumulates on mitochondria in Purkinje neurons lacking Drp1.
-The knockout of p62 slows Purkinje neuron death caused by the lack of Drp1.
-p62 acts downstream of ROS-induced morphological transformation of mitochondria.
References
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- Batlevi Y, La Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis. 2011;43:46–51. doi: 10.1016/j.nbd.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TD. Hereditary Ataxia Overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci. 1978;5:61–69. [PubMed] [Google Scholar]
- Bradshaw TY, Romano LE, Duncan EJ, Nethisinghe S, Abeti R, Michael GJ, Giunti P, Vermeer S, Chapple JP. A reduction in Drp1-mediated fission compromises mitochondrial health in autosomal recessive spastic ataxia of Charlevoix Saguenay. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui HT, Shaw JM. Dynamin assembly strategies and adaptor proteins in mitochondrial fission. Current biology: CB. 2013;23:R891–899. doi: 10.1016/j.cub.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou A, Grady JP, Laude A, Taylor RW, Turnbull DM, Lax NZ. Investigating complex I deficiency in Purkinje cells and synapses in patients with mitochondrial disease. Neuropathol Appl Neurobiol. 2016;42:477–492. doi: 10.1111/nan.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali M, Veneziano L, Sacco T, Boda E, Brussino A, Bonn F, Castellotti B, Baratta S, Mariotti C, Gellera C, Fracasso V, Magri S, Langer T, Plevani P, Di Donato S, Muzi-Falconi M, Taroni F. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42:313–321. doi: 10.1038/ng.544. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J. Pathophysiology of cerebellar ataxia. Movement disorders : official journal of the Movement Disorder Society. 1992;7:95–109. doi: 10.1002/mds.870070202. [DOI] [PubMed] [Google Scholar]
- Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322:254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Fine EJ, Ionita CC, Lohr L. The history of the development of the cerebellar examination. Semin Neurol. 2002;22:375–384. doi: 10.1055/s-2002-36759. [DOI] [PubMed] [Google Scholar]
- Girard M, Lariviere R, Parfitt DA, Deane EC, Gaudet R, Nossova N, Blondeau F, Prenosil G, Vermeulen EG, Duchen MR, Richter A, Shoubridge EA, Gehring K, McKinney RA, Brais B, Chapple JP, McPherson PS. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) Proc Natl Acad Sci U S A. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon RM. The fine structure of the Purkinje cell. J Cell Biol. 1963;18:167–180. doi: 10.1083/jcb.18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends in Cell Biology. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. The EMBO journal. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH, Iijima M, Sesaki H. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. The Journal of Cell Biology. 2012;197:535–551. doi: 10.1083/jcb.201110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer JP. Cellular and molecular control of dendritic growth and development of cerebellar Purkinje cells. Prog Histochem Cytochem. 2004;39:131–182. doi: 10.1016/j.proghi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. Febs J. 2015;282:4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Lax NZ, Hepplewhite PD, Reeve AK, Nesbitt V, McFarland R, Jaros E, Taylor RW, Turnbull DM. Cerebellar ataxia in patients with mitochondrial DNA disease: a molecular clinicopathological study. Journal of neuropathology and experimental neurology. 2012;71:148–161. doi: 10.1097/NEN.0b013e318244477d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016 doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Williams JA, Ding WX. Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med (Maywood) 2013;238:525–538. doi: 10.1177/1535370213489446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195–216. doi: 10.1177/0269215510382495. [DOI] [PubMed] [Google Scholar]
- Mori O, Yamazaki M, Ohaki Y, Arai Y, Oguro T, Shimizu H, Asano G. Mitochondrial encephalomyopathy with lactic acidosis and stroke like episodes (MELAS) with prominent degeneration of the intestinal wall and cactus-like cerebellar pathology. Acta Neuropathol. 2000;100:712–717. doi: 10.1007/s004010000209. [DOI] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes & development. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Laude A, Lightowlers R, Morris CM, Turnbull DM, Lax NZ. Development of passive CLARITY and immunofluorescent labelling of multiple proteins in human cerebellum: understanding mechanisms of neurodegeneration in mitochondrial disease. Scientific reports. 2016;6:26013. doi: 10.1038/srep26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Reddy PH, Iijima M, Sesaki H. Mitochondrial division and fusion in metabolism. Current opinion in cell biology. 2015;33C:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Shirihai OS, Song M, Dorn GW., 2nd How mitochondrial dynamism orchestrates mitophagy. Circulation research. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free radical research. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Itoh K, Sesaki H. SnapShot: Mitochondrial dynamics. Cell. 2011;145:1158, 1158 e1151. doi: 10.1016/j.cell.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer's Disease. J Alzheimers Dis. 2017 doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell T, Willshaw D. Cerebellar cortex: its simulation and the relevance of Marr's theory. Philos Trans R Soc Lond B Biol Sci. 1992;336:239–257. doi: 10.1098/rstb.1992.0059. [DOI] [PubMed] [Google Scholar]
- Vedanarayanan VV. Mitochondrial disorders and ataxia. Semin Pediatr Neurol. 2003;10:200–209. doi: 10.1016/s1071-9091(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Adachi Y, Fukaya M, Iijima M, Sesaki H. Dynamin-Related Protein 1 Deficiency Leads to Receptor-Interacting Protein Kinase 3-Mediated Necroptotic Neurodegeneration. Am J Pathol. 2016;186:2798–2802. doi: 10.1016/j.ajpath.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M, Simonati A, Bindoff LA. Ataxia in mitochondrial disorders. Handb Clin Neurol. 2012;103:359–372. doi: 10.1016/B978-0-444-51892-7.00022-X. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhu Q, Hua T. Aging of cerebellar Purkinje cells. Cell Tissue Res. 2010;341:341–347. doi: 10.1007/s00441-010-1016-2. [DOI] [PubMed] [Google Scholar]
- Zhou W, Yuan J. Necroptosis in health and diseases. Seminars in cell & developmental biology. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]