Abstract
BACKGROUND:
In the setting of severe sepsis and septic shock, mortality increases when lactate levels are ≥ 4 mmol/L. However, the consequences of lower lactate levels in this population are not well understood. The study aimed to determine the in-hospital mortality associated with severe sepsis and septic shock when initial lactate levels are < 4 mmol/L.
METHODS:
This is a retrospective cohort study of septic patients admitted over a 40-month period. Totally 338 patients were divided into three groups based on initial lactate values. Group 1 had lactate levels < 2 mmol/L; group 2: 2–4 mmol/L; and group 3: ≥ 4 mmol/L. The primary outcome was in-hospital mortality.
RESULTS:
There were 111 patients in group 1, 96 patients in group 2, and 131 in group 3. The mortality rates were 21.6%, 35.4%, and 51.9% respectively. Univariate analysis revealed the mortality differences to be statistically significant. Multivariate logistic regression demonstrated higher odds of death with higher lactate tier group, however the findings did not reach statistical significance.
CONCLUSION:
This study found that only assignment to group 3, initial lactic acid level of ≥ 4 mmol/L, was independently associated with increased mortality after correcting for underlying severity of illness and organ dysfunction. However, rising lactate levels in the other two groups were associated with increased severity of illness and were inversely proportional to prognosis.
Keywords: Sepsis, Lactic acid, Emergency medicine
INTRODUCTION
Sepsis remains a substantial source of mortality and health care costs worldwide. In the US, the incidence of sepsis has steadily risen at a rate of 13% annually over the past decade and is estimated to carry a significant burden of healthcare in the future.[1–4] Sepsis-related mortality is reported to be as high as 29.9%.[3,5] From a cost perspective, sepsis is associated with over $24 billion annually in the United States alone.[1–3] Worldwide, the incidence and mortality associated with sepsis continues to climb.[2,6]
The focus of sepsis management has been on early recognition and timely initiation of therapies. Historically, severity of illness was evaluated as a continuum composed of systemic inflammatory response syndrome (SIRS), severe sepsis, and septic shock. Beginning with SIRS, sepsis was defined based on meeting SIRS criteria and having a suspected source of infection. This stage of sepsis carried a 28-day mortality rate of 10%. Severe sepsis suggested a more grave illness and was characterized by organ dysfunction. Septic shock identified the highest risk patients who experience hypotension after adequate volume resuscitation. Mortality associated with severe sepsis and septic shock was 35% and 50%, respectively.[5,6] In 2016, the third international consensus definitions for sepsis and septic shock redefined these terms.[4] Our study was conducted prior to the release and adoption of the new definitions of sepsis. Therefore, all analysis was performed using the previously accepted definitions of sepsis, severe sepsis, and septic shock.
Early investigations into sepsis revealed that a serum lactate level ≥ 4 mmol/L correlated with poor prognosis in critically ill patients.[5–7] This eventually led to a 4 mmol/L lactate threshold as inclusion criteria for patients in many randomized controlled trials evaluating sepsis.[7–10] However, some studies have revealed that lactate values of ≥ 2 mmol/L are associated with increased mortality.[11–14] At present, the risk of mortality associated with lactate values between 2 and 4 mmol/L is not well understood. Further investigation into this group of patients may improve our ability to stratify risk in the setting of sepsis. This study’s aim was to evaluate outcomes in patients with severe sepsis and septic shock categorized into three cohorts based on presenting serum lactate levels of < 2 mmol/L, 2–4 mmol/L, and ≥ 4 mmol/L.
METHODS
This retrospective cohort study was approved for waiver of consent by the McGovern Medical School, UTHealth institutional review board. The study was conducted in an urban, academic tertiary care center with 60,000 annual ED visits.
Severe sepsis was defined as acute organ dysfunction secondary to infection. Septic shock was defined as severe sepsis plus hypotension (systolic blood pressure < 90) that was not reversed by adequate fluid resuscitation.[9,15,16] The criteria for organ dysfunction were as follows: (1) acute respiratory failure: (PaO2/FiO2) < 300; (2) acute renal failure: urine output less than 0.5 mL/(kg·hour) or a creatinine increase > 0.5 mg/dL; (3) acute liver injury: INR > 1.5; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than three times the normal value (120 IU/L); or (4) thrombocytopenia with platelets < 100,000/microL.
The study was limited to patients with severe sepsis or septic shock so that the study population would match the populations found in many large sepsis trials. In addition, restricting the focus to the infected patients with the highest expected mortality rates was an advantage during data analysis when comparing mortality as an outcome of interest.
Once identified, septic patients were divided into three tiers based on initial lactate level. Group 1 presented with serum lactate levels < 2 mmol/L; group 2 had initial levels 2–4 mmol/L; and group 3 had levels ≥ 4 mmol/L. Data were collected on age, gender, race, comorbidities, infection source, SIRS criteria, organ dysfunction, Acute Physiology and Chronic Health Evaluation (APACHE) II score, serum lactate levels, ICU length of stay (ICU LOS), hospital length of stay (HLOS), and in-hospital mortality.
An electronic query of the medical records of all patients hospitalized between October 1, 2010 and December 31, 2013 was performed to identify patients with a discharge diagnosis consistent with an infection- or sepsis-related ICD-9 billing code. The charts of the 1,004 patients identified by the query were reviewed for study eligibility. Patients ≥ 18 years old with an infection, two SIRS criteria and evidence of organ dysfunction were included if they had a serum lactate level obtained within 24 hours of admission. Pediatric patients and those admitted for trauma, out-of-hospital cardiac arrest, or cardiogenic shock were excluded from the study. The primary outcome of interest was in-hospital mortality. Length of stay in the ICU and hospital were evaluated as secondary outcomes.
Demographic continuous variables were summarized by their median and interquartile range (IQR). Categorical variables were described as counts and percentages. Differences in continuous variables, stratified by lactate group, were assessed using the Wilcoxon Rank Sum test, while Chi-square tests were used to analyze the categorical variables. The association between in-hospital mortality and lactate group was assessed using a multivariable logistic regression adjusting for gender, age, white race, pneumonia infection, comorbid End-Stage-Renal-Disease (ESRD), and Apache II score. Similarly for log-transformed hospital LOS and ICU LOS, multivariable regression models were generated and included the same covariate adjustments used for in-hospital mortality. A False Discovery Rate (FDR) multiple testing correction was applied using a 5% significance level. Statistical analyses were carried out in SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Of the 1,004 patients screened for this study, 360 adult patients met criteria for severe sepsis or septic shock that was not caused by another primary process. Of these, 22 patients did not have an initial lactate measured and were excluded from the study. As a result, a total of 338 patients were included in the analysis. Figure 1 shows the enrollment distribution and stratification of all patients evaluated in this study.
Figure 1.
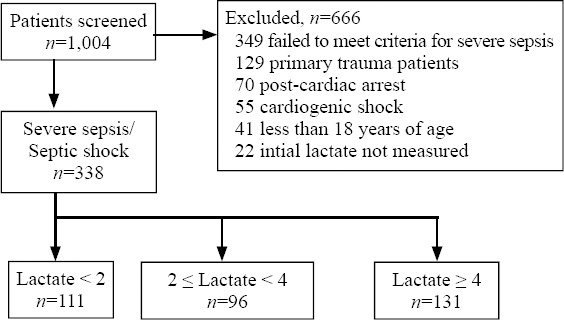
Enrollment and stratification of all patients.
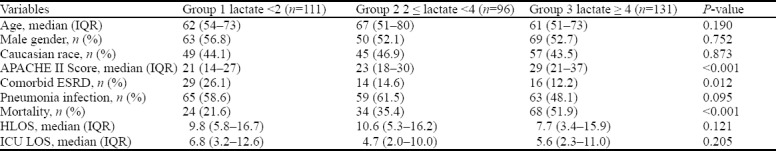
Patients were divided into groups based on lactate level. Group 1 was composed of 111 (32.8%) patients with a serum lactate concentration of < 2 mmol/L. Group 2 had 96 (28.4%) patients with serum lactate values 2–4 mmol/L. Group 3 consisted of 131 (38.8%) patients with an initial lactate ≥ 4 mmol/L. Baseline characteristics, APACHE II Score, pneumonia infection, ESRD, and outcomes for the groups are shown in Table 1. Statistically significant differences between the cohorts were noted in relation to APACHE II score, ESRD, and mortality. The APACHE II score was highest in the third group. ESRD prevalence was highest in the first tier group. The in-hospital mortality rose from group 1 to group 3, with rates of 21.6% for the first group, 35.4% for the second, and 51.9% for the third. These differences were statistically significant on univariate analysis.
Table 1.
Summary statistics of demographic and outcome variables by lactate tier groups
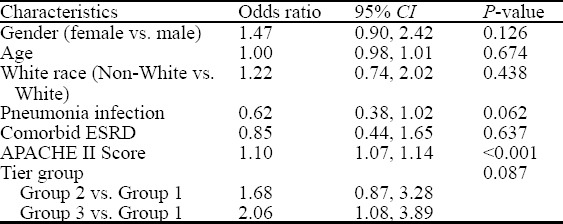
Since there were differences in baseline characteristics between the groups, multivariable logistic regression was performed to adjust for these variations. No statistically significant association between mortality and tiered lactate group was identified after the false discovery rate (FDR) correction, P=0.087. However, there was a statistically significant increased odds of death in group 3 when compared to group 1 (OR=2.05, 95% CI=1.08 to 3.89). Similarly, group 2 demonstrated a trend toward significance for increased mortality compared to group 1 (OR=1.68, 95% CI=0.87 to 3.28, P=0.087). The APACHE II score remained independently associated with mortality (OR=1.10, 95% CI=1.16 to 1.13, P< 0.001) as shown in Table 2.
Table 2.
Mortality by gender, age, white race, pneumonia infection, comorbid ESRD, APACHE II score, and tier groups
Additionally, we generated other multivariable regression models of log-transformed hospital LOS and ICU LOS. Lactate tier group was not found to have a statistically significant correlation with hospital or ICU LOS. The APACHE II score, however, remained statistically significant in the model for HLOS, but not ICU LOS, after the FDR correction (P-value=0.001 and P-value=0.762 respectively).
DISCUSSION
In this study, an intermediate elevation in lactate level, 2–4 mmol/L, was not independently associated with increased mortality as compared to those with lactate < 2 mmol/L. Group 2 did have increased severity of illness and increased mortality as compared to septic patients in group 1. This adds further to the conflicting findings previously reported in septic patients with lactate levels < 4 mmol/L.[11–14]
An important finding of our study is that the mortality rates within each of these groups of patients with severe sepsis and septic shock was high. The overall mortality for this cohort of patients with severe sepsis and septic shock was 37%, with mortality increasing from 21.6% in group 1 to 51.9% in group 3. This represents the mortality of sepsis in the real world setting of a high acuity center, which is different than what has been reported in recent clinical trials where the mortality for the entire group was approximately 20% in the ProCESS trial and 15% in the ARISE trial.[17,18] This can partially be explained by the differences in the severity of illness between the groups in our study versus the severity of illness scores in the aforementioned clinical trials. In addition, as a tertiary care center, we have high risk patient populations, including transplant patients, patients with end-stage liver and renal disease, advanced heart failure, and malignancies. Many of them have relatively or iatrogenically suppressed immune systems.
The fact that patients who meet the criteria for severe sepsis or septic shock have a substantial mortality risk despite normal or intermediate range lactate values is of consequence. This finding draws focus to the need for early identification and aggressive therapy in patients with severe sepsis or septic shock who have lactate values of < 4 mmol/L. This study is not alone in this finding. Emerging evidence suggests that patients who meet criteria for severe sepsis and have lactate values < 4 mmol/L still carry a significant mortality burden and may benefit from vasopressors and aggressive support.[19,20]
Risk-stratifying patients in regard to sepsis is an evolving process. Traditionally when lactate is used as a surrogate marker for end-organ perfusion, it is a binary stratification in which values ≥ 4 mmol/L are considered to require early and aggressive resuscitation whereas values <4 mmol/L are associated with patients who do not require the typical interventions in the early management of sepsis. Our study suggests this is not the case. It is more apparent that just as the systemic response to infection varies by individual, so does lactate expression in sepsis. Rising lactate levels correlate with increasing severity of illness and the associated mortality. Ultimately, patients with normal to mildly elevated lactate in the setting of severe sepsis are still at substantial risk. Lactate values are useful tools in sepsis but should not be used in isolation for risk stratification. As described in our study and previous trials, it is possible to have a grave response to infection in the absence of lactate elevation.[19,20]
Limitations
This study has several limitations. First and foremost, it was a retrospective review of medical records. As such, septic patients who did not have an infection-related discharge diagnosis might not have been included. Also, patient management was not standardized. Therefore, practice variation among providers may have contributed to mortality differences. Information regarding the timing of antibiotic administration, volume of fluid resuscitation, and other interventions were not easily extractable from the charts. As a result, variability in care and possible delays in therapy may have contributed to morbidity and mortality. To minimize mistakes in assessing eligibility and data collection, two study investigators independently reviewed each medical record.
In addition, patients were not followed after discharge. Hence, the study may have underestimated the overall sepsis-related mortality. A recent review article reported that severe sepsis patients who survived to hospital discharge had a 51.4% mortality rate at 1 year and 74.2% at 5 years.[3] Our study was not designed to follow mortality trends after discharge. Lastly, this study was conducted at an academic tertiary care center, and the results may not be widely generalizable to patients presenting to other medical facilities.
CONCLUSIONS
This study demonstrated that patients with severe sepsis and septic shock who had higher initial lactate levels were likely to have a worse prognosis. Although, an intermediate elevation in lactate was not independently associated with mortality, this study found that normal and intermediate lactate tier groups had a high mortality burden. Further study is needed to determine optimal therapeutic strategies to improve the outcomes of these groups of patients.
Footnotes
Funding: The study was funded by Dean’s Summer Research Program Grant and Department of Emergency Medicine at McGovern Medical School.
Ethical approval: This retrospective cohort study was approved for waiver of consent by the institutional review board.
Conflicts of interest: None of the authors have any conflicts of interest as it pertains to this work. PD receives grant funding, which goes to his institution, from Vapotherm Inc.
Contributors: AP and PD conceived the project and supervised the data collection and analysis. AP, KC, BA, BD, AM, MT, and PD participated in the acquisition and interpretation of the data. RB performed the statistical analysis. AP, RB, KC, BA, BD, AM, MT and PD were all involved substantially in the manuscript preparation and revision. All authors approve the submitted version of the manuscript. KC takes responsibility for the paper as a whole.
REFERENCES
- 1.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide Trends of Severe Sepsis in the 21st Century (2000-2007) Chest. 2011;140(5):1223–31. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit Care Med. 2013;41(5):1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 3.Tiru B, DiNino EK, Orenstein A, Mailloux PT, Pesaturo A, Gupta A, et al. The economic and humanistic burden of severe sepsis. Pharmacoeconomics. 2015;33(9):925–37. doi: 10.1007/s40273-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (SEPSIS-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C. The Epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(Suppl 1):S64–74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangel-Fausto MS, Pittet D, Costigan M. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–23. [PubMed] [Google Scholar]
- 8.Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143(3613):1457–9. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21(2):128–40. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 12.Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33(6):970–7. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Kjelland CB, Djogovi D. The role of serum lacate in the acute care setting. J Intensive Care Med. 2010;25(5):286–300. doi: 10.1177/0885066610371191. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 17.ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 18.Pro CESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38(1):4–10. doi: 10.1097/SHK.0b013e318254d41a. [DOI] [PubMed] [Google Scholar]
- 20.Dugas AF, Mackenhauer J, Salciccioli J, Cocchi MN, Gautam S, Donnino MW. Prevalence and characteristics of nonlactate and lactate expressors in septic shock. J Crit Care. 2012;27(4):244–350. doi: 10.1016/j.jcrc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


