Abstract
Sesame (Sesamum indicum L.) is an important oilseed crop. However, multiple abiotic stresses severely affect sesame growth and production. Raffinose family oligosaccharides (RFOs), such as raffinose and stachyose, play an important role in desiccation tolerance of plants and developing seeds. In the present study, three types of key enzymes, galactinol synthase (GolS), raffinose synthase (RafS) and stachyose synthase (StaS), responsible for the biosynthesis of RFOs were identified at the genome-wide scale in sesame. A total of 7 SiGolS and 15 SiRS genes were identified in the sesame genome. Transcriptome analyses showed that SiGolS and SiRS genes exhibited distinct expression profiles in different tissues and seed developmental stages. Comparative expression analyses under various abiotic stresses indicated that most of SiGolS and SiRS genes were significantly regulated by drought, osmotic, salt, and waterlogging stresses, but slightly affected by cold stress. The up-regulation of several SiGolS and SiRS genes by multiple abiotic stresses suggested their active implication in sesame abiotic stress responses. Taken together, these results shed light on the RFOs-mediated abiotic stress resistance in sesame and provide a useful framework for improving abiotic stress resistance of sesame through genetic engineering.
Introduction
Plant growth and productivity are greatly challenged by diverse environmental stresses, such as drought, waterlogging, or high-salinity, for their sessile nature. To cope with these unfavorable conditions, plants have evolved a range of physiological and biochemical responses by activating a large number of stress-responsive genes and synthesizing various functional proteins through an intricate network of signaling cascades1,2. At the cellular level, compatible solutes including sugars (such as oligosaccharides, trehalose, and sorbitol), amines (such as glycine betaine and polyamines), and amino acids (such as proline) accumulate under stress conditions and function as osmolytes or antioxidants that help plants to overcome harmful environmental conditions3.
Raffinose family oligosaccharides (RFOs), such as raffinose (Raf) and stachyose (Sta), are α-1, 6-galactosyl extensions of sucrose (Suc) and ubiquitous in plants4. RFOs (both Raf and Sta) predominantly accumulate in seeds, protecting the embryo against desiccation in seed maturation and thus play a critical role in prolonging seed longevity5,6. Accumulation of Raf is also found in vegetative tissues (leaves and roots) under abiotic stresses and function as an osmolyte, and/or an antioxidant to promote cell survival under adverse growing conditions7–9. Some evidence showed that RFOs may function as signals that mediate stress responses by activating the expression of downstream stress-responsive genes10. In addition, RFOs also participate in several important cellular biological processes, including transport and storage of carbon, and membrane trafficking8,11,12.
The first step in the RFOs biosynthesis starts with the formation of galactinol (Gol; 1-O-α-D-galactopyranosyl-myo-inositol), which is catalyzed by galactinol synthase (GolS, EC 2.4.1.123), using UDP-galactose and myo-inositol (Ino) as substrates13. Then, Raf and Sta are synthesized by stepwise addition of galactosyl units that are catalyzed by raffinose synthase (RafS, EC 2.4.1.82) and stachyose synthase (StaS, EC 2.4.1.67), respectively. RafS transfers a galactosyl unit from Gol to Suc and produces Raf14. Then, StaS further uses Gol as a galactosyl donor to Raf and yields Sta15,16. Both these two reactions are reversible4.
RFOs biosynthesis genes, especially GolS genes, have been well characterized in many plant species, including Arabidopsis7,17, tomato18, rice19, maize20 and poplar21. Most of the GolS genes were reported to be induced by stress stimuli and positively associated with abiotic stress resistance in plants. Among seven GolS genes (AtGolS1-7) identified in Arabidopsis, AtGolS1 and AtGolS2 were up-regulated by salt and drought stresses, while AtGolS3 was up-regulated by cold stress17. AtGolS1- or AtGolS2-overexpressing transgenic Arabidopsis plants with increased endogenous Gol and Raf showed enhanced tolerance to drought, salt, chilling and oxidative stresses7,17. Recently, Selvaraj et al. reported that AtGolS2 was able to confer drought resistance and increase grain yield in two different rice genotypes under dry field conditions22. Two cDNAs encoding GolS from wheat (TaGolS1 and TaGolS2) were transformed into rice. The transgenic plants constitutively overexpressing TaGolS1 or TaGolS2 accumulated significantly higher levels of Gol and Raf, and exhibited enhanced cold stress tolerance compared with the untransformed control plants23. Above results suggest that GolS genes are good targets to improve the abiotic stress tolerance of crop plants through molecular breeding and/or genetic engineering. Compared to GolSs, RafSs and StaSs are poorly characterized in plants. Six putative RafS genes (AtRS1-6) were identified in Arabidopsis. Biochemical and genetic analyses indicated that AtRS5 was the only RafS gene responsible for Raf accumulation in leaves under abiotic stresses14. Another RafS gene, AtRS4, encodes a seed specific multifunctional enzyme with RafS and high affinity StaS activity15.
Sesame (Sesamum indicum L.), an ancient oil crop, is widely grown in tropical and subtropical areas. Sesame is known as an important source of oil with an excellent nutritional quality due to its high content of oil and protein in the seeds, and the balance in composition of oleic and linoleic acids24,25. As an important species from the order Lamiales, the release of the full genome sequence of sesame has provided the useful genomic platform for the genetic improvement of sesame26. Although sesame is considered to be a relatively drought-resistant crop, it is highly sensitive to drought stress during its vegetative stage and its yield potential is often limited by water shortage27,28. RFOs are widely known for their important roles in various abiotic stresses resistance and seed development. However, the accumulation of these oligosaccharides in sesame is largely unknown. In the present study, genes involved in RFOs accumulation, including genes encoding GolS, RafS and StaS, were identified at the genome-wide scale in sesame. Then, a detailed gene structure, phylogenetic analyses were performed. Furthermore, the expression profiles of these genes in different organs and abiotic stresses were assessed, which provided useful information for identification of several SiGolSs and SiRSs as candidate genes for further functional analysis and genetic improvement of sesame.
Results
Identification of raffinose biosynthesis pathway genes in sesame
Seven GolS genes were identified from the sesame genome database (Sinbase, http://ocri-genomics.org/Sinbase/index.html) by a BLAST search using the protein sequence of GolSs from Arabidopsis. As shown in Table 1, we named the obtained GolS sequences SiGolS1 to SiGolS7 according to their positions from the top to the bottom on the sesame linkage groups (LGs). Fifteen putative raffinose synthase genes were also identified in the sesame genome, and designated as SiRS1 to SiRS15. All the identified SiGolSs and SiRSs were checked manually for the presence of Glyco_trans_8 Pfam (PF01501) and Raffinose synthase Pfam (PF05691), respectively. The detailed information of SiGolS and SiRS genes, including locus ID, linkage group distribution, the length of coding sequences, molecular weight (MW), and theoretical isoelectric point (pI) is listed in Table 1.
Table 1.
Raffinose biosynthesis related genes in Sesame.
| Gene name | Locus IDa) | Accession Numberb) | Linkage Group | Start (bp) | End (bp) | Exon number | Protein length (aa) | MW* (kDa) | pI* | Duplications |
|---|---|---|---|---|---|---|---|---|---|---|
| GolS | ||||||||||
| SiGolS1 | SIN_1018104 | XM_011072789 | LG02 | 17749734 | 17751585 | 3 | 339 | 38.41 | 4.82 | Segmental |
| SiGolS2 | SIN_1016153 | XM_011073345 | LG03 | 204802 | 205977 | 4 | 290 | 33.96 | 4.98 | Dispersed |
| SiGolS3 | SIN_1012641 | XM_011081210 | LG06 | 237773 | 239341 | 4 | 340 | 38.71 | 5.79 | Segmental |
| SiGolS4 | SIN_1026653 | XM_011088281 | LG08 | 11164540 | 11166854 | 4 | 333 | 37.97 | 4.94 | Segmental |
| SiGolS5 | SIN_1022773 | XM_011089209 | LG08 | 16345511 | 16347799 | 3 | 336 | 37.70 | 4.96 | Segmental |
| SiGolS6 | SIN_1022774 | XM_011089210 | LG08 | 16353437 | 16354675 | 3 | 306 | 35.07 | 4.75 | Tandem |
| SiGolS7 | SIN_1025925 | XM_011093877 | LG10 | 13337551 | 13339272 | 3 | 322 | 36.92 | 5.44 | Segmental |
| RafS | ||||||||||
| SiRS1 | SIN_1020329 | XM_011073865 | LG03 | 4924917 | 4928632 | 4 | 779 | 86.75 | 6.60 | Dispersed |
| SiRS2 | SIN_1020252 | XM_011073696 | LG03 | 5530701 | 5534982 | 13 | 884 | 97.39 | 6.15 | Segmental |
| SiRS3 | SIN_1020251 | XM_011073698 | LG03 | 5545742 | 5549293 | 14 | 761 | 83.93 | 5.27 | Tandem |
| SiRS4 | SIN_1006202 | XM_011076928 | LG04 | 940755 | 945069 | 12 | 768 | 84.26 | 5.61 | Segmental |
| SiRS5 | SIN_1013540 | XM_011080080 | LG05 | 5970786 | 5974158 | 5 | 865 | 94.85 | 6.07 | Segmental |
| SiRS6 | SIN_1023532 | XM_020693952 | LG05 | 13038353 | 13041972 | 12 | 768 | 84.35 | 5.79 | Segmental |
| SiRS8 | SIN_1007330 | XM_011085808 | LG07 | 6751484 | 6754372 | 4 | 765 | 84.68 | 5.79 | Segmental |
| SiRS9 | SIN_1007333 | XM_011085817 | LG07 | 6786084 | 6789045 | 4 | 755 | 83.69 | 5.97 | Proximal |
| SiRS10 | SIN_1026883 | XM_011087990 | LG08 | 9189292 | 9193596 | 14 | 792 | 87.42 | 5.50 | Dispersed |
| SiRS11 | SIN_1026329 | NA | LG08 | 14107419 | 14108200 | 3 | 198 | 21.36 | 5.12 | Segmental |
| SiRS12 | SIN_1026328 | XM_011088697 | LG08 | 14110902 | 14114536 | 14 | 750 | 82.35 | 5.21 | Tandem |
| SiRS13 | SIN_1014290 | XM_011097309 | LG12 | 1710058 | 1713804 | 12 | 789 | 87.31 | 5.64 | Dispersed |
| SiRS14 | SIN_1007882 | XM_011100134 | LG15 | 130561 | 133205 | 4 | 784 | 86.59 | 5.50 | Dispersed |
| StaS | ||||||||||
| SiRS7 | SIN_1012629 | XM_011081231 | LG06 | 315049 | 317941 | 4 | 862 | 95.64 | 6.07 | Dispersed |
| SiRS15 | SIN_1003607 | XM_011102449 | scaffold00109 | 425302 | 429067 | 4 | 847 | 94.96 | 5.83 | NA |
a)Locus ID was adopted from Sinbase (Sesamum indicum genome database, http://ocri-genomics.org/Sinbase/index.html).
b)Accession Number was adopted from NCBI (The National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/).
*MW: Molecular Weight; pI: Isoelectric point; NA: not available.
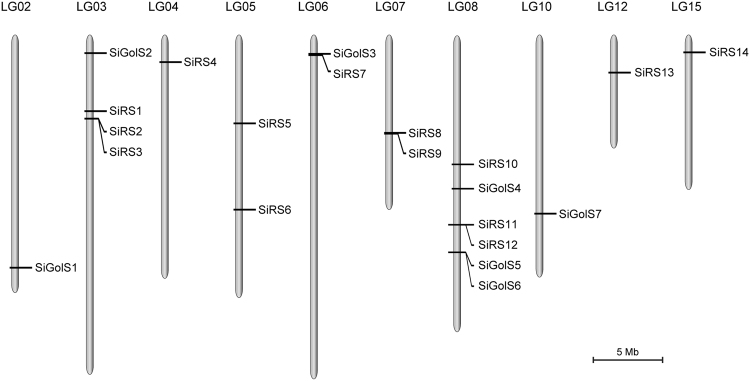
SiGolS and SiRS genes were mapped to the 16 sesame linkage groups based on the coordinates of Sinbase loci. As shown in Fig. 1, all the SiGolS and SiRS genes were unevenly distributed among 10 LGs out of the 16 LGs of the sesame genome, except for SiRS15, which was located on the unanchored scaffold. Sequencing analysis of the sesame genome revealed that the recent sesame whole genome duplication genomic regions covered approximately 50% of the current sesame genome assembly26. We further analyzed the segmental duplication events of SiGolS and SiRS genes. A total of 5 SiGolSs (SiGolS1, 3, 4, 5, and 7) and 6 SiRSs (SiRS2, 4, 5, 6, 8, and 11) were detected as segmentally duplicated genes. As shown in Supplementary Fig. S1, these segmentally duplicated SiGolS and SiRS genes were located on duplicated segments on 8 LGs.
Figure 1.
Linkage group distribution of SiGolS and SiRS genes in sesame. Totally 7 SiGolS and 15 SiRS genes were mapped to the 10 linkage groups (LGs) according to their positions in the sesame genome. The LG number was shown on the top of each LG. The scale bar indicated a LG distance of 5 Mb.
Structural and phylogenetic analyses of SiGolSs
The predicted SiGolS proteins varied from 290 (SiGolS2) to 340 (SiGolS3) amino acids in length. Multiple sequence alignment of SiGolSs along with other reference GolS proteins from representative plant species, such as Arabidopsis, rice, poplar and soybean showed that all the GolS proteins have the glycosyl transferase 8 family domain (PF01501) (Supplementary Fig. S2). Except for SiGolS2, all of the reported GolS and SiGolS proteins contain a putative serine phosphorylation site at position 270 of the SiGolS1 protein. Except for SiGolS2 and SiGolS6, all of the SiGolSs contain the C-terminal hydrophobic pentapeptide (APSAA), a common feature of GolSs. Moreover, SiGolS5 shows a single substitution of proline to serine in APSAA pentapeptide (Supplementary Fig. S2).
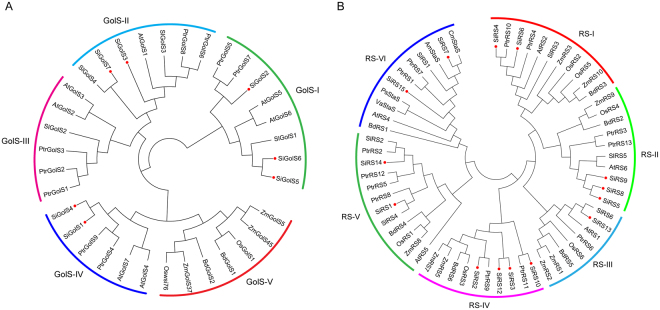
To investigate the evolutionary relationship of GolSs from sesame and other plant species, a Neighbor-joining tree was created based on the protein sequences of 34 GolSs from sesame, Arabidopsis (AtGolS1-7), tomato (SlGolS1-4), maize (ZmGolS1-3), poplar (PtrGolS1-9), rice (Oswsi76 and OsGolS1), and Brachypodium distachyon (BdGolS1 and BdGolS2). GolS proteins could be classified into 5 groups (GolS-I to GolS-V) according to the phylogenetic tree (Fig. 2A). SiGolS proteins were distributed in all groups, except GolS-III and GolS-V. Generally, SiGolSs have a closer relationship with SlGolSs as compared to AtGolSs, in accordance with the current understanding in their evolutionary history26. Notably, in the clade GolS-V, no GolS homologs were found from sesame, Arabidopsis, tomato and poplar (Fig. 2A), suggesting that group GolS-V is specific for monocot species.
Figure 2.
Phylogenetic analysis of GolSs and RSs from sesame and other species. (A) The deduced full-length amino acid sequences of 7 SiGolSs and 27 GolSs from 6 representative sequenced plant species (see Supplementary Table S2) were used for phylogenetic tree construction. (B) The deduced full-length amino acid sequences of 14 SiRSs, 44 RSs from six representative sequenced plant species (see Supplementary Table S3) and 4 referenece StaSs from Vigna angularis (VaStaS, CAB64363), Pisum sativum (PsStaS, CAC38094), Cucumis melo (CmStaS, XP_008451468) and Alonsoa meridionalis (AmStaS, CAD31704) were used for phylogenetic tree construction. The phylogenetic tree was constructed using MEGA 5.05 by the Neighbor-joining method with 1,000 bootstrap replicates. Members of GolS and RS proteins from sesame were denoted in red dots.
To obtain further insight into the structural features of SiGolS genes, the exon/intron organization was analyzed by GSDS v2.0. As shown in Figure S3B, all SiGolSs have 3 or 4 exons. To better understand the evolution of SiGolSs, 10 conserved motifs were captured by MEME v4.11.0 (Supplementary Fig. S3C), and the details of the sequence logo of each motif were presented in Supplementary Fig. S4. Generally, SiGolSs in the same subfamilies showed similar motifs, indicating that the classification of SiGolS families was supported by motif analyses.
Structural and phylogenetic analysis of SiRSs
The amino acid residues, molecular weight (MW) and theoretical isoelectric point (pI) of the 15 SiRS proteins were largely different, ranging from 198 aa/21.36 kDa (SiRS11) to 884 aa/97.39 kDa (SiRS2); 5.12 pI (SiRS11) to 6.60 pI (SiRS1) (Table 1). Among the SiRS genes, SiRS11 only codes for approximately 25% of the length of a RafS or StaS protein (Table 1). Furthermore, the 198 amino acids of SiRS11 correspond to partial Raffinose_synthase domain, and show only 48% identity with its closest relatives, SiRS12. Therefore, SiRS11 and its putative product were not included in further analyses. Multiple sequence alignment of SiRSs along with reference RafS and StaS from other plant species, such as Arabidopsis, rice, Vigna angularis and Cucumis melo showed that all the SiRS protein sequences harbor the conserved domains of the Raffinose_synthase family (PF05691) (Supplementary Fig. S5). Although both RafS and StaS contain the same domain (Raffinose_synthase domain, PF05691), one sequence block of about 80 amino acids length present exclusively in StaS sequences is characteristic for StaS15. Based on the multiple sequence alignment, two SiRSs (SiRS7 and SiRS15), that containing the characteristic insertion of StaS, were identified as putative StaS encoding genes (Supplementary Fig. S5). Other thirteen SiRSs belong to the putative RafS enzyme family.
Phylogenetic analysis based on the full-length amino acid sequences of RSs from sesame and six other plants clearly distinguished RSs into 6 groups (RS-I to RS-VI) (Fig. 2B). SiRS proteins were distributed in all groups. Similar to SiGolSs, SiRSs have a closer relationship with RSs from tomato and poplar. Two putative StaSs in sesame (SiRS7 and SiRS15) were clustered with all other reference StaSs from Arabidopsis (AtRS4), Vigna angularis (VaStaS), Pisum sativum (PsStaS), Cucumis melo (CmStaS) and Alonsoa meridionalis (AmStaS) in group RS-VI15,29,30, indicating the group RS-VI might be specific for StaS enzyme family.
Exon-intron organization of the SiRS family was also investigated to reveal their gene structural diversity (Supplementary Fig. S6B). The numbers of intron of SiRSs varied from 3 to 14. In general, SiRSs clustered in the same group showed similar gene structure (Supplementary Fig. S6B). All SiRS genes in the group RS-II have 4 or 5 exons, while SiRSs in the group IV have 13 or 14 exons. Then, the MEME program was used to predict putative conserved motifs in SiRSs. A total of 20 putative motifs were detected (Supplementary Figs S6C and S7). As expected, SiRSs in the same groups have similar motif organization, indicating the link between evolutionary relationship and conserved motifs.
Expression profiles of SiGolS and SiRS genes in different tissues
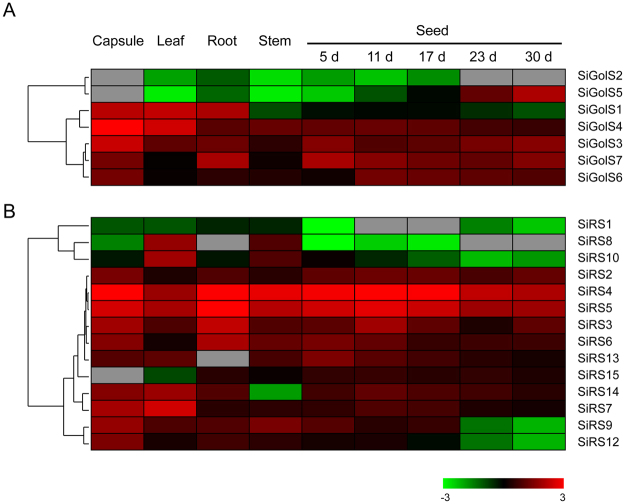
To investigate the expression patterns of SiGolS and SiRS genes, their transcript levels in four tissue samples (capsule, leaf, root and stem) and seed samples at different developmental stages were retrieved from Sesame Functional Genomics Database (SesameFG, http://www.ncgr.ac.cn/SesameFG). Heatmaps were generated according to hierarchical clustering methods based on the RPKM values for each gene (Fig. 3). All SiGolS and SiRS genes displayed very diverse expression in all samples, except for SiRS11, which was not expressed across all tissues.
Figure 3.
Expression profiles of the SiGolS and SiRS genes in different tissues. Transcriptome data downloaded from Sesame Functional Genomics Database (SesameFG, http://www.ncgr.ac.cn/SesameFG) was used to measure the expression level of SiGolS (A) and SiRS (B) genes in capsule, leaf, root, stem and seed at different stage of development. Heat maps were constructed by Cluster 3.0 based on the log10-transformed RPKM values for each gene. Gray box indicated the expression data is not available in this organ or time point. The color scale for expression values is shown.
Among the 7 SiGolS genes, SiGolS3 and SiGolS4 displayed high expression, whereas, SiGolS2 and SiGolS5 showed relatively low expression in almost all tissues (Fig. 3A). SiGolS1 displayed high expression levels in capsule, leaf and root, showed relatively low expression levels in stem and during the seed development. SiGolS6 and SiGolS7 exhibited high expression levels during the seed development and relatively low expression levels in leaf and stem (Fig. 3A).
Concerning the SiRS genes, all of them exhibited high expression levels in all tissues and developing seeds, except that SiRS1, 8 and 10 displayed relatively low expression levels during seed development (Fig. 3B). Especially, SiRS4 and SiRS5 were constitutively expressed at a relatively high level across all tissues. SiRS8 and SiRS10 exhibited specific high expression in leaf and stem. While, SiRS14 and SiRS15 exhibited specific low expression in stem and leaf, respectively. It is worth noting that over half of the SiRS genes displayed lower expression at the late stage of seed development compared to the early stage (Fig. 3B).
Expression profiles of SiGolS and SiRS genes in response to abiotic stresses
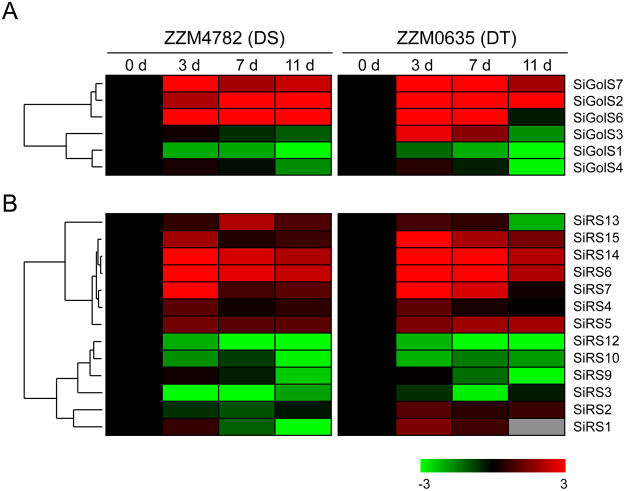
GolS and RS genes have been reported for their responsiveness to various abiotic stresses14,17. Thus, the expression patterns of these genes in response to drought and waterlogging stresses in the root of genotypes with contrasting tolerance levels were firstly revealed by two separate transcriptome analyses31,32. According to the transcriptome data, 6 SiGolSs (except for SiGolS5) and 5 SiGolSs (except for SiGolS2 and SiGolS5) showed the corresponding expression data under drought and waterlogging stresses, respectively. 13 SiRSs (except for SiRS8 and SiRS11) showed the corresponding expression data under both drought and waterlogging stresses. Although some similar expression patterns were exhibited, SiGolSs and SiRSs showed complex expression patterns in response to drought and waterlogging stresses in two contrasting genotypes, as evidenced by the cluster analyses in the heatmaps (Figs 4 and 5).
Figure 4.
Expression profiles of SiGolS and SiRS genes under drought stress. The log2-transformed values (mean of three replicates) of the relative expression levels of the SiGolS (A) and SiRS (B) genes under drought stress in ZZM4782 (drought-sensitive, DS) and ZZM0635 (drought-tolerant, DT) varieties were used for creating the heatmap using Cluster 3.0. Gray box indicated the gene-expression change is not available at this time point. Changes in gene expression are shown in color as the scale.
Figure 5.
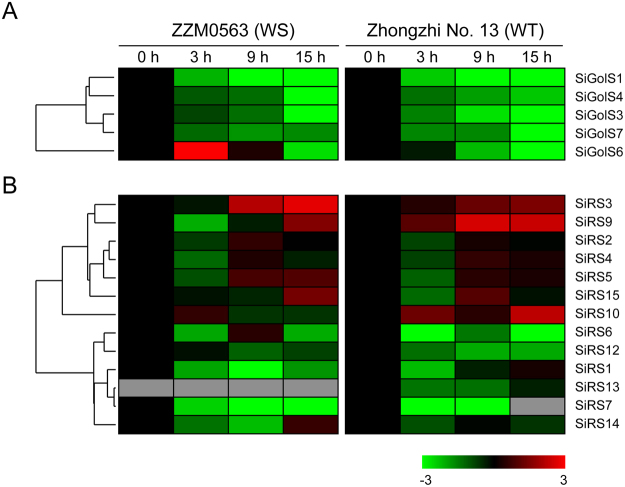
Expression profiles of SiGolS and SiRS genes under waterlogging stress. The log2-transformed values (mean of three replicates) of the relative expression levels of the SiGolS (A) and SiRS (B) genes under waterlogging stress in Zhongzhi No. 13 (waterlogging-tolerant, WT) and ZZM0563 (waterlogging-susceptible, WS) varieties were used for creating the heatmap using Cluster 3.0. Gray box indicated the gene-expression change is not available at this time point. Changes in gene expression are shown in color as the scale.
Most of the SiGolS and SiRS genes showed similar expression pattern in response to drought stress between ZZM4782 (drought-sensitive, DS) and ZZM0635 (drought-tolerant, DT) varieties (Fig. 4). For instance, 3 SiGolSs (SiGolS2, 6 and 7) and 7 SiRSs (SiRS4, 5, 6, 7, 13, 14 and 15) were up-regulated, whereas 2 SiGolSs (SiGolS1 and 4) and 4 SiRSs (SiRS3, 9, 10, and 12) were down-regulated, by drought stress in both genotypes. However, some genes showed different expression pattern between DS and DT varieties. SiGolS3 was significantly induced at 3 d and 7 d after drought stress treatment in DT variety, while, it was slightly down-regulated under drought stress treatment in DS variety (Fig. 4).
Expression of most of the SiGolS and SiRS genes were repressed under waterlogging stress in both the waterlogging-tolerant (WT) variety Zhongzhi No. 13 and the waterlogging-susceptible (WS) variety ZZM0563 (Fig. 5). All the SiGolSs showed decreased transcripts under waterlogging stress at whole time points in the WT and WS varieties, except that SiGols6 was instantaneously up-regulated at 3 h after waterlogging stress in the WT variety. Six SiRSs (SiRS1, 6, 7, 12, 13, and 14) were down-regulated under waterlogging stress in both WT and WS varieties. SiRS3 and 9 were up-regulated at 9 h and 15 h after waterlogging stress in WT and WS varieties. SiRS2, 4 and 5 were down-regulated only at 3 h after waterlogging stress in WT and WS varieties. SiRS10 exhibited relatively higher transcript accumulation at 3 h and 15 h after waterlogging stress in the WT variety, but showed no significant difference in the WS variety (Fig. 5).
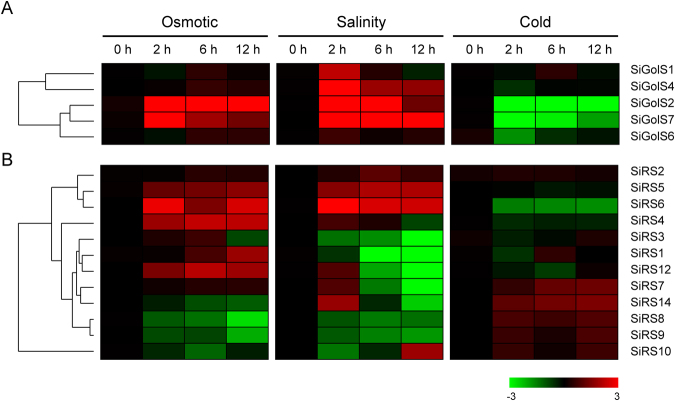
To extend our understanding of SiGolS and SiRS genes in response to other important abiotic stresses impairing the sesame production, 5 SiGolSs (SiGolS1, 2, 4, 6, 7) and 12 SiRSs (SiRS1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14) that showed different expression patterns in different organs, and in response to abiotic stresses based on transcriptome data were chosen for further investigation of their expression patterns in shoot under osmotic, salinity, and cold treatments by qPCR. Under osmotic stress, SiGolS2 and SiGolS7 were significantly up-regulated (fold change > 2) during the whole treated time points, while the other 3 SiGolSs exhibited no significant change (Fig. 6A and Supplementary Fig. S8). Under salt treatment, 3 SiGolSs (SiGolS2, 4, and 7) were significantly induced (fold change > 2) during the treatment period, whereas SiGolS1 was only up-regulated at 2 h treatment. Under cold treatment, SiGolS2 and SiGolS7 were significantly repressed during the whole treated time points. As shown in Fig. 6B and Supplementary Fig. S9, 4 SiRSs (SiRS4, 5, 6, and 12) were significantly (fold change > 2) up-regulated, while SiRS8 was significantly (fold change > 2) down-regulated during the entire osmotic stress time points. SiRS5 and SiRS6 were significantly induced (fold change > 2) during the salt treatment period, whereas SiRS2, 7, 10, 12 and 14 were only up-regulated or down-regulated at particular time points. Under cold treatment, most of the SiRSs were not significantly affected, while SiRS7 and SiRS14 were significantly (fold change > 2) up-regulated. Together, these results indicated that most of the SiGolS and SiRS genes are active in response to osmotic and/or salt stresses, whereas slightly affected by cold stress (Fig. 6, Supplementary Figs S8 and S9).
Figure 6.
Expression profiles of SiGolS and SiRS genes under various abiotic stress treatments. Two-week-old seedlings were subjected to osmotic (15% PEG 6000), salt (150 mM NaCl), and cold (4 °C) stresses. The log2-transformed values (mean of three replicates) of the relative expression levels of the SiGolS (A) and SiRS (B) genes under abiotic stresses based on qPCR were used for creating the heatmap using Cluster 3.0. Changes in gene expression are shown in color as the scale. Original data was shown in Supplementary Figs S8 and S9.
Galactinol and raffinose content in sesame exposed to osmotic stress
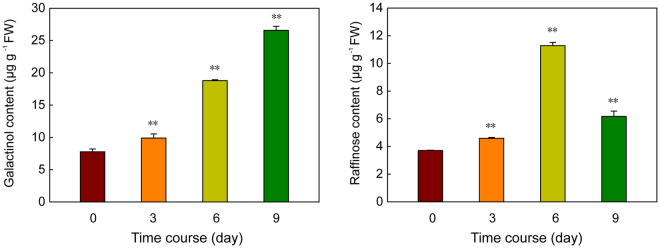
Finally, the changes in the content of galactinol and raffinose in sesame under osmotic stress treatment were investigated. Two-week-old seedlings were treated with 15% PEG 6000, and shoot samples were harvested at 0, 2, 5 and 9 days after treatment. As shown in Fig. 7, the content of both galactinol and raffinose clearly increased under osmotic stress, and the most obvious accumulation in galactinol and raffinose were observed at 6 days after treatment. During the osmotic stress treatment, the amounts of galactinol increased continuously. While the raffinose content peaked at 6 days and decreased subsequently.
Figure 7.
Changes in the content of galactinol and raffinose in sesame under osmotic stress. Two-week-old seedlings were subjected to osmotic (15% PEG 6000) stresses, and shoot samples were harvested at 0, 3, 6 and 9 days after treatment. Content of galactinol and raffinose was determined by LC-MS. Error bars indicate standard deviations based on three replicates. *P < 0.05; **P < 0.01, t test.
Discussion
Sesame is widely grown in arid and semi-arid areas facing frequent occurrences of drought and an increasing soil salinization due to intense use of irrigation and applied fertilizers33. Although sesame is a resilient crop that fairly resistant to several abiotic stresses including drought, salt, heat, it is highly sensitive to environmental stresses during its vegetative stage which directly affects its yield potential27,28,34,35. More importantly, the molecular mechanisms underlying sesame responses to abiotic stresses are poorly understood36. Raffinose family oligosaccharides (RFOs), which accumulate during seed development and plant exposed to abiotic stresses, perform a critical function in desiccation tolerance of developing seeds and plants6,7. Although some RFOs biosynthesis related genes (such as GolSs and RSs) have been studied in many plants7,15,17,37, less information is known about the GolS and RS gene families in sesame. Herein, a total of 7 SiGolSs and 15 SiRSs were genome-wide identified from sesame, which were classified into 5 and 6 subgroups, respectively, according to the phylogenetic relationship (Fig. 2). This classification is consistent with previous studies of GolS family in poplar, tomato and Brachypodium distachyon21,38. Furthermore, 13 RafS and 2 StaS (SiRS7 and 15) were further identified in SiRS gene family based on the existence of characteristic insertion of StaS15. The phylogenetic classification of GolS and RS was also supported by conserved motif and gene structure analyses. Protein and nucleotide sequence analyses showed that GolS and RS gene families harbored similar motifs and exon-intron organizations in the same subgroup (Supplementary Figs S3 and S6). These typical characteristics of these two gene families were also observed in other plants, such as maize, poplar, tomato and Brachypodium distachyon20,21,38. Collectively, similar conserved motifs and exon-intron organizations shared in the same subgroup indicate that SiGolSs and SiRSs in the same group had a closer relationship during the evolution process.
Based on transcriptome data, comprehensive expression profiles of SiGolS and SiRS genes at different developmental stages, or different tissues were revealed (Fig. 3). We found that some SiGolS and SiRS genes exhibited tissue- and developmental stage-specific expression patterns, indicating their possible roles in specific growth or developmental stages. For example, SiRS8 and 10 exhibited specific higher expression in leaf and stem (Fig. 3B). RFOs accumulate during seeds development is thought to be important for desiccation tolerance during seed maturation and longevity in dehydrated state18,39. Seeds of the AtRS4 and AtRS5 double mutant showed a total loss of RFOs and five days delayed germination phenotype in darkness, suggesting that RFOs also act as a galactose store in seeds and are necessary for rapid germination in the dark40. Among sesame GolS and RS genes, 3 SiGolSs (SiGolS3, 6 and 7) and 2 SiRSs (SiRS4 and 5) displayed relatively higher transcripts during seed development, suggesting that these RFOs synthetic genes may be involved in the sesame seed development process.
RFOs were also found accumulated under multiple abiotic stress conditions and function as osmolytes to stabilise cell components, and/or act as reactive oxygen species (ROS) scavengers3,9. We also found galactinol and raffinose significantly accumulated under osmotic stress in sesame. Increasing evidence indicates that RFOs synthesis related genes, especially GolS, are important in the physiology of plant stress resistance. Expression analyses of GolS and RS gene family members in Arabidopsis, rice, maize, poplar, and tomato suggested that many GolS and RS genes showed transcriptional changes under drought, high-salinity, and cold stresses17,19–21. Moreover, transgenic plants analyses revealed special members of GolS and RS gene families as key players in plant abiotic stress resistance. AtGolS2 was up-regulated by drought and salt stresses, overexpression of AtGolS2 not only enhanced tolerance to drought, salt, chilling and oxidative stresses in transgenic Arabidopsis7,17, but also improved drought stress tolerance in the monocot model Brachypodium distachyon and rice22,41. Especially, overexpression of AtGolS2 reduces yield losses under field drought conditions under different environmental conditions and in different rice genetic backgrounds, which suggests that AtGolS2 is a useful biotechnological tool to improve drought tolerance in rice22. Based on in silico analysis and our qPCR analysis, 6, 2, and 4 SiGolSs were regulated by drought stress (4 up-regulated genes and 2 down-regulated genes), osmotic stress (2 up-regulated genes), and salinity stress (4 up-regulated genes), respectively (Figs. 4 and 6). Among the 7 sesame GolS genes, SiGolS2 and SiGolS7 showed a closer phylogenetic relationship with AtGolS2 (Fig. 2A), and exhibited amino acid identities of 81% and 70%, respectively, to the protein encoded by AtGolS2. Moreover, SiGolS4 and SiGolS7 were significantly up-regulated in both osmotic and salinity stresses (Fig. 6A and Supplementary Fig. S8), suggesting that these SiGolSs might be positively involved in drought and salt tolerances of sesame. Our study also found that 10, 8 and 11 SiRSs were regulated by drought stress (5 up-regulated genes and 5 down-regulated genes), osmotic stress (5 up-regulated genes and 3 down-regulated genes) and salt stress (4 up-regulated genes and 7 down-regulated genes), respectively (Figs 4 and 6). Among these genes, SiRS5 and SiRS6 were commonly up-regulated by drought, osmotic, and salinity stresses. On the contrary, SiRS8 and SiRS9 were down-regulated in both osmotic and salinity stresses. Additionally, we found that 3 SiGolSs (SiGolS2, 6 and 7) and 7 SiRSs (SiRS4, 5, 6, 7, 13, 14 and 15) could be induced by drought stress in different genotypes (Fig. 4). All these evidences demonstrated the implication of these genes in response to abiotic stresses in sesame, and therefore, could be further targeted for functional analysis. Interestingly, the expression of SiGolS and SiRS genes was slightly affected by cold stress except SiGolS2 and SiGolS7 (Fig. 6; Supplementary Figs S8 and S9), which could be explained by the fact that sesame was native to warm areas. Sesame is highly susceptible to waterlogging stress, and waterlogging is a significant environmental constraint to sesame production in China and Korea32. However, the expression of genes involved in RFOs biosynthesis under waterlogging stress is largely unknown. Herein, we provide the first insight into waterlogging-responsive of GolS and RS gene family members. Most of the SiGolSs, were down-regulated under waterlogging stress in two genotypes. GolSs act as a switch of inositol metabolism and RFO biosynthesis. Down-regulated of many SiGolS genes under waterlogging stress may divert myo-inositol away from the RFO synthetic pathway, thus participated in O-methyl-inositol (OMI) synthesis and act as a stress tolerance molecule9. These results presented here would be helpful for uncovering the function of RFOs synthetic pathway in abiotic stress resistance in sesame. In conclusion, 7 SiGolS and 15 SiRS genes from sesame have been characterized based on evolutionary, conserved protein motif, and gene structure analyses. The expression profiles of SiGolS and SiRS genes reveal their involvement in sesame seed development and responses to abiotic stresses. Together, these data will supply abundant information for functional characterization of SiGolS and SiRS genes and advance our understanding of RFOs-mediated abiotic stress tolerance in sesame.
Methods
Sequence identification and phylogenetic analysis
Protein sequences of genes involved in raffinose biosynthesis in Arabidopsis, such as AtGolSs and AtRSs, were used as queries to search against the protein database at Sinbase (Sesamum indicum genome database, http://ocri-genomics.org/Sinbase/index.html)42 by using BLASTP. Resulting sequences with an E-value of <1e−100 were analyzed manually in Pfam (http://pfam.sanger.ac.uk/) to validate the presence of Glyco_trans_8 Pfam (PF01501) for GolS proteins, or Raffinose_synthase Pfam (PF05691) for RafS and SatS proteins, respectively. GolS and RS proteins of representative sequenced plant species used in this study were obtained from the respective project databases (see below). Arabidopsis thaliana: The Arabidopsis Information Resource (TAIR), http://www.arabidopsis.org; Oryza sativa subsp. japonica: Rice Genome Annotation Project (RGAP), http://rice.plantbiology.msu.edu; Brachypodium distachyon, Solanum lycopersicum, Populus trichocarpa, and Zea mays: Phytozome, http://www.phytozome.net. The Neighbor-joining (NJ) phylogenetic trees were constructed with Clustalx 1.83 and MEGA5.05 software43,44.
Gene structure and motif identification of SiGolSs and SiRSs
Exon and intron structures of these genes were investigated by comparing the coding sequences with their corresponding genomic sequences from Sinbase database, and visualized by using GSDS 2.0 (http://gsds.cbi.pku.edu.cn/index.php)45. The duplication pattern of each SiGolS and SiRS gene was analyzed using MCScanX software (http://chibba.pgml.uga.edu/mcscan2/) according to the previous description46. Conserved motifs in SiGolSs and SiRSs were identified using MEME v4.11.4 (http://meme-suite.org/tools/meme).
Plant growth and stress treatment
To measure the transcript levels of the sesame GolS and RS family members under various abiotic stresses, seeds of sesame cultivar Zhongzhi No. 13 were germinated and grown hydroponically in a growth chamber with a 16 h light/8 h dark cycle. For osmotic and salt stress treatments, two-week old seedlings were treated with 15% PEG 6000 and 150 mM NaCl. For cold stress, seedlings were transferred to a growth chamber at 4 °C. Shoot samples from five randomly selected plants were collected (as one biological replicate) at 0 h (pretreatment), and at 2 h, 6 h and 12 h after stress treatments. For each treatment and time point, three replicates were used for RNA extraction.
Expression profiles analyses of SiGolSs and SiRSs
Total RNA was isolated using the EASYspin Plus kit (Aidlab, China) according to the manufacturer’s instructions. For real-time quantitative RT-PCR (qPCR) analysis, first-strand cDNAs were synthesized from DNaseI-treated total RNA using the HiScript II 1st Strand cDNA Synthesis kit (Vazyme, China) according to the manufacturer’s instructions. Real-time quantitative RT-PCR was performed on Roche LightCycler 480 real-time PCR system using the ChamQ SYBR qPCR Master Mix (Vazyme, China) according to the manufacturer’s protocol. The sesame Histone H3.3 gene (SIN_1004293) was used as the endogenous control47. The relative expression levels were calculated as described previously48. The qPCR assays were performed with three replicates. The gene-specific primers are listed in Supplementary Table S1.
Expression patterns of SiGolS and SiRS genes in capsule, leaf, root, stem, and seeds at different stages were examined in a set of transcriptome data downloaded from Sesame Functional Genomics Database (SesameFG, http://www.ncgr.ac.cn/SesameFG). Expression data of SiGolS and SiRS genes under drought stress were extracted from the transcriptome data of two sesame varieties (drought-tolerant cultivar ZZM0635 and drought-sensitive cultivar ZZM4782) under drought stress at flowering stage31. Expression data of SiGolS and SiRS genes under waterlogging stress were extracted from the transcriptome data of two sesame varieties (waterlogging-tolerant cultivar Zhongzhi No. 13 and the waterlogging-susceptible cultivar ZZM0563) under waterlogging stress at flowering stage32. The hierarchical cluster analyses of gene expression were performed using Cluster 3.0 software49, and heatmaps were visualized with TreeView50.
Quantification of galactinol and raffinose content
Quantification of galactinol and raffinose content in sesame was performed by liquid chromatography-mass spectrometry (LC-MS) at Wuhan Metware Biotechnology Co.,Ltd (Wuhan, China) as described by51, with small modifications. Briefly, shoot samples from five plants were harvested after stress treatment and immediately frozen in liquid nitrogen. Then, samples were crushed and extracted overnight at 4 °C with 1.0 ml 70% aqueous methanol. After filtering, the extracts were analyzed by LC-MS. Details of the methods for the quantification of galactinol and raffinose content by LC-MS are provided in Supplementary Methods S1.
Electronic supplementary material
Acknowledgements
This work was supported by China Agriculture Research System (no. CARS-14), the Agricultural Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-OCRI), and the National Science-technology Support Plan Project (2013BAD01B03-08).
Author Contributions
J.Y., Y.W., Y.Z. performed the experiments, carried out the analysis and wrote the manuscript. K.D., D.L. and R.Z. participated in data collection and analysis. L.W. and X.Z. supervised the experiments and revised the manuscript. All authors have read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22585-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:Suppl, S165–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- 3.Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta S, Mukherjee S, Basak P, Majumder AL. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015;6:656. doi: 10.3389/fpls.2015.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Souza Vidigal D, et al. Galactinol as marker for seed longevity. Plant Sci. 2016;246:112–118. doi: 10.1016/j.plantsci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Rosnoblet C, et al. The regulatory gamma subunit SNF4b of the sucrose non-fermenting-related kinase complex is involved in longevity and stachyose accumulation during maturation of Medicago truncatula seeds. Plant J. 2007;51:47–59. doi: 10.1111/j.1365-313X.2007.03116.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishizawa A, Yabuta Y, Shigeoka S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008;147:1251–1263. doi: 10.1104/pp.108.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Ende W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013;4:247. doi: 10.3389/fpls.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ElSayed AI, Rafudeen MS, Golldack D. Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol. (Stuttg) 2014;16:1–8. doi: 10.1111/plb.12053. [DOI] [PubMed] [Google Scholar]
- 10.Valluru R, den Ende V. W. Myo-inositol and beyond–emerging networks under stress. Plant Sci. 2011;181:387–400. doi: 10.1016/j.plantsci.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013;4:272. doi: 10.3389/fpls.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thole JM, Nielsen E. Phosphoinositides in plants: novel functions in membrane trafficking. Curr. Opin. Plant Biol. 2008;11:620–631. doi: 10.1016/j.pbi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu JJ, Odegard W, de Lumen BO. Galactinol synthase from kidney bean cotyledon and zucchini leaf. Purification and N-terminal sequences. Plant Physiol. 1995;109:505–511. doi: 10.1104/pp.109.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egert A, Keller F, Peters S. Abiotic stress-induced accumulation of raffinose in Arabidopsis leaves is mediated by a single raffinose synthase (RS5, At5g40390) BMC Plant Biol. 2013;13:218. doi: 10.1186/1471-2229-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangl R, Behmuller R, Tenhaken R. Molecular cloning of AtRS4, a seed specific multifunctional RFO synthase/galactosylhydrolase in Arabidopsis thaliana. Front. Plant Sci. 2015;6:789. doi: 10.3389/fpls.2015.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterbauer T, Richter A. Galactosylononitol and stachyose synthesis in seeds of adzuki bean. Purification and characterization of stachyose synthase. Plant Physiol. 1998;117:165–172. doi: 10.1104/pp.117.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taji T, et al. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 18.Downie B, et al. Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented. Plant Physiol. 2003;131:1347–1359. doi: 10.1104/pp.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito M, Yoshida M. Expression analysis of the gene family associated with raffinose accumulation in rice seedlings under cold stress. J. Plant Physiol. 2011;168:2268–2271. doi: 10.1016/j.jplph.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ML, et al. Genome-wide identification of genes involved in raffinose metabolism in Maize. Glycobiology. 2012;22:1775–1785. doi: 10.1093/glycob/cws121. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, et al. Responses of Populus trichocarpa galactinol synthase genes to abiotic stresses. J. Plant Res. 2014;127:347–358. doi: 10.1007/s10265-013-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvaraj, M. G. et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol J. (2017). [DOI] [PMC free article] [PubMed]
- 23.Shimosaka E, Ozawa K. Overexpression of cold-inducible wheat galactinol synthase confers tolerance to chilling stress in transgenic rice. Breed Sci. 2015;65:363–371. doi: 10.1270/jsbbs.65.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Were BA, Onkware AO, Gudu S, Welander M, Carlsson AS. Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over 3 years. Field Crop. Res. 2006;97:254–260. doi: 10.1016/j.fcr.2005.10.009. [DOI] [Google Scholar]
- 25.Johnson LA, Suleiman TM, Lusas EW. Sesame protein: a review and prospectus. J. Am. Oil Chem. Soc. 1979;56:463–468. doi: 10.1007/BF02671542. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014;15:R39. doi: 10.1186/gb-2014-15-2-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassanzadeh M, et al. Effects of water deficit on drought tolerance indices of sesame (Sesamum indicum L.) genotypes in Moghan Region. Res. J. Environ. Sci. 2009;3:116–121. doi: 10.3923/rjes.2009.116.121. [DOI] [Google Scholar]
- 28.Boureima S, Eyletters M, Diouf M, Diop TA, Van Damme P. Sensitivity of seed germination and seedling radicle growth to drought stress in sesame (Sesamum indicum L.) Res. J. Environ. Sci. 2011;5:557–564. doi: 10.3923/rjes.2011.557.564. [DOI] [Google Scholar]
- 29.Peterbauer T, et al. Stachyose synthesis in seeds of adzuki bean (Vigna angularis): molecular cloning and functional expression of stachyose synthase. Plant J. 1999;20:509–518. doi: 10.1046/j.1365-313X.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 30.Peterbauer T, Mucha J, Mach L, Richter A. Chain Elongation of raffinose in pea seeds. Isolation, characterization, and molecular cloning of mutifunctional enzyme catalyzing the synthesis of stachyose and verbascose. J. Biol. Chem. 2002;277:194–200. doi: 10.1074/jbc.M109734200. [DOI] [PubMed] [Google Scholar]
- 31.Dossa K, et al. Transcriptomic, biochemical and physio-anatomical investigations shed more light on responses to drought stress in two contrasting sesame genotypes. Sci. Rep. 2017;7:8755. doi: 10.1038/s41598-017-09397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, et al. Tolerant and susceptible sesame genotypes reveal waterlogging stress response patterns. PLoS One. 2016;11:e0149912. doi: 10.1371/journal.pone.0149912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhakrishnan R, Lee I-J. Penicillium–sesame interactions: A remedy for mitigating high salinity stress effects on primary and defense metabolites in plants. Environ. Exp. Bot. 2015;116:47–60. doi: 10.1016/j.envexpbot.2015.03.008. [DOI] [Google Scholar]
- 34.Koca H, Bor M, Özdemir F, Türkan İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007;60:344–351. doi: 10.1016/j.envexpbot.2006.12.005. [DOI] [Google Scholar]
- 35.Bazrafshan AH, Ehsanzadeh P. Growth, photosynthesis and ion balance of sesame (Sesamum indicum L.) genotypes in response to NaCl concentration in hydroponic solutions. Photosynthetica. 2014;52:134–147. doi: 10.1007/s11099-014-0015-z. [DOI] [Google Scholar]
- 36.Dossa K, et al. The Emerging Oilseed Crop Sesamum indicum Enters the “Omics” Era. Front. Plant Sci. 2017;8:1154. doi: 10.3389/fpls.2017.01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuo C, et al. A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiol. Plant. 2013;149:67–78. doi: 10.1111/ppl.12019. [DOI] [PubMed] [Google Scholar]
- 38.Filiz E. Ozyigit, II & Vatansever, R. Genome-wide identification of galactinol synthase (GolS) genes in Solanum lycopersicum and Brachypodium distachyon. Comput. Biol. Chem. 2015;58:149–157. doi: 10.1016/j.compbiolchem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Salvi P, et al. Differentially expressed galactinol synthase(s) in chickpea are implicated in seed vigor and longevity by limiting the age induced ROS accumulation. Sci. Rep. 2016;6:35088. doi: 10.1038/srep35088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gangl R, Tenhaken R. Raffinose Family Oligosaccharides Act As Galactose Stores in Seeds and Are Required for Rapid Germination of Arabidopsis in the Dark. Front. Plant Sci. 2016;7:1115. doi: 10.3389/fpls.2016.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Himuro Y, et al. Arabidopsis galactinol synthase AtGolS2 improves drought tolerance in the monocot model Brachypodium distachyon. J. Plant Physiol. 2014;171:1127–1131. doi: 10.1016/j.jplph.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Yu J, Li D, Zhang X. Sinbase: an integrated database to study genomics, genetics and comparative genomics in Sesamum indicum. Plant Cell Physiol. 2015;56:e2. doi: 10.1093/pcp/pcu175. [DOI] [PubMed] [Google Scholar]
- 43.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic. Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B, et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic. Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei L, et al. Identification and testing of reference genes for Sesame gene expression analysis by quantitative real-time PCR. Planta. 2013;237:873–889. doi: 10.1007/s00425-012-1805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 50.Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 51.Chen W, et al. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol. Plant. 2013;6:1769–1780. doi: 10.1093/mp/sst080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.