Abstract
The current study aimed at exploring the diversity of bacterial lactase genes in the intestinal mucosa of mice with dysbacterial diarrhea induced by antibiotics and to provide experimental basis for antibiotics-induced diarrhea. Mice model of dysbacterial diarrhea was established by gastric perfusion with mixture of cephradine capsules and gentamicin sulfate (23.33 mL kg−1 d−1), twice a day and continuously for 5 days. Intestinal mucosa from jejunum to ileum was collected, and bacterial metagenomic DNA was extracted for Miseq metagenome sequencing to carry out diversity analysis. The results showed that specific operational taxonomic units (OTUs) were 45 in the control group and 159 in the model group. The Chao1, ACE, Shannon and Simpson indices in model group were significantly higher (P < 0.01 or P < 0.05) than control group. Principal component analysis (PCA) and box chart of the control group were relatively intensive, while in the model group, they were widely dispersed. Furthermore, the inter-group box area was higher than that in the intra-group. Compared with the model group, the abundance of bacterial lactase genes in Proteobacteria from the intestinal mucosa of the control group was higher, but lower in Actinobacteria and unclassified bacteria. At the genus level, the relative abundance of bacterial species and taxon units in model group was obviously increased (P < 0.05). Our results indicate that antibiotics increased the diversity and abundance of bacterial lactase genes in the intestinal mucosa, as the abundance of Betaproteobacteria, Cupriavidus, Ewingella, Methyloversatilis, Rhodocyclaceae and Rhodocyclales. In addition, antibiotics become an additional source for lactase genes of Ewingella, Methyloversatilis, Mycobacterium, Microbacterium, Beutenberqia and Actinomyces.
Keywords: Dysbacterial diarrhea, Lactase gene, Intestinal mucosa, Gene diversity, High-throughput sequencing, Antibiotics
Introduction
A large number and wide variety of microorganisms inhabit in human and animal intestinal tract. Intestinal microbiotas in the intestinal contents and mucosa are mainly composed of bacteria and fungi, and they are the main contributor to various reactions in the intestine (Cao et al. 2014). Usually, the intestinal microbiota lives in a dynamically balanced ecological environment and plays an irreplaceable role in antagonizing pathogens, regulating immunity, maintaining intestinal mucosal barrier integrity and so on (Guo 2001; Li et al. 2007; Lu 2001).
Animal intestinal mucosa is the direct contact surface to intestinal material and also the main interface between the immune system and external environment. Intestinal mucosa plays a major role in the absorption of nutrients and acts as an important barrier in the prevention of inflammation by preventing intestinal pathogens infection. Clinically, various symptoms are associated with intestinal mucosal immune system, such as various pathogen infections, inflammatory bowel disease and celiac disease (Merga et al. 2014; Parvin et al. 2013). The immune defense mechanism of intestinal mucosal is tightly related to intestinal microbiota, which promotes the production and maturation of intestinal mucosa lymphoid tissue, boosts the synthesis of secretory immunoglobulin A (sIgA), participates in the development of intestinal mucosal immune system and interacts with intestinal immune cells to maintain the stability of the intestinal environment (Hong and Zhan 2014). For example, intestinal symbiotic microbiota inhibit the growth of pathogens by competing consumption of nutrients and restrain the translocation of toxin in the intestine by decomposing metabolic carbohydrate to obtain short-chain fatty acids (mainly acetic acid) (Fukuda et al. 2011).
Long-term antibiotic exposure can induce damage in intestinal mucosal barrier by leading to intestinal dysbacteriosis, which is manifested in the abnormal changes in number, type, proportion, location and biological characteristics of intestinal microbiota (Tan et al. 2013; Liu et al. 2015). On the other hand, dysbacteriosis will cause body diarrhea by affecting the body’s absorption of nutrients, inhibiting intestinal mucosal lactase activity and weakening intestinal mucosal barrier (Chen et al. 2014; Liu et al. 2015). A variety of diarrhea can be treated by supplementing lactic acid bacteria or lactase (Luo et al. 2016). Lactase is an important functional enzyme in the intestine associated with diarrhea, distributed in the intestinal contents and mucosa, and can be secreted through intestinal microorganisms and intestinal mucosa. Many bacteria living in the intestinal tract also produce lactase, such as Lactobacillus sp., Bifidobacterium sp., Bacillus sp., Escherichia coli (Juajun et al. 2011; Rhimi et al. 2009). The diversity of lactase-producing bacteria leads to the change of lactase gene diversity and activity.
In our preliminary research, we found that antibiotics reduced the diversity of bacterial lactase genes in intestinal contents and transformed their structure (Long et al. 2017a). The current research aimed to further reveal the correlation between diarrhea and bacterial lactase genes in intestinal mucosa, to explain the mechanism of antibiotics-associated diarrhea treated with traditional Chinese medicine, and more importantly, to understand the mucosal bacterial composition in detail. Therefore, we shall investigate the diversity of bacterial lactase genes in intestinal mucosa of mice with dysbacterial diarrhea induced by antibiotics.
Materials and methods
Materials
Animals
Twelve mature Kunming mice (six males and six females) weighing 20 ± 2 g were purchased from Hunan Slaccas Jingda Laboratory Animal Company (Hunan, China) with license number SCXK (Xiang) 2013-0004. All procedures involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committee of Hunan University of Chinese Medicine.
Reagents
Cephradine capsules were purchased from Suzhou Zhonghua Pharmaceutical Industry Co., Ltd, product batch number: 151101. Gentamicin sulfate injection was purchased from Yichang Renfu Pharmaceutical Co., Ltd, product batch number: 5150307. Solutions like protease K, lysozyme, TE buffer and Tris-saturated phenol–chloroform–isoamyl alcohol (25:24:1) were purchased from Beijing Ding-guo Biotechnology Co., Ltd. Other solutions such as 10% SDS, 0.1 mol L−1 PBS buffer, CTAB/NaCl and 5 mol L−1 NaCl were prepared in the laboratory.
Methods
After 2 days of adaptive feeding, mice were randomly selected as control group (lcm) and model group (lmm), six mice (three males and three females) in each group. The mice in lmm were administered 0.35 mL antibiotics mixture at the concentration of 62.5 g L−1 composed of gentamycin sulfate and cefradine (Zeng et al. 2012; Zhang et al. 2014). The mice in lcm were treated with 0.35 mL sterile water, twice a day for 5 days. Mice in both lmm and lcm were maintained under controlled conditions (23–25 °C, humidity 50–70%). When symptoms, such as fecal material wet, cold limbs, curled up, arched back trembling and poor appetite appear in mice, it indicates the success of establishing diarrhea model. Then the mice were killed using cervical dislocation; test specimens from jejunum to ileum were selected. On a sterile operation platform, after squeezing out the chymus, cutting open the intestinal tract and cleaning the intestinal wall with saline, intestinal mucosa was scraped with coverslips and added 2 times weight of saline, then intestinal mucosa samples were immediately frozen and stored at 4 °C for DNA extraction (Jin et al. 2012).
Metagenome extraction and purification
Metagenome DNA was extracted from intestinal mucosal microorganism according to our previous publication (Wu et al. 2012; Long et al. 2017b). Two grams of intestinal mucosa was collected in a sterile environment, and homogenized in 5 mL of 0.1 mol L−1 phosphate buffer solution (PBS), followed by centrifugation at 200g for 2 min. After being washed twice with PBS, the supernatant was transferred into new germ-free tubes and centrifuged for 8 min at 10,000g. The new sediments were then collected, washed once with PBS, twice with acetone and three times with PBS, then resuspended in 4 mL TE buffer. After sample pretreatment, 500 μL of spreadhead were added with 45 μL TE buffer, 20 μL lysozyme and 5 μL proteinase K and homogenized in 1.5 mL germ-free Eppendorf tubes. Samples were incubated at 37 °C for 30 min and mixed with 30 μL of 10% SDS, followed by incubation at 37 °C for 40 min, with vortexing once every 10 min. Then 80 μL of CTAB/NaCl and 100 μL of 5 mol L−1 NaCl were mixed. The mixture was vortexed at 65 °C for 10 min. An equal volume of Tris-saturated phenol–chloroform–isoamyl alcohol (25:24:1) then was added to the sample, mixed well and centrifuged at 10,000g for 3 min. The supernatant was transferred to new germ-free tubes, mixed with an equal volume of chloroform–isoamyl alcohol (24:1), centrifuged at 10,000g for 3 min. The supernatant was transferred into fresh germ-free tubes and mixed with an equal volume of chloroform–isoamyl alcohol (24:1) again. After centrifugation at 10,000g for 3 min, the supernatant was transferred into fresh germ-free tubes; 10−1 volume of 3 mol L−1 sodium acetate and double volume of absolute ethyl alcohol were added, and precipitated at − 20 °C for about 12 h. Samples were centrifuged at 10,000g for 3 min. The acquired sediment were washed with 70% ethanol, dried and eventually dissolved in 50 μL TE buffer for DNA metagenome extraction.
PCR amplification and Miseq metagenome sequencing
A pair of degenerate primers was designed to amplify the DNA according to the conserved region of the beta-galactosidase (lacZ) gene nucleotide sequence of Lactobacillus and E. coli reported by NCBI. The upstream primer was: 5′-TRRGCAACGAATACGGSTG-3′ and the downstream primer was: 5′-ACCATGAARTTSGTGGTSARCGG-3′. Universal primers were designed and synthesized by Shanghai Personal Biotechnology Co., Ltd. PCR mixture (25 µL) contained 5× reaction buffer 5.0 μL, 5× GC buffer 5.0 μL, 10 mmol L−1 dNTP 0.5 μL, 10 µmol L−1 forward primer 1.0 μL, 10 µmol L−1 reverse primer 1.0 μL, DNA template 1.0 μL, sterilized ddH2O 11.25 μL, Q5 high-fidelity DNA polymerase 0.25 µL. The PCR conditions were as follows: initial denaturation at 98 °C for 30 s, followed by denaturation at 98 °C for 15 s, annealing at 46 °C for 30 s, extension at 72 °C for 30 s and then at 72 °C for 5 min, repeated for 32 cycles (Long et al. 2017b). Miseq metagenome sequencing was completed by Shanghai Persional Biotechnology Co., Ltd.
Bioinformatic and statistical analysis
Alpha diversity analysis which contained Chao1, ACE, Simpson, and Shannon indices was applied to identify the population of intestinal mucosal bacterial lactase genes by determining operational taxonomic units (OTUs) (Shannon 1997; Mahaffee and Kloepper 1997; Pitta et al. 2010, 2014). Chao1 and ACE abundance indices, Simpson and Shannon diversity indices were calculated using Qiime (v1.8.0, http://qiime.org/) software (Caporaso et al. 2010). Principle component analysis (PCA) (Ramette 2007) and Lefse analysis (Segata et al. 2011) were used to analyze the main distribution characteristics of community samples based on linear discriminant analysis (LDA) effect size. Uclust sequence alignment tool in Qiime software was applied to classify high-quality sequences and divide OTUs (Blaxter et al. 2005) according to similarity over 97%. The SPSS 21.0 software (IBM Corp, Armonk, NY, USA) was used to analyze the measurement data. Pairwise samples t test was applied to compare the statistical significance of differences, with P < 0.05 or P < 0.01.
Results
Effects of antibiotics on the OTUs number of bacterial lactase genes in the intestinal mucosa
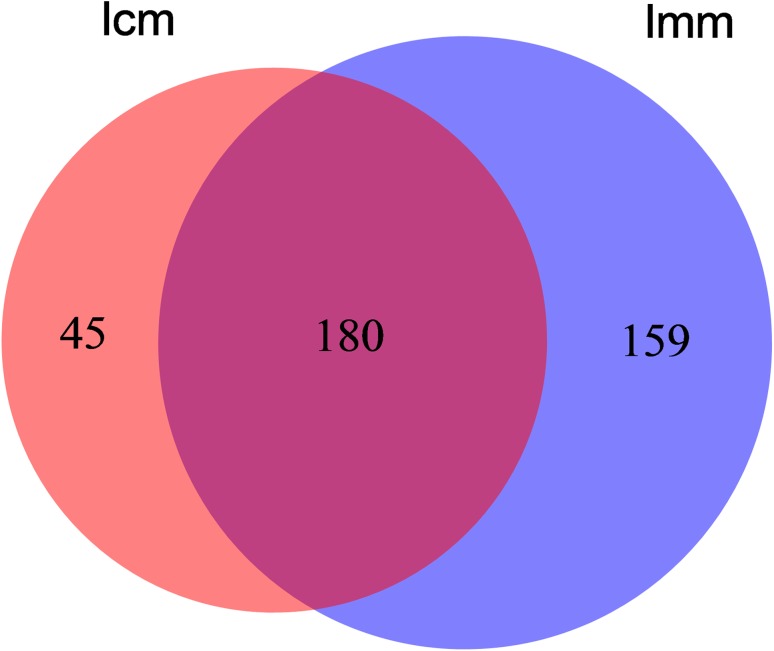
In total, 270,653 effective sequences were obtained, over 90% of them were high-quality sequences. Among the high-quality sequences, their sequence length was concentrated at 361 bp, which can be used for subsequent information analysis. Based on sequence homogeneity, the sequences with over 97% similarity were aligned and grouped into individual OTUs using Qiime software. There were 339 and 225 OTUs expressed in mice from model group and control group, respectively. Among them, 180 were identical. In addition, the numbers of unique OTUs identified from model group and control group were 159 and 45 (Fig. 1). It indicates that there was significantly difference in intestinal mucosa bacteria lactase genes between model group mice and control group mice.
Fig. 1.
Effects of antibiotics on the intestinal mucosa bacterial lactase genes OTUs number. lcm control group, lmm model group
Effect of antibiotics on the diversity of bacterial lactase genes in the intestinal mucosa
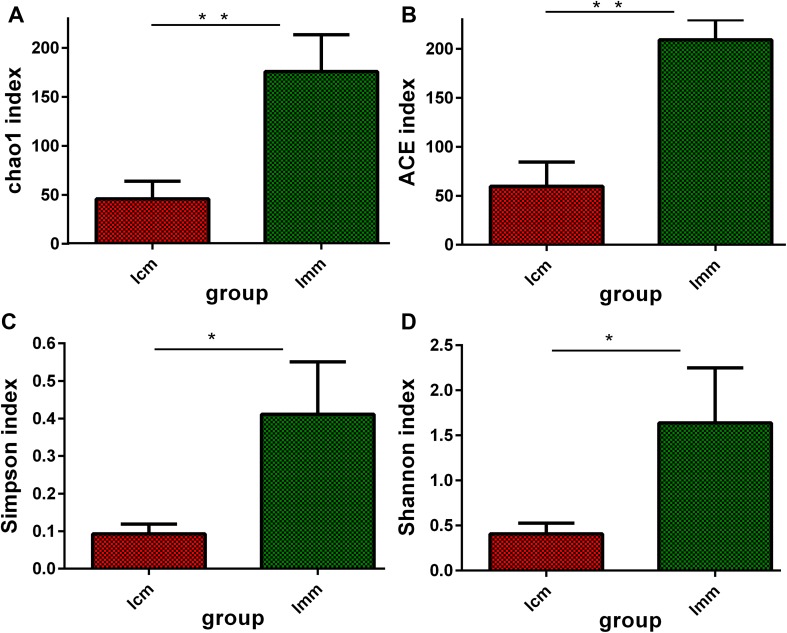
Alpha diversity was estimated by four indices which contained Chao1, ACE, Simpson and Shannon. According to the definition, a higher value of the Chao1 or ACE index indicates greater abundance of a bacterial population, and higher Shannon and Simpson indices suggests a more diverse bacterial population. We found that Chao1, ACE, Simpson and Shannon indices were significantly higher in the model group than those in the control group (P < 0.01 or P < 0.05; Fig. 2), indicating that antibiotics increased the abundance and diversity of bacterial lactase genes in the intestinal mucosal community.
Fig. 2.
Effects of antibiotics on the intestinal mucosa bacterial lactase genes diversity *P < 0.05, **P < 0.01
Effects of antibiotics on the similarity of bacterial lactase genes in the intestinal mucosa
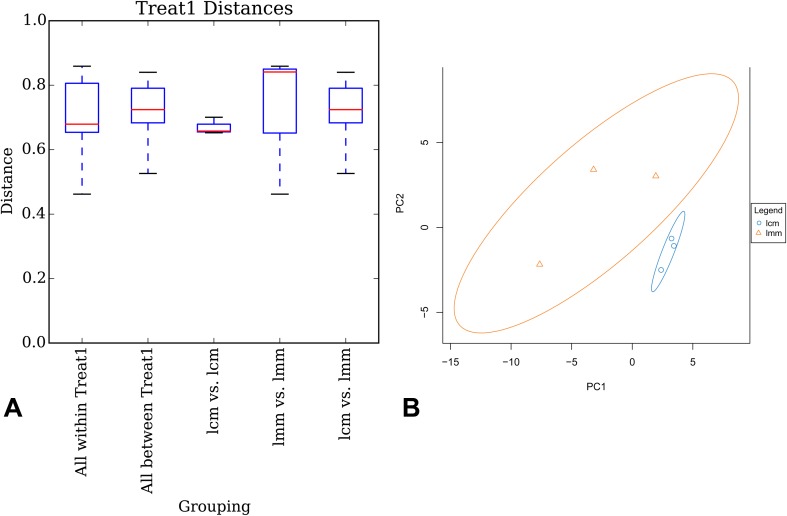
A box plot for multi-group comparison can present the specific distribution characteristics of each sample data according to minimum value, lower quartile, median value, upper quartile and maximum value based on UniFrac distance. The abscissa indicates the comparisons in inter-group or in intra-group between two samples, and the ordinate represents the corresponding distance values, while the size of the box is in response to the difference size of sample gene sequence. In general, the distance between samples at the same group reflects the difference in inter-group. The differences in intra-group is significantly higher than in inter-group, indicating that there is statistical difference between samples of two groups. The results showed that the difference in inter-sample was minimum in the control group, and the differences in intra-group was higher than that in inter-group (Fig. 3a).
Fig. 3.
Effects of antibiotics on the intestinal mucosa bacterial lactase genes similarity. lcm control group, lmm model group
Each point represented one sample, and the points with identical color belonged to the same group. The closer the distance between two points was, the higher the similarity of gene sequence between two samples, and the smaller the difference was. The distance of samples from the control group was tightly concentrated compared to the samples from the model group (Fig. 3b). It indicates that the similarity of lactase genes sequence in control group mice is high, and antibiotics may destroy the structure of intestinal mucosa lactase genes.
Effects of antibiotics on the abundance of bacterial lactase genes in the intestinal mucosa
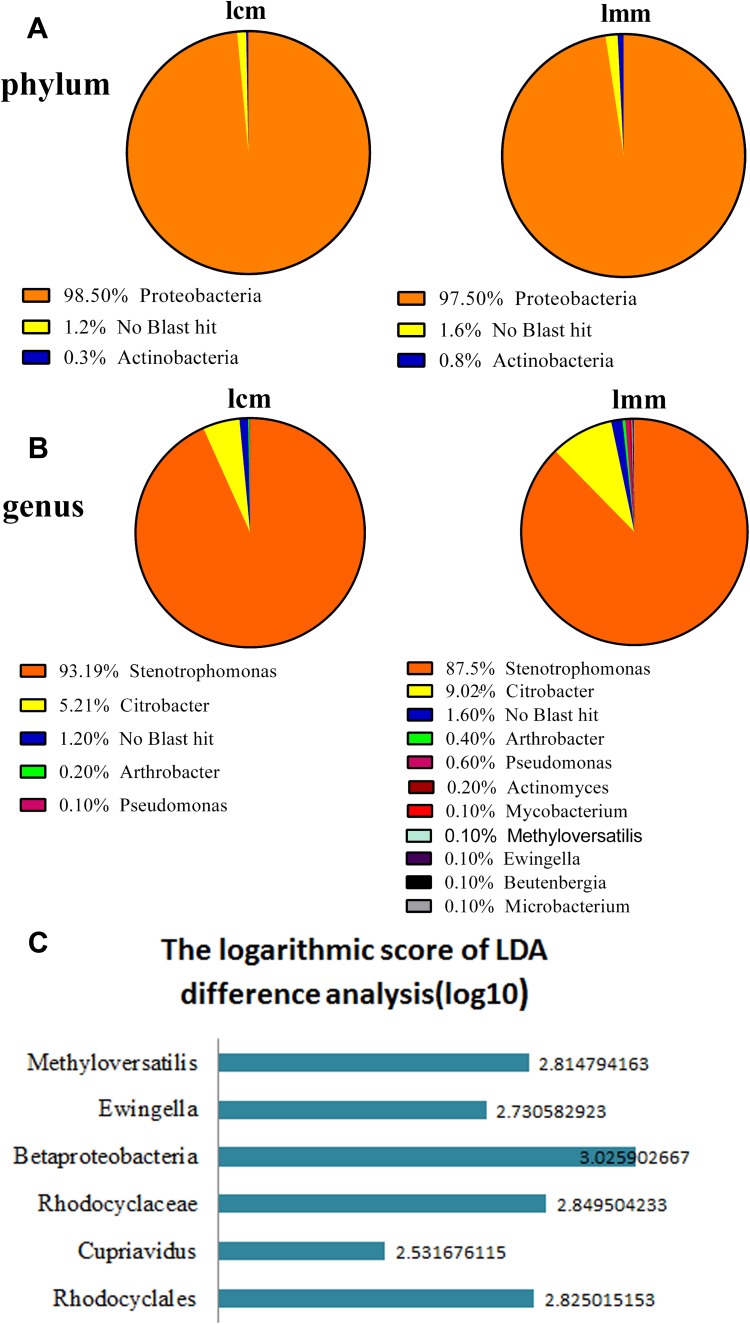
The intestinal mucosa bacterial lactase genes were mainly originated from Proteobacteria, Actinobacteria and unclassified bacteria at the phylum level, and the Proteobacteria was the most abundant. After being induced by antibiotics, the relative abundance of Actinobacteria and unclassified bacteria increased and the abundance of Proteobacteria decreased (Fig. 4a). At the genus level, based on the source of lactase genes, the quantity of them was lower in the control group than in the model group. Except for Stenotrophomonas, the relative abundance of other bacterial lactase genes increased in the model group. Furthermore, Ewingella, Methyloversatilis, Mycobacterium, Microbacterium, Beutenberqia and Actinomyces were only found in the model group (Fig. 4b). In Fig. 4c, the ordinate indicates taxonomic units with significant differences between control group and model group. The abscissa shows the logarithm scores of the LDA difference analysis corresponding to the taxonomic units based on bar graphs and sorted by score values to describe the difference between groups. The longer the bar length, the more the corresponding difference of taxonomic unit became. As indicated in Fig. 4c the abundance of Betaproteobacteria, Cupriavidus, Ewingella, Methyloversatilis, Rhodocyclaceae and Rhodocyclales in model group was significantly higher than that in control group.
Fig. 4.
Effects of antibiotics on the intestinal mucosa bacterial lactase genes abundance. lcm control group, lmm model group
Discussion
Antibiotics significantly increased intestinal mucosa bacterial lactase genes diversity
In recent years, the diversity of enzymes and their functional genes have drawn great attentions from scholars (Yang et al. 2015). It has become a hot issue to explore the microbial ecological function and community diversity from enzyme functional genes (Amann et al. 1995; Zhang et al. 2006; Edgar 2010). In addition to intestinal mucosa, intestinal microbes are also the main source of lactase. To investigate the diversity of bacterial lactase genes in the intestinal mucosa, we applied Miseq metagenome sequencing of beta-galactosidase gene from antibiotic-induced mice for the first time. The results show that the alpha diversity index of bacterial lactase genes in the intestinal mucosa was significantly increased after being induced by antibiotics (P < 0.05 or P < 0.01). From the total number of OTUs, there was a significant difference between control group mice and model group mice, indicating the poor similarity between them. These results are in sharp contrast to our previous study in intestinal contents, the number of OTUs, chao1 index and ACE index of bacterial lactase genes in the intestinal contents were significantly decreased in model group mice treated with antibiotics (Long et al. 2017a). A possible reason may be closely related to the different bacterial composition between intestinal contents and intestinal mucosa. In the known species, Firmicutes is a major phylum in intestinal contents (Zhao et al. 2016). However, there is few Firmicutes in intestinal mucosa.
Antibiotics increased intestinal mucosa bacterial lactase genes abundance
Bacterial lactase genes from the intestinal mucosa were mainly constituted of Proteobacteria, Actinobacteria and unclassified bacteria at the phylum level. Of them, Proteobacteria was the most abundant phylum, followed by unclassified bacteria and Actinobacteria. At the genus level, the difference was more apparent. For example, Ewingella, Methyloversatilis, Mycobacterium, Microbacterium, Beutenberqia and Actinomyces were commonly found in the intestinal mucosa of model mice. However, the lactase genes from these bacteria were not detectable in control mice. Furthermore, in the Lefse analysis, some critical microbial community of lactase-producing gene could be consistently detected in model mice treated with antibiotics including Betaproteobacteria, Cupriavidus, Ewingella, Methyloversatilis, Rhodocyclaceae and Rhodocyclales. And the abundance of these taxonomic units in model group was significantly higher than that in control group. In our previous study, bacterial lactase genes in intestinal contents were mainly derived from Actinobacteria, Probeobacteria, Firmicutes and unclassified bacteria. There were 79.33 ± 0.58 and 76.00 ± 4.58 different genera in the control group and model group, respectively (Long et al. 2017a), far beyond the genera of bacterial lactase from intestinal mucosa, indicating that there are more lactase-producing strains in the intestinal contents. Among the known intestinal microbiota, their relative proportions are very low in the intestinal contents (Long et al. 2017a). Moreover, Streptococcus, Escherichia, Clostridium and Enterococcus are the dominant genera in intestinal contents (Zhao et al. 2016). Maybe it is related to the liquidity of the contents and the effect of antibiotics on species activity. Moreover, we found difference in the main source of lactase-producing strains between contents and mucosa. In our opinion, when compared to intestinal contents, bacteria in intestinal mucosa are more stable. In contrast, bacteria in intestinal contents are more susceptible to be affected by external factors, such as diet and antibiotics. In addition, the intestinal mucosa has the function of self-immunity, which can maintain the stability of microbiota by self-regulation.
Antibiotics changed the community structure of intestinal mucosa bacterial lactase-producing genes
At present, a few researchers tried to explore the gastrointestinal bacterial community diversity of mammalian by using intestinal microbial function enzyme gene. The differences in enzyme gene diversity affect the activity level of functional enzyme, mainly in the type and quantity of functional enzyme strains, even the variation of functional enzyme gene structure coming from the same strain also has a certain relevance to the enzyme activity (Jiang et al. 2014; Tan and Shi 2008; Tan et al. 2009; Wang et al. 2014). From our results, there were significant differences in intestinal mucosa bacterial lactase genes between control and model groups by PCA analysis. In box plot, the size was smaller in inter-group and larger in intra-group, suggesting the poor similarity between control and model groups. We have got the same result in the intestinal contents, but different in forms. Abuse of antibiotics, which damaged intestinal mucosal brush-like lactase, affected the rapid regeneration of intestinal mucosa and decreased its activity, leading to diarrhea (Peng and Ren 2011). But for intestinal contents, some of the sensitive bacterial lactase genes were inhibited or killed, and insensitive genes were reproduced, mainly changed the number, source and structure of lactase-producing strains, and decreased their activity, leading to diarrhea (Long et al. 2017a).
In summary, antibiotics-induced diarrhea increased the diversity and abundance of bacterial lactase genes in the intestinal mucosa and decreased some key lactase-producing strains activity by destroying the community structure of bacterial prolactinase genes or by undermining the encoding function of normal lactase gene, leading to diarrhea. Further study of gene expression would be needed for verifying it.
Acknowledgements
Thanks are extended to the National Natural Science Foundation of China for research funding (no. 81573951).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest related to this article.
Contributor Information
Nenqun Xiao, Phone: (+86) 13974954942, Email: xiaonenqun@sohu.com.
Zhoujin Tan, Phone: (+86) 13974954942, Email: tanzhjin@sohu.com.
References
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial-cells without cultivation. Microbiol Res. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci. 2005;360:1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang H, Guo KX, Peng MJ, He YS, Zhang QL, Peng CY, Tan ZJ. Effects of ultra-micro Qiweibaizhusan on disaccharides metabolism of intestinal microbiotia in diarrheal mice with dysbacteriosis. Int J Curr Microbiol Appl Sci. 2014;3:446–457. [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peṅa AG, Goodrich JK, Gordon JI, Hutley GA, Kelley ST, Knights D, Koeniq JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knignt R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Huang XJ, Shi DY, Guo SN. Research progress on interaction of Chinese materia medica with intestinal flora. Chin Tradit Herbal Drugs. 2014;45:1031–1036. [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Guo BH. Physiological function of intestinal flora. J Chin Dairy Ind. 2001;29:4. [Google Scholar]
- Hong N, Zhan XB. Correlation of gut microflora and intestinal mucosal immunity. J Med Postgra. 2014;27:444–446. [Google Scholar]
- Jiang XD, Guo GG, Zhang J. Association of genetic diversity for Amy6-4 gene with α-amylase activity in germplasm of barley. Acta Agron Sin. 2014;40:205–213. doi: 10.3724/SP.J.1006.2014.00205. [DOI] [Google Scholar]
- Jin L, Yang XH, Ren JL, Li JL, Guo XY, Cao P, Wang Z. Effect of dietary compound probiotics on disaccharidase in small intestine mucosa of layer breeders. China Poultry. 2012;34:14–17. [Google Scholar]
- Juajun O, Nguyen TH, Maischberger T, Iqbal S, Haltrich D, Yammabhai M. Cloning, purification, and characterization of β-galactosidase from Bacillus licheniformis DSM 13. Appl Microbiol Biotechnol. 2011;89:645–654. doi: 10.1007/s00253-010-2862-2. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao XH, Jin C, Li ZL, Luo Y. Chinese medicinal compound and modulation of intestinal microecology. Chin J Integr Tradit West Med. 2007;27:466–469. [PubMed] [Google Scholar]
- Liu QS, Liu H, Peng W, She Y, Tan ZJ. The influence of antibiotic modeling on intestinal mucosa in dysbacteriotic diarrhea mice. Chin J Microecol. 2015;27:501–504. [Google Scholar]
- Long CX, He L, Guo YF, Liu YW, Xiao NQ, Tan ZJ. Diversity of bacterial lactase genes in intestinal contents of mice with antibiotics-induced diarrhea. World J Gastroenterol. 2017;23:7584–7593. doi: 10.3748/wjg.v23.i42.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CX, He L, Liu YJ, Hui HY, Tan ZJ, Li DD. Universal primer for analysis of the diversity of intestinal bacterial lactase gene. Chin J Appl Environ Biol. 2017;23:758–763. [Google Scholar]
- Lu DY. Medical microbiology. Beijing: People’s Medical Publishing House; 2001. p. 89. [Google Scholar]
- Luo WX, Xie M, Gao LW. Observation on the therapeutic effect of lactase on lactose intolerance in infants with diarrhea. Strait Pharm J. 2016;28:153–154. [Google Scholar]
- Mahaffee WF, Kloepper JW. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L) Microb Ecol. 1997;34:210–223. doi: 10.1007/s002489900050. [DOI] [PubMed] [Google Scholar]
- Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- Parvin R, Louie T, Pitchumoni CS. Infectious complications of acute pancreatitis. Infect Dis Clin Pract. 2013;21:94–104. doi: 10.1097/IPC.0b013e3182769586. [DOI] [Google Scholar]
- Peng HZ, Ren LH. Relationship between antibiotic associated diarrhea and lactose intolerance. Chin Gen Pract. 2011;14:2999–3006. [Google Scholar]
- Pitta DW, Pinchak E, Dowd SE, Osterstock J, Gontcharova V, Youn E, Dorton K, Yoon I, Min BR, Fulford JD, Wickersham TA, Malinowski DP. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol. 2010;59:511–522. doi: 10.1007/s00248-009-9609-6. [DOI] [PubMed] [Google Scholar]
- Pitta DW, Parmar N, Patel AK, Induqu N, Kumar S, Prajapathi KB, Patel AB, Reddy B, Joshi C. Bacterial diversity dynamics associated with different diets and different primer pairs in the rumen of Kankrej cattle. PLoS One. 2014;9:e111710. doi: 10.1371/journal.pone.0111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62:142–160. doi: 10.1111/j.1574-6941.2007.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhimi M, Aghajari N, Jaouadi B, Juy M, Boudebbouze S, Maquin E, Haser R, Bejar S. Exploring the acidotolerance of beta-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus: an attractive enzyme for lactose bioconversion. Res Microbiol. 2009;160:775–784. doi: 10.1016/j.resmic.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:1–18. doi: 10.1186/gb-2011-12-S1-P1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. The mathematical theory of communication. 1963. MD Comput Comput Med Pract. 1997;14:306–317. [PubMed] [Google Scholar]
- Tan CC, Shi L. Diversity of Helicobacter pylori clinical isolates by the method of polymerase chain reaction-restriction fragment length polymorphism. Mod Dig Int. 2008;13:18–21. [Google Scholar]
- Tan ZY, Peng GX, Xu PZ, Ai SY, Tang SH, Zhang GX, Zeng FY. Diversity and nitrogenase activity of endogenous azotobacteria in Oryza rufipogon. Chin Sci Bull. 2009;54:1885–1893. doi: 10.1007/s11434-009-0625-1. [DOI] [Google Scholar]
- Tan ZJ, Zhang HL, Zhou SN, Yu WY, Zeng A, Cai Y, Cai GX. Change of intestinal microbes in dysbacteriosis-modeled mice treated with ultra-micro powder of Qiweibaizhusan. Chin J Appl Environ Biol. 2013;19:449–453. doi: 10.3724/SP.J.1145.2013.00449. [DOI] [Google Scholar]
- Wang JZ, Liang JR, Qiu HY, Duan R, Xiao YC, Wang X, Jing HQ. Analysis on polymorphism of Yersinia enterocolitica urease gene and urease activity. Chin J Zoon. 2014;30:140–145. [Google Scholar]
- Wu H, Zhou SN, Guo C, Tan ZJ, Cai GX, Zeng A, Zhang HL. A metagenome DNA extracting method of intestinal flora in mice for molecular diversity analysis based on PCR technology. Chin J Microecol. 2012;24:648–651. [Google Scholar]
- Yang Y, Huang L, Yang L, Xie D, Wang T. Reduction characteristics and differential expression of Acidiphilium cryptum XTS Cr(VI)-reduced related gene. Microbiol China. 2015;42:64–73. [Google Scholar]
- Zeng A, Zhang HL, Tan ZJ, Cai Y, Cai GX, Zhou SN. The construction of mice diarrhea model due to dysbacteriosis and curative effect of ultra-micro Qiweibaizhusan. Microbiol China. 2012;39:1341–1348. [Google Scholar]
- Zhang J, Zhang HW, Li XY, Su ZC, Zhang CG. Soil microbial ecological process and microbial functional gene diversity. Chin J Appl Ecol. 2006;17:1129–1132. [PubMed] [Google Scholar]
- Zhang HL, Cai Y, Tan ZJ, Zhou SN, Guo KX, She Y, Cai GX. Effects of ultra-micro powder Qiweibaizhusan on metabolism diversity of intestinal microflora in diarrhea mice with dysbacteriosis. Chin J Appl Environ Biol. 2014;1:93–100. [Google Scholar]
- Zhao WJ, Liu SY, Ding JM, Dai RH, Meng H. Metagenomic sequencing of gut microbiota along the intestinal tracts and feces in mice. J Shanghai Jiaotong Univ (Agric Sci) 2016;34:15–21. [Google Scholar]