Abstract
This study highlights the anti-oxidant and anti-cancer activities of bio-functionalized Thymus vulgaris silver nanoparticles (TVAgNPs) and bioactive compounds were compared using the human breast cancer T47D cell line. The aqueous ethanolic extract of T. vulgaris evaluated for chemical composition using the gas chromatography–mass spectrometer (GC–MS) analysis. The prepared TVAgNPs were determined by means of UV–Vis spectroscopy, FTIR spectroscopy, zeta potential, scanning electron microscopy, transmission electron microscopy, and energy-dispersed spectroscopy analysis. The T. vulgaris extract and TVAgNPs were studied for their in vitro anti-oxidant property by 2, 2-diphenyl, 1-picryl hydrazyl (DPPH) assay. Microscopic observations indicated spherical shaped and monodispersed nanoparticles and the average size of the nanoparticles was about 30 nm. Regarding the elemental composition profile of the TVAgNPs, the highest signal of silver (89.30%) was detected followed by other elements. An absorption peak was registered at 440 nm according to surface plasmon resonance (SPR) of the TVAgNPs in solution. A zeta potential of fabricated nanoparticles was approximately − 12.6 mV, indicating higher stability of the bio-functionalized TVAgNPs. The T. vulgaris extract and synthesized TVAgNPs were evaluated for their anti-cancer activity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay and Annexin V double staining with propidium iodide (PI) flow cytometric analysis toward T47D cells. The cytotoxicity properties of the bio-functionalized T. vulgaris AgNPs revealed that the sensitivity of T47D human breast cancer cells is high compared with T. vulgaris extract. The Annexin V/PI staining indicated that the fabricated TVAgNPs shows increased apoptosis in T47D cells as compared to untreated cells. Besides, the anti-oxidant activity of the TVAgNPs clarified a higher anti-radical-scavenging activity compared to Thymus vulgaris extract. Our data show that the potential biological activities of the bioactive constituents of T. vulgaris can be enhanced through bio-functionalized T. vulgaris AgNPs due to the bioorganic compounds that exist in the extract.
Keywords: Anti-cancer, Anti-oxidant, Silver nanoparticles, T47D, Thymus vulgaris
Introduction
Metallic nanoparticles have become progressively popular because of their application potential in electrical, optical, catalysis, magnetic, photonic, and chemical processes (Barabadi et al. 2017; Parameshwaran et al. 2013; Shenya et al. 2011). Among different metal nanoparticles, silver nanoparticles (AgNPs) have attracted more attention of researchers due to their broad scope for investigation in terms of catalysis, anti-oxidant, anti-fungal, anti-microbial, anti-platelet, anti-viral, and anti-cancer activities (Asgary et al. 2016; Zayed et al. 2012; Ghanbar et al. 2017; Gopinath et al. 2013). Different chemical and physical methods have been used for the synthesis of AgNPs (Ramteke et al. 2013; Roopana et al. 2013). However, the main disadvantage with these reported methods is the use of hazardous reagents, high-energy requirement, difficulty in purification, and high synthesis costs. Methods that are safe, easy-to-handle, and possess potential for scaling up are also preferred. Nowadays, the biologically green synthesized AgNPs using plant extracts have received significant importance over physical and chemical strategies due to environment friendliness and the absence of toxic chemicals (Satyavani et al. 2012; Mason et al. 2012; Sadat Shandiz et al. 2017; Jeyaraj et al. 2013). Medicinal plants have been used for the development of some treatment proposes, particularly in alternative medicine, pharmaceuticals, food preservation, and natural therapies. Various studies have shown that extracts from medicinal plant species are resources for new drugs and it is necessary to investigate medicinal plants scientifically, as they have led to improvements in present chemotherapeutic quality (Atanasov et al. 2015).
The genus Thymus has around 300 species of aromatic, perennial herbs, and small sub-shrubs, and is commonly found in North Africa, the Southern Europe Mediterranean region, and Asia (Maksimovic et al. 2008; Agili 2014). The genus Thymus, belonging to the Lamiaceae family, is widely considered as folk medicine due to its antiseptic, anti-microbial, anti-virotic, anti-fungal, and sedative effects (Al-Shahrani et al. 2017). Besides use in the biological activities of plant extracts, it can be used as a capping and reducing agent in the fabrication of metallic nanoparticles. Reports show that the flavonoids glycosides, phenolic acids, and terpenoids were found in Thymus spp (Khalilnezhad et al. 2015).
Thus, this work focuses on green synthesis of AgNPs from ethanol extracts of T. vulgaris, which is a reducing and capping agent. Morphological and chemical determination of the bio-functionalized T. vulgaris AgNPs was examined in this study. In addition, the evaluation of the anti-cancer activity of the bio-functionalized T. vulgaris AgNPs in T47D breast cancer cell line was another aim of this study. The phyto-compounds of the extract, as well as the anti-oxidant properties of T. vulgaris and bio-functionalized T. vulgaris AgNPs, were also explained.
Materials and methods
Preparation of aqueous ethanolic extract and synthesis of AgNPs
Thymus vulgaris was collected from Rasht in the Guilan province of Iran and registered with the voucher specimen number of IBRC P1007241. The fresh leaf of T. vulgaris was washed thoroughly several times with double-distilled water (DDW) and dried in the shade at room temperature for 5 days. A total of 20 g of dried leaf powder was stirred with a 1:1 ratio of 100 mL ethanol and 100 mL of deionized water followed by boiling for 30 min. After filtering the crude extract using Whatman No. 1 filter paper, the extract was kept inside a refrigerator until further application.
The AgNPs were fabricated using 10 mL of T. vulgaris aqueous ethanolic extract solution with silver nitrate (AgNO3) (Darmstadt, Germany) aqueous solution to prepare a final volume of 200 mL. Fabrication of AgNPs owing to the reduction of Ag+ ions into silver nanoparticles Ag° via the active bio-molecules exists in T. vulgaris aqueous ethanolic extract at room temperature (Ovais et al. 2016). Thereafter, the formation of particles was characterized by spectrophotometric analysis.
Gas chromatography–mass spectrometric analysis (GC–MS)
GC–MS analysis of the T. vulgaris extract was done with an Agilent 7890 GC–MS system (JMS 700 M Station, Jeol Ltd., Peabody, MA, USA) equipped with 0.25 μm film thickness, HP DB-5 capillary column of 30 m × 0.25 mm i.d., and ionization potential energy of 70 eV. These were used for interpretation of the compounds. The recent work has indicated the operation conditions (Salehi et al. 2016a).
Characterization of TVAgNPs
The surface morphology and size determination of bio-functionalized T. vulgaris AgNPs were analyzed by employing SEM (Zeiss, Germany) and transmission electron microscopy (TEM). In addition, the appearance of elemental silver signal was examined by an energy-dispersed spectroscopy (EDS) analysis system. The identification of bio-molecules present in the TVAgNPs from the aqueous ethanolic extract of T. vulgaris was carried out by Fourier transform infrared (FTIR) spectroscopy using a spectrum RX 1 spectrophotometer at a wavelength of 400–4000 cm−1. Examination of net surface charge of TVAgNPs was further evaluated using zetasizer Nano ZS (Nano ZS90 Malvern Instruments Ltd., UK) instrument. In addition, the optical absorption spectra were used to confirm the bio-reduction of AgNO3 into the nanoparticle using the UV–Vis spectrophotometer (Bio-Tek, Winooski, VA, USA) from 250 to 600 nm.
In vitro anti-cancer activity
Cell culture and cytotoxicity assay
The T47D cells were obtained from the National Cell Bank of Iran (NCBI) and the Pasteur Institute of Iran, and cultured in the standard conditions in Dulbecco modified Eagle Medium (DMEM) containing 10 % fetal bovine serum (FBS), 1 mM sodium pyruvate, 2 mM l-glutamine, and 1% penicillin–streptomycin solution at 37 °C under 5% of CO2. To detect cell cytotoxicity, T. vulgaris aqueous ethanolic extract and bio-functionalized T. vulgaris AgNPs were measured by 3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay. In this regard, the cell suspensions (104 cells/well) were seeded in 96 well plates with various amounts of samples (12.5–200 μg/mL) for 24 h. The cells were incubated in MTT solution (5 mg/mL in PBS) for 4 h. Finally, 100 μL of DMSO was added and optical density (OD) calculated at 570 nm using microplate Autoreader (Bio-Tek Instruments, Inc., Vinooski, VT, USA). The percentage of cell viability was measured using the ratio of OD of treated to untreated cells as a control.
Flow cytometry analysis
Apoptosis and necrosis T47D cells were examined by annexin V-Fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining kit. The T47D cells were incubated with Thymus vulgaris aqueous ethanolic extract and bio-functionalized T. vulgaris AgNPs and were washed three times with ice-cold PBS, trypsinized and centrifuged at RCF of 1006×g for 5 min. Then, the cells were re-suspended in 20μL of FITC-annexin V/PI and incubated in the dark room for 15 min. Finally, to determine the cell death mechanism, the samples were analyzed immediately on the flow cytometer (FCAS Calibur, BD).
Evaluation of anti-oxidant activity using DPPH assay
The anti-oxidant properties of T. vulgaris extract and TVAgNPs were quantitatively evaluated using free radicals of stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. Twenty microliters of samples were added to three milliliters of DPPH solution (0.1 mM). In addition, ascorbic acid was used as a positive control. After 30 min in the dark, the reduction capacity of DPPH radical was calculated at 517 nm using spectrophotometer (HACH 4000 DU UV–visible spectrophotometer) (Adersh et al. 2015). The scavenging activity was measured using the following equation:
Statistical analysis
All experiments were repeated three times and the data obtained were analyzed with a one-way analysis of variance (ANOVA) followed by a t test at 5% level of significance (p ≤ 0.05).
Results and discussion
GC/MS analysis results
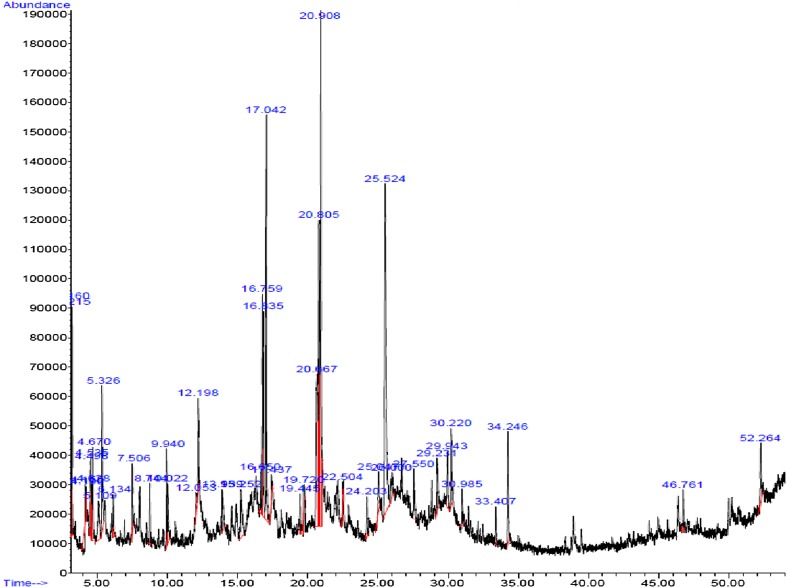
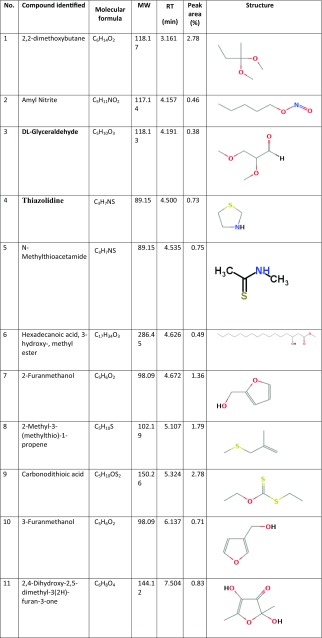
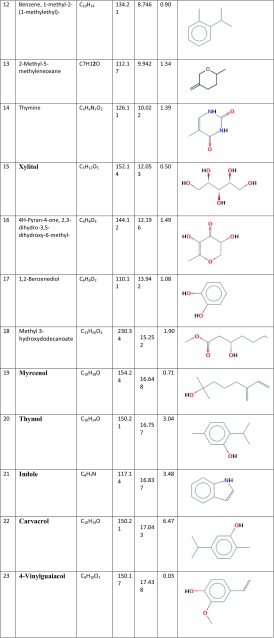
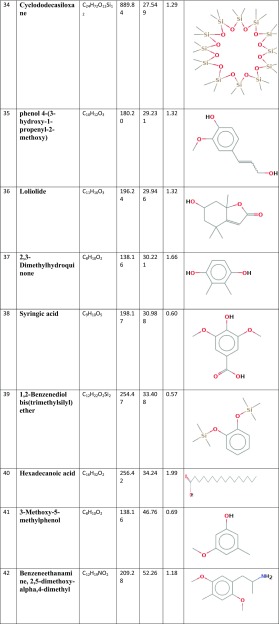
Figure 1 presents the GC/MS analysis of all chemicals clarified in the Thymus vulgaris leaf ethanolic extract. Based on Table 1, 42 different compounds were found in the T. vulgaris extract. Among the phyto-compounds identified, the dominant compounds were quininic acid (12.7%), carvacrol (6.47%), indole (3.48%), thymol (3.04%), and 2-methoxy-4-methylphenol (10.01%). The mass spectra of main phytochemicals constituents identified in T. vulgaris extract are indicated in Fig. 2. T. vulgaris is a plant and is full of polyphenolic compounds with different anti-oxidants anti-cancer and anti-bacterial properties (Roby et al. 2013; Teixeira et al. 2013). The different phytochemicals with medicinal activities in T. vulgaris extract are shown in Table 2. Due to the reducing and capping agents of the main phytochemicals constituents present in the T. vulgaris leaf extract, a cap was formed around Ag+ of the bio-functionalized T. vulgaris AgNPs which was stable.
Fig. 1.
GC–MS chromatogram of Thymus vulgaris extract
Table 1.
Chemical constituents of Thymus vulgaris extract, based on GC–MS analysis
Fig. 2.
Mass spectrum and structure of active phytocomponents identified by GC–MS in the ethanolic extracts of Thymus vulgaris
Table 2.
Bioactivity of main phytocomponents identified in the extracts of T. vulgaris by GC–MS
| No. | RT | Name of the compound | Nature of compound | Therapeutic application | References |
|---|---|---|---|---|---|
| 1 | 25.523 | Quininic acid | Phenolic acid derivatives | Anti-cancer | Inbathamizh and Padmini (2013) |
| 2 | 17.043 | Carvacrol | Monoterpenoid phenol | Antitumor | Fan et al. (2015) |
| 3 | 16.837 | Indole | Heterocyclic organic compound | Anti-cancer | Ahmad et al. (2010) |
| 4 | 16.757 | Thymol | Phenolic compounds | Anti-cancer, anti-oxidant anti-microbial | Kang et al. (2016) |
| 5 | 20.911 | 2-Methoxy-4-methylphenol (MMP) | Phenolic compounds | Anti-cancer and anti-inflammatory | Garg et al. (2001) |
Characterization of bio-functionalized T. vulgaris AgNPs
In this study, the confirmation of phyto-synthesized AgNPs prepared by the eco-friendly green procedure using T. vulgaris extract is given. The T. vulgaris extract behaved as a capping and reducing agent for TVAgNPs. Many biologically natural bio-molecules present in the plant extracts are reported as stabilizing and reducing agents for the fabrication of silver nanoparticles (Salehi et al. 2016b).
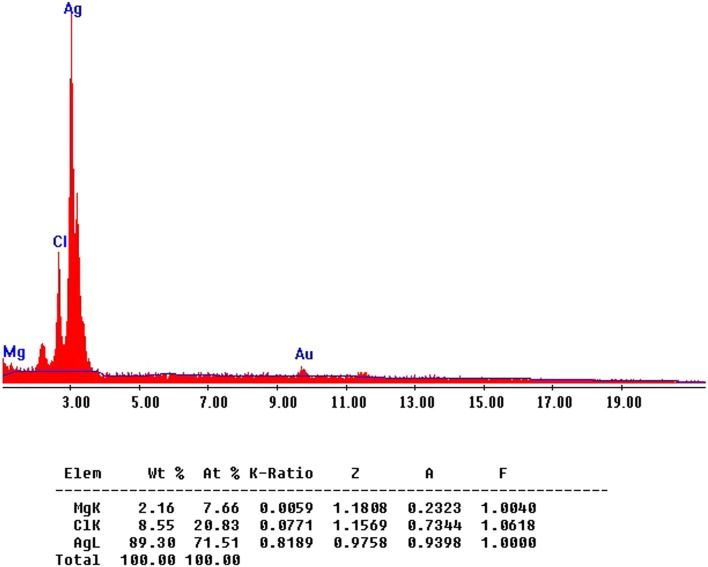
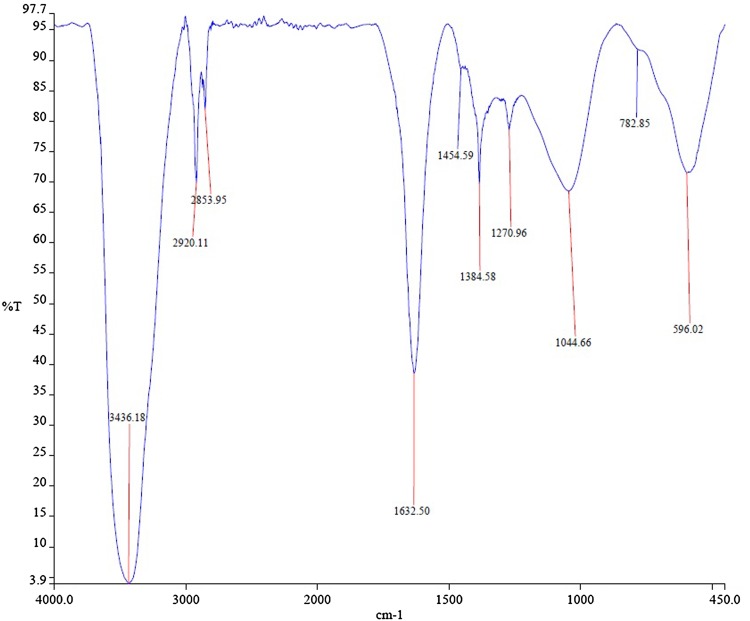
The fabricated TVAgNPs were determined by TEM analysis for characterization of morphology and exact particle size. As shown in Fig. 3, TEM results revealed that the average size of the prepared nanoparticles was about 30 nm. Furthermore, SEM observations indicated that T. vulgaris-synthesized AgNPs were monodispersed, and it is evident that the size and morphology of the synthesized AgNPs are spherical (Fig. 4). To determine the presence of elemental silver signal, EDS spectrum analysis was studied (Fig. 5). The table below Fig. 5 reveals the elemental composition profile of the silver particles that the highest signal of silver (89.30%) is followed by other elements. The appearance of other signals such as magnesium and chlorine indicates the appearance of organic moieties from the plant extract, which is attributed to the biomaterials that were stabilizing over the fabricated AgNPs. In general, the intense peak located at ~ 3 keV indicates surface plasmon resonance of silver nanostructures (Bar et al. 2008; Magudapathy et al. 2001). The FTIR spectrum was clarified to hypothesize the existence of possible active bio-molecules in the T. vulgaris extract responsible for the capping agent of TVAgNPs, which may interact between nanoparticles (Balaji et al. 2009; Shaligram et al. 2009). The FTIR spectra of TVAgNPs revealed seven main peaks at 3436, 2920, 2853, 1632, 1384, 1270, 1044, and 596 cm−1 attributed to the O–H, C–H, C=C, C–C, O–C, and C–N groups, respectively (Fig. 6). Most of the paper reported that biological compounds have pivotal play as stabilizing and capping agents in the reduction of silver salt (Subbaiya et al. 2017; Kasithevar et al. 2017). Regarding the FTIR spectrum results of fabricated TVAgNPs, it was suggested that the ethanolic extract of T. vulgaris contain active bio-molecules such as an amino acids and phenol that could be responsible for bio-reduction of Ag+ during the synthesis of AgNPs. The bio-functionalized T. vulgaris AgNPs were also characterized by UV–visible spectrometry. An absorption peak was registered at 440 nm according to surface plasmon resonance (SPR) of the AgNPs in solution (Fig. 7). Many researchers have approved similar UV–Vis spectra results for the stabilizing effect of natural plant extracts in the synthesis of stable metal nanoparticles (Kotakadi et al. 2015; Yin et al. 2004; Jeyaraj et al. 2013; Emmanuel et al. 2017).
Fig. 3.
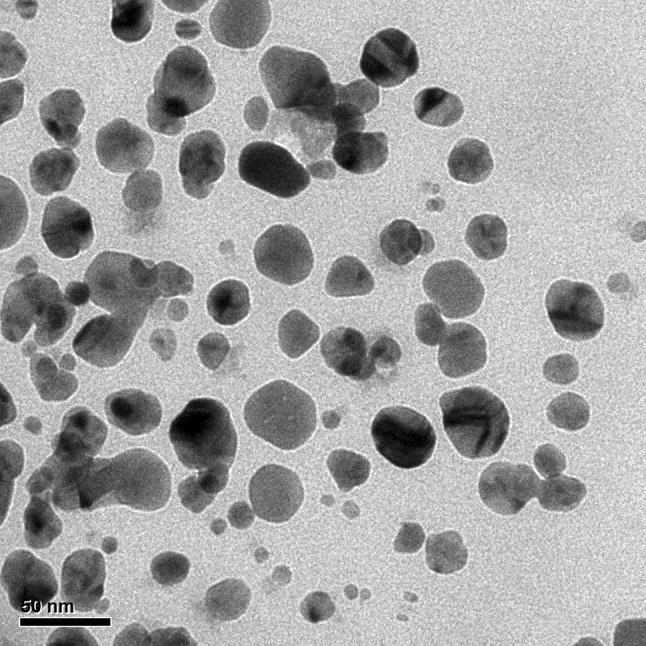
TEM micrograph of synthesized TVAgNPs
Fig. 4.

SEM image of Ag nanoparticles fabricated by reduction of aqueous AgNO3 ions using ethanolic extract of Thymus vulgaris
Fig. 5.
Energy-dispersed spectroscopy (EDS) analysis of TVAgNPs
Fig. 6.
FTIR spectrum of silver nanoparticles synthesized using leaf extracts of Thymus vulgaris
Fig. 7.
Ultraviolet–visible spectra of Thymus vulgaris silver nanoparticles (TVAgNPs) prepared via phyto-synthesis
In the current study, assessment of TVAgNPs surface charges was recorded by the zeta potentiometry. A zeta potential of fabricated nanoparticles was approximately − 12.6 mV, indicating higher stability of the bio-functionalized TVAgNPs (Fig. 8). The greater negative surface charge potential value can support high dispersity and long-term stability (Ahire et al. 2012; Bhadra et al. 2014; Atale et al. 2016).
Fig. 8.
Zeta potential of fabricated TVAgNPs
Cytotoxicity effect of TVAgNPs
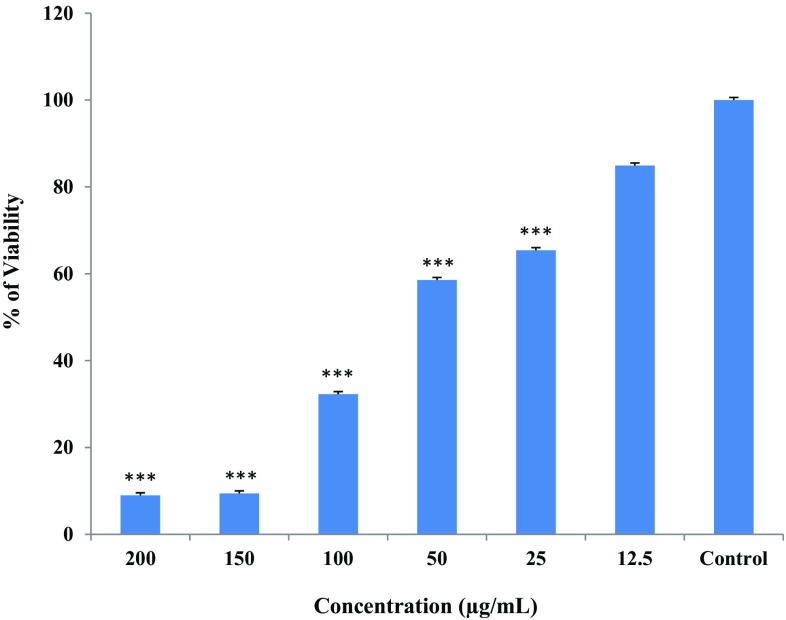
In vitro cytotoxic activity of T. vulgaris and TVAgNPs were performed using an MTT assay (Figs. 9, 10). The T47D cell lines were treated with various concentrations of samples ranging from 12.5 to 200μg/mL to assess the cytotoxicity percentage toward cells. The synthesized TVAgNPs trigger cell death by inhibiting was about 90% in T47D cells at the highest concentration of 200 μg/mL, whereas T. vulgaris plant extracts showed about 75% cell mortality.
Fig. 9.
Cytotoxic effects of cell viability on treatment with varying concentrations of Thymus vulgaris aqueous ethanolic extract on the T47D cells calculated by MTT assay. Results are expressed as mean ± standard error as calculated from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 shows significant difference between controls versus Thymus vulgaris aqueous ethanolic extract
Fig. 10.
Cytotoxic effects of cell viability on treatment with varying concentrations of TVAgNPs on the T47D cells calculated by MTT assay. Results are expressed as mean ± standard error as calculated from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 shows significant difference between controls versus TVAgNPs
We showed that the T. vulgaris extract is less toxic on T47D cells compared to TVAgNPs. When we checked the effectiveness of TVAgNPs toward T47D, we found a dramatic reduction in viability of the cells, and indicate a dose-dependent pattern. We believe that this is the first comparative report to indicate cytotoxic effect of T. vulgaris plant-mediated silver nanoparticles against T47D cancer cells. In fact, the high cytotoxic activity of the fabricated TVAgNPs against T47D cells may be due to potential bioorganic groups capped on the surface of nanoparticles. Those compounds may enhance toxicity. Therefore, TVAgNPs may have potential as inhibitor of T47D cancer cell proliferation.
From various studies, it is reported that silver nanoparticles contribute to the cytotoxicity of cancer cells (Du and Singh 2016; Arunachalam et al. 2015). In addition, the previous studies suggested that one of the probable mechanisms of action of AgNPs on cancerous cells could be enhancing reactive oxygen species (ROS) generation and damaging cell integrity, which leads to cell death (Ovais et al. 2017; Du and Singh 2016; Ahmed et al. 2016; Piao et al. 2011).
Determination of apoptosis and necrosis
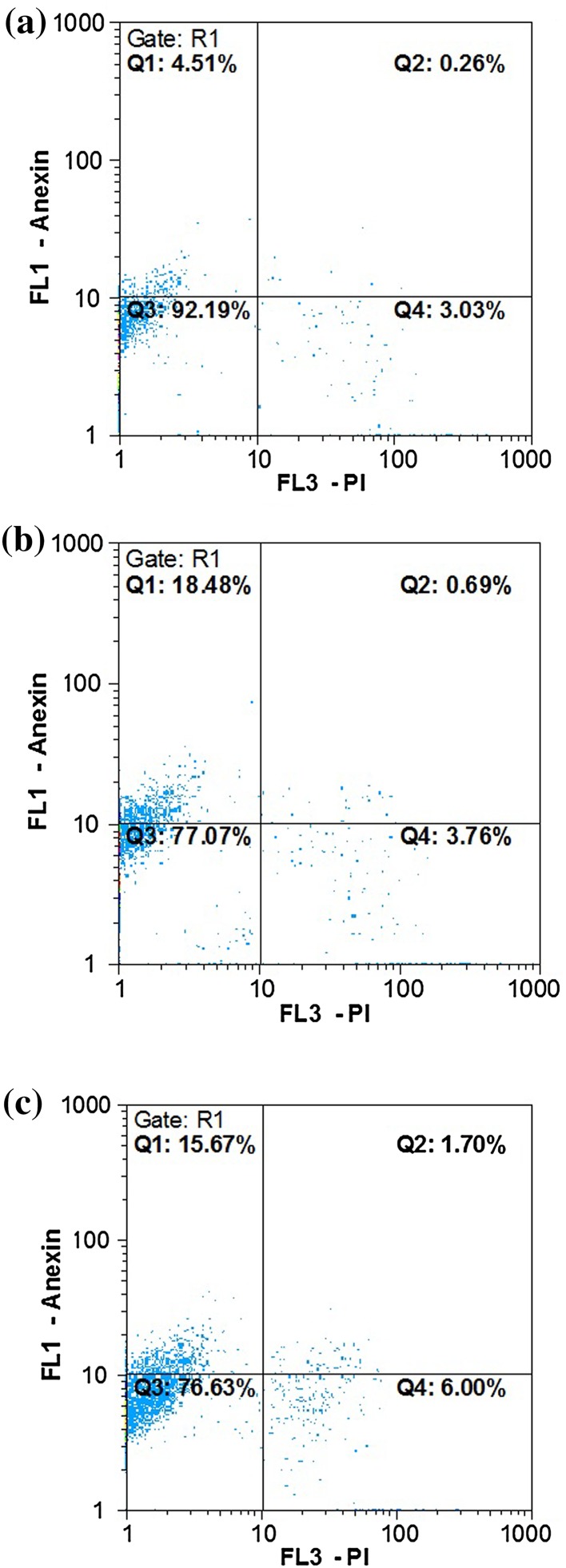
The translocation of phosphatidylserine (PS) to the outer cell membrane is a significant stage in the apoptosis pathway of cells. Annexin V binding is extremely specific to PS, which is expressed on the outer cell surface, while PI does not incorporate into the cell with the wholesome cell membrane. The effect of apoptosis and necrosis in cancer cells was tested for 24 h (incubation), as presented in Fig. 11. We hinted at the ability of T. vulgaris and TVAgNPs to induce apoptosis according to flow cytometry analysis. Apoptosis and necrosis were assessed via Annexin V coupled with FITC to determine apoptotic cells and PI to identify cell nuclei and then subjected to flow cytometry. Dot plots of annexin V/PI staining are depicted in Fig. 11a for untreated T47D cells. The T47D cells treated with IC50 concentration of fabricated TVAgNPs significantly exhibited 18.40% early apoptosis (Annexin V+/PI−) and showed 0.69% late apoptosis/dead cells (Annexin V+/PI+). Treatment of IC50 concentration of T. vulgaris extract showed 15.67% early apoptosis and 1.70% late apoptosis/dead cells (Fig. 11b, c). Flow cytometry analysis indicated that TVAgNPs could trigger translocation of PS from the inner membrane as compared to untreated cells indicating apoptosis pathway rather than necrosis.
Fig. 11.
Apoptosis induction assay by flow cytometry. Representative dot plots are shown where the percentage of viable cells, early apoptosis, late apoptosis and necrosis cells are evident for Untreated T47D cells (a) and after exposure to IC50 concentrations of TVAgNPs (b) and T. vulgaris extract (c)
Further investigations have to be done to clarify the nature of proliferation and apoptosis or necrosis of cells caused by TVAgNPs using T. vulgaris extract.
DPPH radical-scavenging assay
DPPH is a stable free radical and is commonly carried out to determine the radical-scavenging properties of anti-oxidant compounds. The IC50 values of T. vulgaris extract and TVAgNPs were 443 and 44.72 µg/mL, while that of ascorbic acid was 11.28 µg/mL. Similar results have been reported with improved DPPH radical-scavenging potential by different metal nanoparticles (Bhuvaneswari et al. 2016). Mohanta et al. (2017) studied the anti-oxidant potential of green synthesized AgNPs from Erythrina suberosa extract using a DPPH assay with IC50 of 30.04 µg/mL (Mohanta et al. 2017). It was suggested that the application of TVAgNPs as promising anti-oxidant agents was due to the existing phytochemical constituents of the T. vulgaris extract identified on the surface of nanoparticles. Moreover, during nanoparticle synthesis, the interaction of plant bioactive components with metal ions may result in enhanced free radical-scavenging compounds.
Conclusion
The current study reports the successful synthesis of silver nanoparticles via green synthesis as a fast, environmentally benign, and cost-effective method using T. vulgaris leaf extract. Owing to the capping and reducing agent of the main phytochemicals constituents present in the T. vulgaris leaf extract, a cap was formed around Ag+ of the bio-functionalized T. vulgaris AgNPs which was stable. The morphology and chemical compositions of the silver nanoparticles were characterized by various techniques, i.e., SEM, EDS, zeta potential, TEM, FTIR, and UV–Vis spectroscopy. The T. vulgaris extract and TVAgNPs were compared and demonstrated promising anti-cancer properties against T47D cells. Moreover, anti-oxidant activity of the TVAgNPs clarified a higher anti-radical-scavenging activity compared to Thymus vulgaris extract. Based on current data, use of such silver synthesized nanoparticles using the plant in future therapeutic systems is suggested.
Acknowledgements
The authors would like to acknowledge Islamic Azad University, Astaneh Ashrafiyeh Branch (Iran), for financial support of this research.
Compliance with ethical standards
Conflict of interest
All of the authors have declared that no competing interests exist.
References
- Adersh A, Ghosh S, More P, Chopade BA, Gandhi MN, Kulkarni AR. Surface defect rich ZnO quantum dots as antioxidant inhibiting α-amylase and α-glucosidase: a potential anti-diabetic nanomedicine. J Mater Chem B. 2015;3:4597–4606. doi: 10.1039/C5TB00407A. [DOI] [PubMed] [Google Scholar]
- Agili FA. Chemical composition, antioxidant and antitumor activity of Thymus vulgaris L. essential oil. Middle-East J Sci Res. 2014;21:1670–1676. [Google Scholar]
- Ahire M, Pardesi K, Bellare J, Dhavale DD, Jabgunde A, Balu A. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomed. 2012;7:483–496. doi: 10.2147/IJN.S24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010;11:652–666. doi: 10.2174/138945010791170923. [DOI] [PubMed] [Google Scholar]
- Ahmed KBA, Mahapatra SK, Raja MRC, Subramaniam S, Sengan M, Rajendran N, Das SK, Haldar K, Roy S, Sivasubramanian A, Anbazhagan V. Jacalin-capped silver nanoparticles minimize the dosage use of the anticancer drug, shikonin derivatives, against human chronic myeloid leukemia. Rsc Adv. 2016;6:18980–18989. doi: 10.1039/C5RA27952F. [DOI] [Google Scholar]
- Al-Shahrani MH, Mahfoud M, Anvarbatcha R, Athar MT, Al Asmari A. Evaluation of antifungal activity and cytotoxicity of Thymus vulgaris essential oil. Pharmacogn Commn. 2017;7:34–40. doi: 10.5530/pc.2017.1.5. [DOI] [Google Scholar]
- Arunachalam KD, Arun LB, Annamalai SK, Arunachalam AM. Potential anticancer properties of bioactive compounds of Gymnemasylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed. 2015;10:31–41. doi: 10.2147/IJN.S71182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgary V, Shoari A, Baghbani-arani F, Sadat Shandiz SA, Khosravi MS, Janani A, Bigdeli R, Bashar R, Ahangari Cohan R. Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. Int J Nanomdicine. 2016;11:3597–3605. doi: 10.2147/IJN.S109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atale N, Saxena S, Nirmala JG, Narendhirakannan RT, Mohanty S, Rani V. Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: a green approach. Appl Biochem Biotechnol. 2016;181:1140. doi: 10.1007/s12010-016-2274-6. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Waltenberger B, Wenzig EMP, Linder T, Wawrosch C, Uhrin P, Temml V. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji D, Basavaraja S, Deshpande R, Bedre M, Prabhakara B, Venkataraman A. Colloids Surf B. 2009;68:88. doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Colloids Sur A Physicochem Engin Asp. 2008;339:134. doi: 10.1016/j.colsurfa.2009.02.008. [DOI] [Google Scholar]
- Barabadi H, Ovais M, Khan Shinwari Z, Saravanan M. Anti-cancer green bionanomaterials: present status and future prospects. Green Chem Lett Rev. 2017;10:285–314. doi: 10.1080/17518253.2017.1385856. [DOI] [Google Scholar]
- Bhadra MP, Sreedhar B, Patra CR. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system) Theranostics. 2014;4:316–335. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari R, John Xavier R, Arumugam M (2016) Facile synthesis of multifunctional silver nanoparticles using mangrove plant Excoecaria agallocha L. for its antibacterial, J Parasit Dis 41:180 [DOI] [PMC free article] [PubMed]
- Du J, Singh THY. Antibacterial, anti-biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioprocess Biosyst Eng. 2016;39:1923–1931. doi: 10.1007/s00449-016-1666-x. [DOI] [PubMed] [Google Scholar]
- Emmanuel R, Saravanan M, Ovais M, Padmavathy S, Shinwari ZK, Prakash P. Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: a nanoantibiotic approach. Microb Pathog. 2017;113:295–302. doi: 10.1016/j.micpath.2017.10.055. [DOI] [PubMed] [Google Scholar]
- Fan K, Li X, Cao Y. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs. 2015;26:813–823. doi: 10.1097/CAD.0000000000000263. [DOI] [PubMed] [Google Scholar]
- Garg R, Kapur S, Hansh C. Radical toxicity of phenols: a reference point for obtaining perspective in the formulation of QSAR. Med Res Rev. 2001;21:73–82. doi: 10.1002/1098-1128(200101)21:1<73::AID-MED3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ghanbar F, Mirzaie A, Ashrafi F, Noorbazargan H, Dalirsaber Jalali M, Salehi S, Sadat Shandiz SAS. Antioxidant, antibacterial and anti-cancer properties of phyto-synthesized Artemisia quttensis Podlech extract mediated silver nanoparticles. IET Nanobiotechnol. 2017;11:485–492. doi: 10.1049/iet-nbt.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Gowri Sh, Arumugam A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostruct Chem. 2013;3:68. doi: 10.1186/2193-8865-3-68. [DOI] [Google Scholar]
- Inbathamizh L, Padmini E. Quinic acid as a potent drug candidate for prostate cancer—a comparative pharmacokinetic approach. Asian J Pharm Clin Res. 2013;6:106–112. [Google Scholar]
- Jeyaraj M, Sathishkumar G, Sivanandhan G, Mubarakali D, Rajesh M, Arun R, et al. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surf B. 2013;106:86–92. doi: 10.1016/j.colsurfb.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kim YS, Kim EK, Hwang JW, Jeong JH, Dong X, Lee JW, Moon SH, Jeon BT, Park PJ. Anticancer effect of thymol on AGS human gastric carcinoma cells. J Microbiol Biotechnol. 2016;26:28–37. doi: 10.4014/jmb.1506.06073. [DOI] [PubMed] [Google Scholar]
- Kasithevar M, Saravanan M, Prakash P, Kumar H, Ovais M, Barabadi H, Shinwari ZK. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J Interdiscip Nanomed. 2017;2:131–141. doi: 10.1002/jin2.26. [DOI] [Google Scholar]
- Khalilnezhad F, Torabi S, Larijany K, Khosrowshahli M. Nano silver particle synthesis using leaf extract of pharmaceutical plant Thymus vulgaris. Int J Biosci. 2015;6:192–196. doi: 10.12692/ijb/6.4.192-196. [DOI] [Google Scholar]
- Kotakadi S, Gaddam VA, Venkata SK, Parasad S, Gopal S. Ficus fruit-mediated biosynthesis of silver nanoparticles and their antibacterial activity against antibiotic resistant E. coli strains. Curr Nanosci. 2015;11:527–538. doi: 10.2174/1573413711666150126225951. [DOI] [Google Scholar]
- Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. Phys B Condensed Matter. 2001;299:142. doi: 10.1016/S0921-4526(00)00580-9. [DOI] [Google Scholar]
- Maksimovic Z, Stojanovic D, Sostaric I, Dajic Z, Ristic M. Composition and radical scavenging activity of Thymus glabrescens Willd. (Lamiaceae) essential oil. J Sci Food Agr. 2008;88:2036–2041. doi: 10.1002/jsfa.3311. [DOI] [Google Scholar]
- Mason C, Vivekanandhan S, Misra M, Mohanty AK. Switchgrass (Panicum virgatum) extract mediated green synthesis of silver nanoparticles. World J Nano Sci Engin. 2012;2:47–52. doi: 10.4236/wjnse.2012.22008. [DOI] [Google Scholar]
- Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, Mohanta TK (2017) Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.) Front Mol Biosci 4:14 [DOI] [PMC free article] [PubMed]
- Ovais M, Khalil TA, Reza A, Adeeb Khan M, Ahmad I, Ulislam N, et al. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine. 2016;11:3157–3177. doi: 10.2217/nnm-2016-0279. [DOI] [PubMed] [Google Scholar]
- Ovais M, Reza A, Naz S, Ul Islam N, Khalil AT, Ali S, Adeeb Khan M, Khan Shinwari Z. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl Microbiol Biotechnol. 2017;101:3551–3565. doi: 10.1007/s00253-017-8250-4. [DOI] [PubMed] [Google Scholar]
- Parameshwaran R, Kalaiselvamb S, Jayavel R. Green synthesis of silver Nanoparticles using Beta vulgaris: role of process conditions on size distribution and surface structure. Mater Chem Phys. 2013;140:135–147. doi: 10.1016/j.matchemphys.2013.03.012. [DOI] [Google Scholar]
- Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201:92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Ramteke Ch, Chakrabarti T, Sarangi BK, Pandy RA. Synthesis of silver nanoparticles from aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem. 2013 [Google Scholar]
- Roby MHH, Sarhan MA, Selim KH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod. 2013;43:827–831. doi: 10.1016/j.indcrop.2012.08.029. [DOI] [Google Scholar]
- Roopana SM, Rohita Madhumithaa G, Rahuman AA, Kamaraj C, Bharathi A, Surendraa TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod. 2013;43:631–635. doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- Sadat Shandiz SA, Shafiee Ardestani M, Shahbazzadeh D, Assadi A, Ahangari Cohan R, Asgary V, Salehi S. Novel imatinib loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Art Cells Nanomed Biotechnol. 2017;45:1082–1091. doi: 10.1080/21691401.2016.1202257. [DOI] [PubMed] [Google Scholar]
- Salehi S, Mirzaie A, Sadat Shandiz SA, Noorbazargan H, Rahimi A, Yarmohammadi S, Ashrafi F. Chemical composition, antioxidant, antibacterial and cytotoxic effects of Artemisia marschalliana Sprengel extract. Nat Prod Res. 2016;31:469. doi: 10.1080/14786419.2016.1174234. [DOI] [PubMed] [Google Scholar]
- Salehi S, Shandiz SAS, Ghanbar F, Darvish MR, Ardestani MS, Mirzaie A, Jafari M. Phyto-synthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial parts extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomed. 2016;11:1835–1846. doi: 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyavani K, Gurudeeban S, Ramanathan T, Balasubramanian T. Toxicity study of silver nanoparticle synthesis from suaeda monoica on Hep-2 Cell Line. Avicenna J Med Biotech. 2012;4:35–39. [PMC free article] [PubMed] [Google Scholar]
- Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, Pandey A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009;44:939–943. doi: 10.1016/j.procbio.2009.04.009. [DOI] [Google Scholar]
- Shenya DS, Mathewa J, Philip D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardiumocci dentale. Spectrochim Acta Part A. 2011;79:254–262. doi: 10.1016/j.saa.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M, Balajee R, Barabadi H. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017;11:965–972. doi: 10.1049/iet-nbt.2016.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira B, Marques A, Ramos SC, Serrano C, Matos O, Nenq NR, Nogueira JM, Saraiva JA, Nunes ML. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric. 2013;93:2707–2714. doi: 10.1002/jsfa.6089. [DOI] [PubMed] [Google Scholar]
- Yin H, Yamamoto T, Wada Y, Yanagida SH. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Mater Chem Phys. 2004;83:66–70. doi: 10.1016/j.matchemphys.2003.09.006. [DOI] [Google Scholar]
- Zayed MF, Eisa WH, Shabaka AA. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2012;98:423–428. doi: 10.1016/j.saa.2012.08.072. [DOI] [PubMed] [Google Scholar]