ABSTRACT
Reactive oxygen species such as peroxides play an important role in plant development, cell wall maturation, and defense responses. During nodulation with the host plant Medicago sativa, Sinorhizobium meliloti cells are exposed to H2O2 in infection threads and developing nodules (R. Santos, D. Hérouart, S. Sigaud, D. Touati, and A. Puppo, Mol Plant Microbe Interact 14:86–89, 2001, https://doi.org/10.1094/MPMI.2001.14.1.86). S. meliloti cells likely also experience oxidative stress, from both internal and external sources, during life in the soil. Here, we present microarray transcription data for S. meliloti wild-type cells compared to a mutant deficient in the key oxidative regulatory protein OxyR, each in response to H2O2 treatment. Several alternative sigma factor genes are upregulated in the response to H2O2; the stress sigma gene rpoE2 shows OxyR-dependent induction by H2O2, while rpoH1 expression is induced by H2O2 irrespective of the oxyR genotype. The activity of the RpoE2 sigma factor in turn causes increased expression of two more sigma factor genes, rpoE5 and rpoH2. Strains with deletions of rpoH1 showed improved survival in H2O2 as well as increased levels of oxyR and total catalase expression. These results imply that ΔrpoH1 strains are primed to deal with oxidative stress. This work presents a global view of S. meliloti gene expression changes, and of regulation of those changes, in response to H2O2.
IMPORTANCE Like all aerobic organisms, the symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti experiences oxidative stress throughout its complex life cycle. This report describes the global transcriptional changes that S. meliloti makes in response to H2O2 and the roles of the OxyR transcriptional regulator and the RpoH1 sigma factor in regulating those changes. By understanding the complex regulatory response of S. meliloti to oxidative stress, we may further understand the role that reactive oxygen species play as both stressors and potential signals during symbiosis.
KEYWORDS: OxyR, RpoH, Sinorhizobium meliloti, catalase, oxidative stress, sigma factors, transcriptome
INTRODUCTION
Reactive oxygen species (ROS) are omnipresent for aerobic organisms. Enzyme function and the imperfect transfer of electrons to oxygen during aerobic respiration contribute to intracellular production of ROS (reviewed in reference 1). Bacteria also encounter environmental sources of ROS. Soil bacteria might experience ROS from redox-cycling compounds secreted by neighboring cells or from exposure to metals and other compounds in the environment (2).
The majority of the toxicity of ROS such as superoxide and H2O2 does not appear to arise from direct damage to amino acids, lipids, or nucleic acids. Rather, damage is primarily caused by inactivation of iron cofactors in proteins and, through production of other radicals, by damage to DNA (reviewed in reference 3). For example, H2O2 reacts with Fe2+ via the Fenton reaction to produce hydroxyl radicals, resulting in severe DNA damage (4).
As a result of the ubiquity of ROS in aerobic life, numerous cellular defenses against oxidative stress, including those represented by catalases, peroxidases, glutathione, and ascorbate, have developed (2). Replacement and repair of iron-sulfur clusters, increased expression of alternative proteins utilizing different metal cofactors, and downregulation of metabolism are some additional mechanisms used to survive H2O2-dependent damage (5, 6). Similarly, regulation of iron transport to limit exposure of iron to H2O2 is a common response to ROS stress. Increased expression of genes that encode DNA repair proteins is also a common response to oxidative stress (2).
The symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti exhibits a complex lifestyle: it undergoes free-living growth as a soil saprophyte, but it also elicits and inhabits root nodules of legumes of Medicago spp. S. meliloti is exposed to ROS in both of these environments (7–11). Plants use H2O2 as a signal and may transport extracellular H2O2 into adjacent cells to facilitate signaling (12). ROS play a major role in cell wall formation and in defense reactions in plants (13, 14). Additionally, early events in symbiosis are characterized by suppression followed by enhancement of ROS production in the host plant (11, 15, 16).
As enzymatic defenses against ROS, S. meliloti is predicted to have two monofunctional catalases, KatA (encoded on the chromosome) and KatC (encoded on pSymB), a biofunctional catalase-hydroperoxidase, KatB (encoded on pSymA), and a predicted ahpC-type alkyl hydroperoxidase, SMb20964 (17). These catalases are expressed in distinct environmental conditions and at specific stages in symbiosis. In culture, the katA gene is expressed in exponential growth whereas katC is expressed in late stationary phase (18). The genes also respond in various ways to heat shock, osmotic shock, and acid shock and are differentially expressed in nodulation. Individual katA or katC mutations have no impact on nodulation, but double mutants show lowered nitrogen fixation (18, 19). Among these catalases, KatA is the only one whose expression is increased in response to the presence of H2O2, and its expression is dependent on the OxyR transcriptional regulator (19, 20).
In Escherichia coli and other proteobacteria, OxyR is the primary transcriptional regulator of the H2O2 stress response (21, 22). OxyR directly senses the redox status in the cell (23, 24). When oxidative stress levels are low, OxyR is in a reduced form that allows it to bind to two adjacent major grooves in DNA, preventing RNA polymerase (RNAP) from recognizing a promoter. When levels of oxidative stress increase, disulfide bridges form within the OxyR protein, leading to a conformational change (23, 24) that allows OxyR to shift its binding upstream to four adjacent major grooves in DNA and to directly interact with RNAP to activate transcription of target genes (25).
In E. coli, OxyR regulates the transcription of at least 40 genes (21), including genes involved in reducing levels of ROS in the cell (encoding catalase, alkyl hydroperoxidase, and glutaredoxin 1), genes whose effect is to minimize free iron and diminish DNA damage (such as genes encoding iron-binding proteins and fur, encoding a transcription repressor of iron import functions), and genes involved in protein repair (encoding proteins with functions such as Fe-S cluster assembly and chaperone regulation) (5, 21, 22). The sequence of OxyR is well conserved in proteobacterial species (26). S. meliloti and E. coli share the same genomic context of oxyR in that oxyR is divergently transcribed from katA (27). In E. coli, OxyR negatively regulates its own expression and positively regulates the expression of the bifunctional catalase/peroxidase gene katG, which is induced by H2O2 (28). Similarly, S. meliloti OxyR negatively regulates its own expression. Unlike E. coli, however, S. meliloti OxyR both represses and activates katA expression depending on its oxidation state (27). oxyR is specifically expressed during symbiosis in the nitrogen-fixing zone (27). While ROS appears to play a significant role under free-living and symbiotic conditions, the S. meliloti transcriptional response to H2O2 has not as yet been reported.
One way that bacteria can express large-scale transcriptional change is through the use of alternative sigma factors (σs) (29–32). Sigma factors are subunits of RNAP necessary for promoter recognition and transcription initiation. Under normal conditions, a housekeeping σ (sigma70, encoded by rpoD) recognizes and drives transcription of a large number of genes. Alternative σs recognize distinct promoter sequences, generally driving transcription of genes of shared function. To change the cell's transcriptional program, alternative σs compete with the housekeeping σ for core RNAP. Changing the availability of alternative σs to interact with RNAP regulates which promoters are recognized and thus which genes are expressed (reviewed in reference 33).
Bacteria that engage in complex lifestyles tend to have greater numbers of alternative sigma factors than those with simpler lifestyles (34). Fourteen alternative σs are encoded in the S. meliloti genome; this abundance is consistent with the idea that S. meliloti experiences a variety of different environments and stresses in the soil and during nodulation. However, the role of alternative σs during different life stages and under different stress conditions in S. meliloti is not well known and is an active area of research (35–40).
RpoE2 is an extracytoplasmic function-type (ECF-type) σ thought to function as the general stress response sigma factor (36). While RpoE2 is not required for symbiosis, expression of rpoE2 is increased in response to many different stresses, including stationary-phase growth, heat shock, osmotic stress, and microaerobic stress (36, 37, 41, 42). RpoE2 directly or indirectly controls 346 genes (39), including katC and alternative sigma factor genes rpoE5 and rpoH2 (36, 43).
The RpoH1 σ regulates genes in response to heat shock, stationary phase (37, 40), and pH stress (38) and is required for symbiosis (40, 44, 45). Although both RpoE2 and RpoH1 respond to stress, the two regulons appear to be largely independent, with few overlapping genes (37).
To understand more fully the responses of S. meliloti to H2O2, we performed whole-genome transcriptional analysis of wild-type (WT) and oxyR mutant S. meliloti strains exposed to two concentrations of H2O2. We also explored the regulation of the H2O2 response using S. meliloti strains with deletions of alternative σ genes. This allowed us to define the S. meliloti transcriptional response to oxidative stress induced by H2O2, to show its dependence on OxyR, and to reveal the role of alternative σ RpoH1 in regulating that response.
RESULTS
H2O2 induces a large number of transcriptional changes in S. meliloti.
Expression of katA is induced by H2O2 and correlates with oxidative stress levels (20). In order to define the response of S. meliloti to H2O2 challenge, whole-genome transcriptional profiling was performed. All experiments were done in a background of S. meliloti CL150, a WT derivative of strain Rm1021, corrected for the function of anti-sigma factor EcfR1 and high-affinity phosphate transporter PstC (39). We chose this strain in case ECF sigma factor RpoE1 played a role in the ROS response. Additionally, we used a katA::uidA transcriptional fusion to ascertain the conditions with maximal induction of katA expression. Comparing expression of katA in the CL150 background to that occurring in the WT Rm1021 background, we saw that the basal expression of katA in the CL150 background was nearly half that in the WT Rm1021 background. With these lower basal levels of katA expression, CL150 showed a nearly 4-fold-larger increase in katA expression after H2O2 exposure (data not shown). The initial conditions used for transcriptome profiling were mid-exponential-phase S. meliloti CL150 (optical density at 600 nm [OD600] = 0.3 to 0.5) grown in rich medium (TY) treated with 1 mM H2O2 for 30 min. These were the time and concentration that showed maximum expression of katA using the katA::uidA fusion (data not shown).
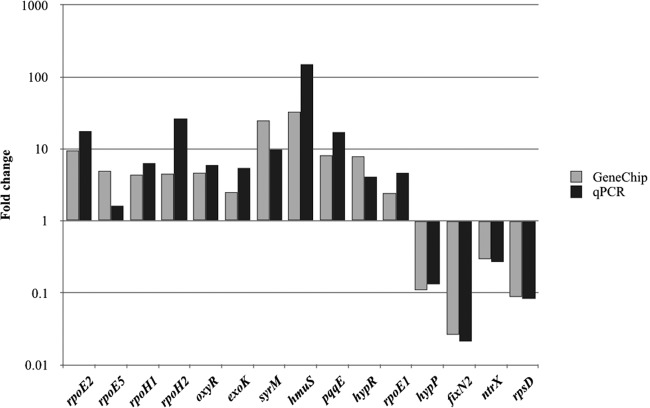
In total, 1,370 genes were differentially regulated by H2O2: 789 genes were upregulated (see Table S1 in the supplemental material), and 581 genes were downregulated (Table S2). Fifteen genes were selected for testing differential expression by reverse transcription-PCR (RT-qPCR) to confirm the Affymetrix GeneChip results (Fig. 1). All of these genes showed similar expression changes in tests using RT-qPCR and Affymetrix GeneChip.
FIG 1.
Comparison qPCR and chip expression data. Data represent fold changes in gene expression of selected genes differentially regulated by the use of 1 mM H2O2 as determined in three replicates each using Affymetrix GeneChip and qPCR. Raw data and statistical analysis are shown in Table S4.
The tripartite S. meliloti genome (6.69 Mb in size) is composed of a 3.65-Mb chromosome (55%), 1.35-Mb pSymA (20%), and 1.68-Mb pSymB (25%). While genes whose expression was increased in response to H2O2 were distributed fairly evenly (chromosome, 60%; pSymA, 20%; pSymB, 20%), those whose expression was decreased were biased toward the chromosome (93%), with only 1% on pSymA and 6% on pSymB. This strong chromosomal bias of downregulated genes corresponds broadly to decreased expression of genes encoding housekeeping functions. For example, expression of the gene set encoding translation machinery was sharply downregulated in response to H2O2 (Table 1). As expected, several genes whose expression was upregulated in response to H2O2 have known antioxidant activities (Table 1).
TABLE 1.
Functional categories of genes differentially regulated by H2O2a
| Gene category | No. of genes with: |
|
|---|---|---|
| Increased expression | Decreased expression | |
| Amino acid transport and metabolism | 26 | 44 |
| Carbohydrate transport and metabolism | 5 | 15 |
| Cell cycle control, mitosis, and meiosis | 2 | 2 |
| Cell motility | 0 | 2 |
| Cell wall/membrane biogenesis | 13 | 11 |
| Coenzyme transport and metabolism | 7 | 7 |
| Energy production and conversion | 32 | 32 |
| Function unknown | 3 | 4 |
| Hypothetical protein | 129 | 45 |
| Inorganic ion transport and metabolism | 16 | 5 |
| Lipid transport and metabolism | 14 | 12 |
| Nitrogen regulation | 1 | 0 |
| Nucleotide transport and metabolism | 7 | 28 |
| Posttranslational modification, protein turnover, chaperones | 17 | 13 |
| Replication, recombination, and repair | 26 | 9 |
| RNA | 0 | 15 |
| Secondary metabolite biosynthesis, transport, and catabolism | 9 | 4 |
| Secretion | 0 | 8 |
| Signal transduction mechanisms | 9 | 4 |
| Stress | 3 | 0 |
| Toxin | 0 | 1 |
| Transcription | 36 | 11 |
| Translation | 3 | 86 |
Functional gene categories were determined using clusters of orthologous groups. Categories were refined when possible based on reported gene functions.
The OxyR regulon in S. meliloti.
To delineate the part of the S. meliloti H2O2 response that requires OxyR, an insertion mutant in oxyR was generated. An S. meliloti mutant in oxyR (in strain Rm1021) was previously reported to be more sensitive to H2O2 (27, 46). To determine optimal conditions for transcriptional analysis of the CL150 oxyR mutant strain, its survival was tested upon challenge with various concentrations of H2O2 (data not shown). Since mid-exponential-phase wild-type and oxyR mutant cells showed similar levels of survival after a 30-min exposure to 0.5 mM H2O2, this nonlethal level of H2O2 was used for direct comparison of these strains via Affymetrix GeneChip analysis.
When WT cells were exposed to 0.5 mM H2O2, 396 genes showed increased expression and 420 showed decreased expression. Most of these genes (358 whose expression was upregulated and 358 whose expression was downregulated) were also found in the set of genes differentially regulated by exposure to 1 mM H2O2 (Fig. 2). The oxyR mutant, on the other hand, showed increased expression of 236 genes, and the expression of 300 genes was decreased by H2O2 treatment. The majority of these genes were also differentially regulated by H2O2 in wild-type S. meliloti (Fig. 2); thus, while these genes are differentially expressed in response to H2O2 exposure, that expression is not dependent on OxyR.
FIG 2.
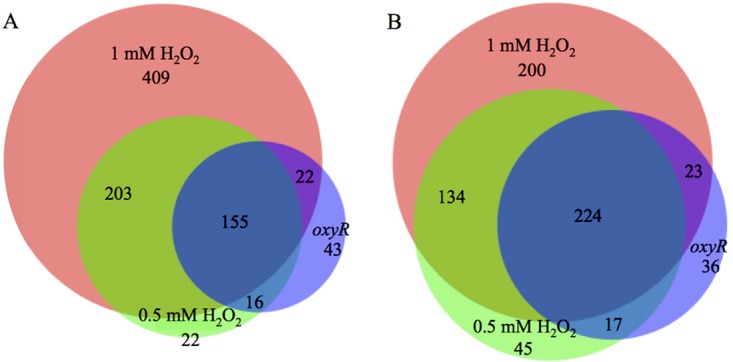
Overlap of genes differentially regulated in response to two concentrations of H2O2 and in the oxyR mutant. (A) Genes upregulated in response to H2O2 in wild-type CL150 (treated with 1 mM or 0.5 mM H2O2) and in the oxyR mutant (treated with 0.5 mM H2O2). (B) Genes downregulated in response to H2O2 in wild-type CL150 (treated with 1 mM or 0.5 mM H2O2) and in the oxyR mutant (treated with 0.5 mM H2O2).
OxyR is required for part of the response to H2O2.
OxyR-dependent genes were defined by comparing genes differentially regulated in the wild-type strain (after treatment with both 1 mM and 0.5 mM H2O2) but not in the oxyR mutant (Table S3). In total, transcription of 203 genes (57% of the genes upregulated by both 1 mM and 0.5 mM H2O2) was increased after H2O2 addition only in wild-type S. meliloti, and expression of 134 (37% of the genes downregulated by both levels of H2O2) genes was decreased. These regulatory changes are presumed to be directly or indirectly dependent on OxyR.
rpoE2 expression was induced by H2O2 in an OxyR-dependent manner, but expression of rpoH1 was increased irrespective of the oxyR genotype. Expression levels for several genes encoding antioxidant functions were increased in response to H2O2, and the changes were dependent on wild-type oxyR. The products encoded by those genes included SMb20964, the ahpC-type alkyl hydroperoxidase, and three other catalases and peroxidases (Table 2 and Table S3). Increased expression of katA and oxyR also depended on WT OxyR. This serves as a useful internal control, since OxyR is reported to downregulate its own expression and that of katA. In an oxyR mutant (27), the repression of katA and oxyR is relieved. Our interpretation is that expression of these two genes in the oxyR mutant is higher prior to addition of H2O2 such that any further increase in expression in response to H2O2 is not significant.
TABLE 2.
Representative genes regulated by H2O2
| Gene | Designation | Description of product | SLR |
||
|---|---|---|---|---|---|
| 1 mM CL150 | 0.5 mM CL150 | 0.5 mM oxyR mutant | |||
| Antioxidants and regulators | |||||
| katA | SMc00819 | Catalase | 8.7 | 6.16 | |
| cpo | SMc01944 | Nonheme chloroperoxidase F (chloride peroxidase; CPO-F) | 4.46 | 2.25 | |
| SMb20054 | Chloride peroxidase | 4.22 | 1.95 | ||
| SMb20964 | Peroxidase, AhpC family | 2.18 | 1.5 | 0.65 | |
| oxyR | SMc00818 | Regulator of kat genes | 2.23 | 3.38 | |
| Sigma factors | |||||
| rpoH1 | SMc00646 | RNA polymerase sigma factor (sigma-32) | 2.15 | 1.61 | 1.37 |
| rpoE2 | SMc01506 | RNA polymerase sigma-E factor (sigma-24) | 3.27 | 1.91 | |
| rpoH2 | SMc03873 | RNA polymerase sigma factor (sigma-32) | 2.19 | ||
| rpoE5 | SMb21484 | Putative RNA polymerase sigma-E factor (sigma-24) protein | 2.3 | ||
| Degradation | |||||
| paaA | SMb21640 | Putative phenylacetic acid degradation protein | 3.75 | 4.09 | 2.4 |
| paaB | SMb21639 | Putative phenylacetic acid degradation protein | 6.28 | 4.84 | 4.85 |
| Manganese regulation | |||||
| sitA | SMc02509 | Manganese ABC transporter periplasmic substrate binding protein | 2.01 | 2.31 | 1.62 |
| sitB | SMc02508 | Manganese ABC transporter ATPase | 2.56 | 2.64 | 1.81 |
| sitC | SMc02507 | Manganese ABC transporter permease | 2.29 | 2.62 | 1.82 |
| sitD | SMc02506 | Manganese ABC transporter permease | 2.3 | 2.57 | 1.85 |
| Miscellaneous | |||||
| exoP | SMb20961 | Protein tyrosine kinase, involved in succinoglycan chain-length determination | 1.97 | ||
| glgA1 | SMc03924 | Probable glycogen synthase (starch [bacterial glycogen] synthase) protein | 1.09 | ||
| SMc01113 | Conserved hypothetical protein | 1.01 | 0.99 | ||
A ΔrpoH1 mutant shows increased survival in H2O2.
Expression levels for the alternative sigma factor genes rpoH1, rpoH2, rpoE2, and rpoE5 are increased in response to H2O2 (Table 2). To see if these genes play a functional role in the cell's response to H2O2, S. meliloti mutants with complete deletions of these alternative sigma factor genes were tested for survival after a 30-min exposure to 1 mM H2O2. To ensure that the anti-sigma factors that regulate RpoE2 and RpoE5 did not interfere with the activity of other ECF sigma factors in the cell, deletion constructs for rpoE2 and rpoE5 also included deletion of the putative cognate anti-sigma factor gene. The rpoE2 and rpoE5 deletion mutants tended to survive exposure to H2O2 less well than the wild-type strain, but this difference was not significant in three independent experiments (Fig. 3 and data not shown). However, a mutant carrying a complete deletion of rpoH1 (ΔrpoH1 and ΔrpoH1H2) showed a slight but significant improvement in H2O2 survival compared to the wild-type strain (Fig. 3).
FIG 3.
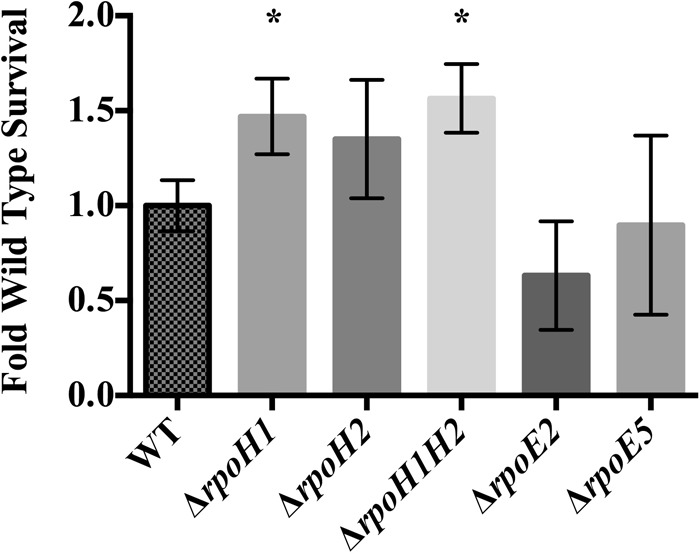
Effect of H2O2 on S. meliloti mutants with complete deletions of alternative sigma factors. Survival rates were determined after 30 min of exposure to H2O2 of S. meliloti strains deleted for alternative sigma factors implicated in the response to H2O2. Survival was normalized to wild-type CL150 survival (10.3% ± 1.6%). Error bars indicate standard deviations; *, P ≤ 0.05. Experiments were performed in triplicate; two other independent experiments were performed with comparable results.
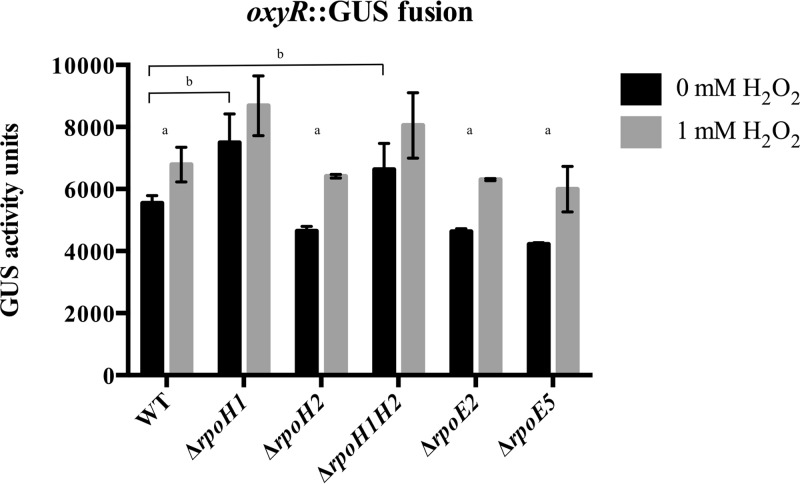
Baseline oxyR expression is increased in ΔrpoH mutants.
One possible explanation for the improved survival of the ΔrpoH1 mutant upon H2O2 treatment is higher expression of genes that protect against oxidative stress. To test if the levels of expression of oxyR differed in these mutants, a uidA transcriptional fusion to oxyR was generated and inserted into the alternative sigma factor deletion strains. Baseline expression of oxyR was higher in both rpoH1 deletion mutants. Figure 4 shows that transcription of oxyR increased significantly in the wild-type strain and in the ΔrpoE2 and ΔrpoE5 mutants after exposure to H2O2. In contrast, oxyR expression levels did not increase significantly above the already elevated baseline in the ΔrpoH1 and ΔrpoH1H2 mutants.
FIG 4.
Transcription of oxyR in various S. meliloti mutants deleted for alternative sigma factors. Data represent expression of a oxyR-uidA transcriptional fusion with and without 30 min of treatment with 1 mM H2O2 in mutants lacking various S. meliloti alternative sigma factors. Error bars indicate standard deviations. “a” indicates a P value of ≤0.05 compared to 0 mM H2O2. “b” indicates a P value of ≤0.05 comparing the activity of the mutant strain at 0 mM H2O2 to the activity of the wild-type strain at 0 mM H2O2. Experiments were performed in triplicate.
Total catalase activity is increased in a ΔrpoH1 mutant.
To check if increased expression of oxyR in the sigma factor deletion mutants resulted in increased antioxidant activity, total catalase activity was tested using Amplex Red, a stable, highly sensitive probe for H2O2. As expected, exposure to H2O2 increased overall catalase activity in all mutants tested. Similarly to the pattern observed with oxyR expression, ΔrpoH1 and ΔrpoH1H2 mutants had higher catalase activity before exposure to H2O2 (Fig. 5B). Catalase expression levels were similar whether normalized to OD600 or to total cell protein (data not shown).
FIG 5.
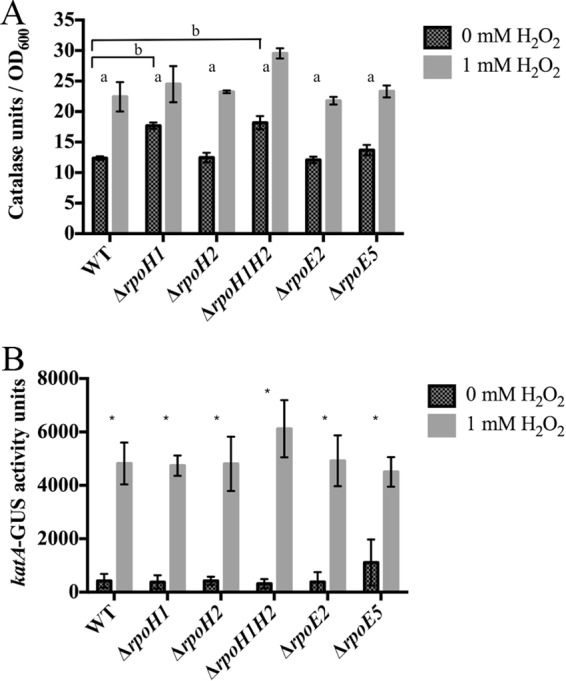
Catalase activity in S. meliloti stains deleted for alternative sigma factors. (A) Total catalase activity of various alternative sigma factor deletions with and without 30 min of treatment with 1 mM H2O2. Catalase activity was normalized to OD600. Error bars indicate standard deviations. “a” indicates a P value of ≤0.05 compared to 0 mM H2O2. “b” indicates a P value of ≤0.05 comparing the activity of the mutant strain at 0 mM H2O2 to the activity of the wild-type strain at 0 mM H2O2. Experiments were performed in triplicate. (B) Activity of katA-uidA transcriptional fusion with and without 30 min of treatment with 1 mM H2O2. Error bars indicate standard deviations. *, P ≤ 0.05 (comparing the activity of the mutant strain at 0 mM H2O2 to the activity of the wild-type strain at 0 mM H2O2). Experiments were performed in triplicate.
The S. meliloti genome includes three catalase genes (katA, katB, and katC) as well as at least one ahpC-type alkyl hydroperoxidase (SMb20964). To determine whether transcription of katA was responsible for the increased enzyme activity in the ΔrpoH1 and ΔrpoH1H2 mutants, we generated and tested katA-uidA transcriptional fusions. We observed no significant difference in katA expression levels in any of the sigma factor deletion strains with or without H2O2 treatment (Fig. 5B). RT-qPCR was used to test expression of the other catalases, using primers specific for katB, katC, and SMb20964. Expression of these catalase/peroxidase genes was not significantly changed in any strain (data not shown).
DISCUSSION
S. meliloti responds to H2O2 stress with differential expression of over 600 genes, a set that includes both expected and novel candidates. We defined the relationship of this gene set with transcription control by the LysR-type regulator, OxyR, and discovered an OxyR-independent connection between the RpoH1 sigma factor and response to oxidative stress.
We tested only a limited subset of oxidative stress conditions (using a single oxidant, one time point, and just two concentrations). However, despite these limitations, this work agrees well with other studies characterizing the S. meliloti response to ROS. For example, three genes upregulated by H2O2 in our data set (exoP, glgA1, and sitA) correspond to H2O2-sensitive mutants discovered by Davies and Walker (47); these three mutants were also defective in symbiosis. Our upregulated gene set also included a gene encoding a secreted peroxidase (SMc01944) and a gene of unknown function (SMc01113) whose expression has been previously shown to be induced by H2O2 (48, 49).
Role of OxyR.
Genes that were upregulated in response to H2O2 in wild-type S. meliloti but not in the oxyR mutant (57% of the genes generally upregulated by H2O2) are presumed to be directly or indirectly dependent on the OxyR transcription factor. The OxyR-dependent transcripts are distributed proportionally across all three replicons of the S. meliloti genome and include some functions expected as part of an oxidative stress response. The monofunctional catalase gene (katA) was previously reported to be divergently transcribed from and dependent on oxyR (20, 27). In our data, katA was the transcript most strongly upregulated in wild-type cells in response to H2O2 exposure, and that upregulation was dependent on OxyR. In E. coli, AhpC, an alkyl hydroperoxide reductase, is a primary antioxidant expressed in response to H2O2, and its expression is induced in an OxyR-dependent manner (1, 21). We found that expression of the putative S. meliloti ortholog SMb20964 is likewise induced by H2O2 and dependent on OxyR. Similarly, the H2O2-induced increase in the levels of transcripts for genes encoding the stress-associated ECF-type sigma factor RpoE2 (36) showed dependence on OxyR. This study did not address which transcripts are directly or indirectly regulated via OxyR. Future work may be able to elucidate the more direct role of OxyR in the S. meliloti transcriptional response to H2O2.
Numerous genes were differentially regulated by H2O2 independently of OxyR, including those involved in manganese transport (sitABCD) and those predicted to be involved in phenylacetic acid degradation (paaA and paaB genes). The S. meliloti sit genes are among those that have already been found to be involved in ROS resistance (50). Genes in the paa operon are differentially expressed during nodulation (51, 52) and are part of the general stress response regulon mediated by RpoE2 (36). Expression of rpoE2 itself appears to be OxyR dependent. However, H2O2-induced expression of rpoH1 and of RpoH1-dependent genes still occurs in the oxyR mutant strain. This implies that H2O2 stress is sensed and transduced to RpoH1 through some mechanism that is independent of OxyR.
Sigma factors.
Because alternative sigma factors change the recognition of promoters, the consequent changes in gene expression are often numerous. Upregulation of the two alternative sigma factor genes rpoH1 and rpoE2 in response to H2O2 may reflect a need for broad changes in gene expression. Flechard et al. (43) reported that RpoE2 activity was relevant to ROS stress in stationary-phase cells. While 1 mM H2O2 induced expression of four alternative sigma factor genes (rpoH1, rpoH2, rpoE2, and rpoE5), only deletion of rpoH1 had an effect on survival in 1 mM H2O2. While deletion of rpoE2 and rpoE5 did seem to reduce survival in 1 mM H2O2, this reduction was not significant. It is possible that these deletion mutants are indeed more sensitive to either other ROS or different concentrations of H2O2. Investigating the role of RpoE2 and RpoE5 in the S. meliloti ROS response may be a fruitful area for future work.
Because of the evidence showing a positive role for RpoH in gene expression related to stress resistance (37) and because Martínez-Salazar et al. (53) showed that Rhizobium etli rpoH mutants are more sensitive to H2O2, we were surprised that deletion of rpoH1 in S. meliloti resulted in increased survival after H2O2 challenge. The increase in the level of survival was slight, but significant, and was similar to the improved levels of survival seen when wild-type S. meliloti cells were pretreated with low concentrations of H2O2.
Strains lacking rpoH1 may experience higher levels of oxidative stress than wild-type S. meliloti under normal growth conditions because they do not express baseline defenses against endogenous stresses (i.e., under conditions without imposed external stress). Consequently, this postulated higher degree of stress may “prime” the cells to deal more effectively with later increased levels of external H2O2. The idea of priming is consistent with the observed higher levels of both oxyR expression and catalase activity in ΔrpoH1 strains than in the wild-type strain under normal culture conditions. Perhaps more OxyR is in an oxidized state and oxyR expression is derepressed in strains lacking rpoH1 (46). We have shown that OxyR regulates, directly or indirectly, almost half of the response to H2O2 in S. meliloti, and some of these genes may be responsible for improved survival of ΔrpoH1 mutants in H2O2.
Interaction of RpoH1 with ROS stress responses.
The higher total catalase activity seen in the rpoH1 deletion mutant correlates with higher oxyR expression. Which of the three known S. meliloti catalases might be responsible for the increased activity is uncertain. OxyR regulates expression of katA and katB (46), and katB may also be regulated by ActR/ActS. The katC gene is primarily expressed during the stationary phase (18), and its expression is also RpoE2 dependent. Disruption of OxyR function in S. meliloti leads to higher levels of expression of katA and katB but lower overall catalase activity (46), indicating that OxyR positively influences the activity of other catalases in the cell.
Expression of SMb20964, the ahpC-type alkyl hydroperoxidase, also appears to be unchanged in these sigma factor deletion mutants. We found expression of this gene to be increased by exposure to H2O2 in an OxyR-dependent manner, while katA was the only annotated catalase gene upregulated in response to H2O2 (also in an OxyR-dependent manner). katB and katC were not differentially regulated by H2O2 in either the wild-type strain or the oxyR mutant. RT-qPCR detection of gene expression in S. meliloti mutants after exposure to H2O2 showed that SMb20964 expression does increase in response to H2O2, and there were similar levels of increased expression after H2O2 treatment in all strain backgrounds. Perhaps higher levels of OxyR, as found in ΔrpoH1 and ΔrpoH1H2 mutants, increase total catalase activity either by acting on other, unknown catalases or peroxidases or by a combination of small increases in the activities of all catalases. Future work will be needed to differentiate between these possibilities (or to identify additional causes of this increased basal catalase activity).
While RpoH1-dependent genes corresponding to heat shock, stationary phase, and acid stress have been defined previously (37, 38, 40), additional RpoH1-dependent genes may be activated during H2O2 stress. Based on the increased survival of ΔrpoH1 mutants seen under conditions of H2O2 challenge, it seems possible that wild-type cells express some unknown RpoH1-dependent genes at low levels prior to encountering any external source of stress. When this low background expression of presumed RpoH1-dependent genes is missing (as in the case of the ΔrpoH1 mutant), the cells might lack some of the defenses against endogenously produced ROS, leading to a higher basal level of ROS stress even in the absence of environmentally introduced ROS. This, in turn, might lead to activation of ROS responses that normally would be expressed only after exposure to higher levels of ROS. In sum, we infer that when cells are missing RpoH1-dependent responses, both oxyR expression and catalase activity increase. Future work will be needed to confirm this hypothesis and to identify which, if any, RpoH1-dependent genes are responsible for the improved survival of the ΔrpoH1 mutant in H2O2.
Transcriptional profiling of an rpoH1H2 double mutant showed some genes with increased expression in the mutant, prominently including predicted transporters, other membrane proteins, and enzymes for small-molecule metabolism. de Lucena et al. (38) reported that S. meliloti rpoH1 mutants cultured under nonstressed conditions display an increase in rhizobactin synthesis gene expression compared to the wild type, as measured by hybridization to spotted arrays and by rhizobactin production. Our data determined using the Affymetrix GeneChip employed here do not show a rhizobactin expression increase for a double ΔrpoH1 ΔrpoH2 mutant under heat shock or nonstressed conditions, similar to results found in other rpoH1H2 mutants (37; M. J. Barnett and S. R. Long, unpublished data). This difference may arise from the differing characteristics of the two genotypes or from possible differences in conditions. It is not apparent from the transcription profiles alone which might be the genes responsible for improved tolerance of peroxide treatment.
This analysis of the transcriptional changes induced by H2O2 in S. meliloti reveals connections to the major regulator OxyR and points to a role for RpoH1 in maintaining the redox status of the cell. We see these connecting circuits as clues to the important and complex role that oxidative stress plays in the soil and endosymbiotic lives of S. meliloti. It is possible that the presence of reactive oxygen species represents both an environmental stress to contend with and also a key signal element that helps determine bacterial fate.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains are described in Table 3. LB or tryptone-yeast extract (TY) medium was used for bacterial growth (58). The following antibiotics were used: streptomycin (Sm; 500 μg/ml), tetracycline (Tet; 10 μg/ml), and hygromycin (Hy; 50 μg/ml). For Affymetrix GeneChip assays, S. meliloti bacteria were grown in triplicate at 30°C in liquid TY medium without antibiotics to mid-exponential phase (OD600 = 0.3 to 0.4). Cultures were split, and H2O2 was added to half of the cultures to reach a final concentration of either 1 mM or 0.5 mM. At 30 min after H2O2 addition, cells were harvested as described previously (51). β-Glucuronidase (GUS) assays were performed in triplicate as described previously (59).
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR | 54 |
| E. coli MT616 | MM294A pRK600 (Cmr) | 55 |
| S. meliloti CL150 | Wild type (Smr, corrected ecfR1 and pstC) | 39 |
| S. meliloti RFF155 | ΔrpoH2 (CL150, Smr) | R. Fisher |
| S. meliloti RFF157 | ΔrpoH1 (CL150, Smr) | R. Fisher |
| S. meliloti RFF164 | ΔrpoE2, rsiA1 (CL150, Smr) | R. Fisher |
| S. meliloti RFF231 | ΔrpoH1, rpoH2 (CL150, Smr) | R. Fisher |
| S. meliloti RFF272 | ΔrpoE5, Smb21687 (CL150, Smr) | R. Fisher |
| S. meliloti APL91 | CL150 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL92 | RFF155 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL93 | RFF157 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL94 | RFF164 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL95 | RFF231 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL96 | RFF272 pAPL56 oxyR-uidA fusion | This study |
| S. meliloti APL44 | CL150 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL81 | RFF155 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL82 | RFF157 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL46 | RFF164 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL83 | RFF231 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL84 | RFF272 pAPL10 katA-uidA fusion | This study |
| S. meliloti APL97 | CL150 pAPL57 oxyR mutant | This study |
| Plasmids | ||
| pDW33 | Terminator and polylinker preceding uidA (Apr Hyr) | 56 |
| pAPL10 | PkatA in pDW33 (Apr Hyr) | 57 |
| pAPL56 | PoxyR in pDW33 (Apr Hyr) | This study |
| pAPL57 | Internal fragment of oxyR in pDW33 (Apr Hyr) | This study |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance.
To generate an oxyR insertion plasmid, a fragment consisting of bp 303 to 649 of the oxyR open reading frame (ORF) was amplified by PCR using primers with SpeI/XhoI sites. The digested PCR fragment was ligated with pDW33, generating pAPL57. To generate an oxyR-uidA (encoding β-glucuronidase) transcriptional fusion plasmid, a fragment consisting of 25 bp upstream through the first 296 bp of the oxyR ORF was amplified via PCR using primers with SpeI/XhoI sites. pAPL56 was generated by ligating the PCR fragment into pDW33.
pAPL10 (57) and pAPL56 were integrated into the S. meliloti genome via single crossover following conjugation by triparental mating to generate katA and oxyR uidA fusion strains in various S. meliloti backgrounds (Table 3). GUS assays were performed in triplicate as described previously (59). pAPL57 was integrated into CL150 via single crossover following conjugation by triparental mating (59), generating oxyR insertion mutant APL97.
RNA purification, cDNA synthesis, labeling, and hybridization.
RNA was purified as described in the supplemental material of reference 51. cDNA first-strand synthesis was performed using Invitrogen SuperScript III and random hexamers as primers. cDNA fragmentation, labeling, and hybridization to custom S. meliloti Affymetrix symbiosis chips were performed as described previously (51).
Data analysis was performed as described previously (51). Comparison expression analysis was used where an experimental array was compared to a baseline array: three biological replicates each of control (no H2O2 exposure) and experimental (H2O2 exposure) strains yielded nine pairwise comparisons. The level of a given gene's expression was considered increased or decreased if the average signal log ratio (SLR) was ≥0.98 with a P value of ≤0.05 in all nine comparisons. A total of three sets of nine pairwise comparisons were performed as follows: 1 mM H2O2 exposure compared to no exposure in wild-type CL150, 0.5 mM H2O2 exposure compared to no exposure in wild-type CL150, and 0.5 mM H2O2 exposure compared to no exposure in oxyR insertion mutant APL97. Functional categories for differentially expressed genes were determined using clusters of orthologous groups (COGs) and annotation from http://iant.toulouse.inra.fr/S.meliloti. Venn diagrams were produced using BioVenn (60).
RT-qPCR.
Real time RT-qPCR was performed as described previously (61) using a Bio-Rad CFX96 system. A 0.5 μM concentration of each primer and 10 ng of cDNA were used in each reaction. The internal control was uppS (SMc02097) (51). Three technical replicates of each of three biological replicates were performed. Data analysis was performed as described previously (62).
Hydrogen peroxide sensitivity assay.
H2O2 sensitivity was determined as described previously (57) with a minimum of 3 replicates per experiment. Briefly, mid-exponential-phase free-living cells were diluted 1:100 in LB and the reaction mixtures were split in half. H2O2 was added to one half of the cultures to reach a final concentration of 1 mM. After 30 additional minutes at 30°C, cultures were diluted and a 100-μl aliquot of each dilution was plated on LB plates containing selective antibiotics. Colonies were counted after 3 to 4 days at 30°C, and levels of CFU per milliliter were calculated to determine percent survival of treated versus untreated cultures. Percent survival was then normalized to the survival rate of the wild-type strain.
Catalase assay.
To determine total catalase activity, overnight cultures of S. meliloti were diluted to an OD600 of 0.1 in LB medium without antibiotics and grown with shaking at 30°C to mid-exponential phase (OD600 = 0.3 to 0.4). Cultures were split in half, H2O2 was added to one of the halves, and both halves were returned to 30°C for 30 min. A 1-ml volume of treated and untreated cultures was harvested by centrifugation for 1 min. Cell pellets were resuspended in 1 ml lysis buffer (50 mM sodium phosphate [pH 7], 0.1% Triton X-100) and diluted 5-fold to 10-fold in 1× Amplex Red reaction buffer (Invitrogen catalog no. A22180).
A 25-μl volume of each diluted cell lysate was incubated with 20 μM H2O2 (final concentration) in a 96-well black microtiter plate with a final volume of 50 μl. Plates were incubated for 30 min at room temperature. Amplex Red reagent (final concentration of 50 μM) and horseradish peroxidase (final concentration fo 0.2 U/ml) in 1× reaction buffer were added to cell lysates (final volume of 100 μl), and the reaction mixtures were incubated at 37°C for 30 min in the dark. Plates were read as previously described (15). A standard curve of catalase activity was used to determine equivalent catalase units in each S. meliloti sample. Catalase activity was normalized to either the OD600 of cultures at the time of lysis or to total cell protein data, as determined by the use of a modified Bradford assay (Bio-Rad protein assay). All assays were performed at least in triplicate.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NIH (R01 GM093628).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00622-17.
REFERENCES
- 1.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 4.Touati D. 2000. Iron and oxidative stress in bacteria. Arch Biochem Biophys 373:1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 5.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon J-M, Lee H-I, Donati AJ, So J-S, Emerich DW, Chang W-S. 2011. Whole-genome expression profiling of Bradyrhizobium japonicum in response to hydrogen peroxide. Mol Plant Microbe Interact 24:1472–1481. doi: 10.1094/MPMI-03-11-0072. [DOI] [PubMed] [Google Scholar]
- 7.Santos R, Hérouart D, Sigaud S, Touati D, Puppo A. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant Microbe Interact 14:86–89. doi: 10.1094/MPMI.2001.14.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Oger E, Marino D, Guigonis J-M, Pauly N, Puppo A. 2012. Sulfenylated proteins in the Medicago truncatula-Sinorhizobium meliloti symbiosis. J Proteomics 75:4102–4113. doi: 10.1016/j.jprot.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Andrio E, Marino D, Marmeys A, De Segonzac MD, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N. 24 January 2013. Hydrogen peroxide-regulated genes in the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol doi: 10.1111/nph.12120. [DOI] [PubMed] [Google Scholar]
- 10.Damiani I, Drain A, Guichard M, Balzergue S, Boscari A, Boyer J-C, Brunaud V, Cottaz S, Rancurel C, Da Rocha M. 7 June 2016. Nod factor effects on root hair-specific transcriptome of Medicago truncatula: focus on plasma membrane transport systems and reactive oxygen species networks. Front Plant Sci doi: 10.3389/fpls.2016.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiani I, Pauly N, Puppo A, Brouquisse R, Boscari A. 8 April 2016. Reactive oxygen species and nitric oxide control early steps of the legume-rhizobium symbiotic interaction. Front Plant Sci doi: 10.3389/fpls.2016.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H. 4 March 2016. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol doi: 10.1104/pp.15.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien JA, Daudi A, Butt VS, Bolwell GP. 2012. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779. doi: 10.1007/s00425-012-1696-9. [DOI] [PubMed] [Google Scholar]
- 14.Kärkönen A, Kuchitsu K. 2015. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112:22–32. doi: 10.1016/j.phytochem.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Shaw S, Long S. 2003. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol 132:2196–2204. doi: 10.1104/pp.103.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cárdenas L, Martínez A, Sánchez F, Quinto C. 2008. Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J 56:802–813. doi: 10.1111/j.1365-313X.2008.03644.x. [DOI] [PubMed] [Google Scholar]
- 17.Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Dreano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Golding B, Gouzy J, Gurjal M, Hernandez-Lucas I, Hong A, Huizar L, Hyman RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thebault P, Vandenbol M, et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- 18.Sigaud S, Becquet V, Frendo P, Puppo A, Herouart D. 1999. Differential regulation of two divergent Sinorhizobium meliloti genes for HPII-like catalases during free-living growth and protective role of both catalases during symbiosis. J Bacteriol 181:2634–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamet A, Sigaud S, Van de Sype G, Puppo A, Hérouart D. 2003. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol Plant Microbe Interact 16:217–225. doi: 10.1094/MPMI.2003.16.3.217. [DOI] [PubMed] [Google Scholar]
- 20.Hérouart D, Sigaud S, Moreau S, Frendo P, Touati D, Puppo A. 1996. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J Bacteriol 178:6802–6809. doi: 10.1128/jb.178.23.6802-6809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang SM, Schellhorn HE. 2012. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys 525:161–169. doi: 10.1016/j.abb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Imlay JA. 2015. Transcription factors that defend bacteria against reactive oxygen species. Annu Rev Microbiol 69:93–108. doi: 10.1146/annurev-micro-091014-104322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M, Aslund F, Storz G. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 24.Storz G, Tartaglia LA, Ames BN. 1990. The OxyR regulon. Antonie Van Leeuwenhoek 58:157–161. doi: 10.1007/BF00548927. [DOI] [PubMed] [Google Scholar]
- 25.Toledano MB, Kullik I, Trinh F, Baird PT, Schneider TD, Storz G. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897–909. doi: 10.1016/S0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakjarung K, Mongkolsuk S, Vattanaviboon P. 2003. The oxyR from Agrobacterium tumefaciens: evaluation of its role in the regulation of catalase and peroxide responses. Biochem Biophys Res Commun 304:41–47. doi: 10.1016/S0006-291X(03)00535-7. [DOI] [PubMed] [Google Scholar]
- 27.Jamet A, Kiss E, Batut J, Puppo A, Hérouart D. 2005. The katA catalase gene is regulated by OxyR in both free-living and symbiotic Sinorhizobium meliloti. J Bacteriol 187:376–381. doi: 10.1128/JB.187.1.376-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christman MF, Storz G, Ames BN. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A 86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staroń A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. 2009. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol Microbiol 74:557–581. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 30.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feklístov A, Sharon BD, Darst SA, Gross CA. 2014. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol 68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 32.Sineva E, Savkina M, Ades SE. 2017. Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Curr Opin Microbiol 36:128–137. doi: 10.1016/j.mib.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Österberg S, del Peso-Santos T, Shingler V. 2011. Regulation of alternative sigma factor use. Annu Rev Microbiol 65:37–55. doi: 10.1146/annurev.micro.112408.134219. [DOI] [PubMed] [Google Scholar]
- 34.Gruber TM, Gross CA. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol 57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 35.Bastiat B, Sauviac L, Picheraux C, Rossignol M, Bruand C. 2012. Sinorhizobium meliloti sigma factors RpoE1 and RpoE4 are activated in stationary phase in response to sulfite. PLoS One 7:e50768. doi: 10.1371/journal.pone.0050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauviac L, Philippe H, Phok K, Bruand C. 2007. An extracytoplasmic function sigma factor acts as a general stress response regulator in Sinorhizobium meliloti. J Bacteriol 189:4204–4216. doi: 10.1128/JB.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnett MJ, Bittner AN, Toman CJ, Oke V, Long SR. 2012. Dual RpoH sigma factors and transcriptional plasticity in a symbiotic bacterium. J Bacteriol 194:4983–4994. doi: 10.1128/JB.00449-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lucena DKC, Pühler A, Weidner S. 2010. The role of sigma factor RpoH1 in the pH stress response of Sinorhizobium meliloti. BMC Microbiol 10:265. doi: 10.1186/1471-2180-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlüter J-P, Reinkensmeier J, Barnett MJ, Lang C, Krol E, Giegerich R, Long SR, Becker A. 2013. Global mapping of transcription start sites and promoter motifs in the symbiotic α-proteobacterium Sinorhizobium meliloti 1021. BMC Genomics 14:156. doi: 10.1186/1471-2164-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki S, Minamisawa K, Mitsui H. 2016. A Sinorhizobium meliloti RpoH-regulated gene is involved in iron-sulfur protein metabolism and effective plant symbiosis under intrinsic iron limitation. J Bacteriol 198:2297–2306. doi: 10.1128/JB.00287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobik C, Meilhoc E, Batut J. 2006. FixJ: a major regulator of the oxygen limitation response and late symbiotic functions of Sinorhizobium meliloti. J Bacteriol 188:4890–4902. doi: 10.1128/JB.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domínguez-Ferreras A, Pérez-Arnedo R, Becker A, Olivares J, Soto MJ, Sanjuán J. 2006. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J Bacteriol 188:7617–7625. doi: 10.1128/JB.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flechard M, Fontenelle C, Trautwetter A, Ermel G, Blanco C. 2009. Sinorhizobium meliloti rpoE2 is necessary for H(2)O(2) stress resistance during the stationary growth phase. FEMS Microbiology Lett 290:25–31. doi: 10.1111/j.1574-6968.2008.01401.x. [DOI] [PubMed] [Google Scholar]
- 44.Oke V, Long SR. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol Microbiol 32:837–849. doi: 10.1046/j.1365-2958.1999.01402.x. [DOI] [PubMed] [Google Scholar]
- 45.Oke V, Rushing BG, Fisher EJ, Moghadam-Tabrizi M, Long SR. 2001. Identification of the heat-shock sigma factor RpoH and a second RpoH-like protein in Sinorhizobium meliloti. Microbiology 147:2399–2408. doi: 10.1099/00221287-147-9-2399. [DOI] [PubMed] [Google Scholar]
- 46.Luo L, Qi M-S, Yao S-Y, Cheng H-P, Zhu J-B, Yu G-Q. 2005. Role of oxyR from Sinorhizobium meliloti in regulating the expression of catalases. Acta Biochim Biophys Sin 37:421–428. doi: 10.1111/j.1745-7270.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- 47.Davies BW, Walker GC. 2007. Identification of novel Sinorhizobium meliloti mutants compromised for oxidative stress protection and symbiosis. J Bacteriol 189:2110–2113. doi: 10.1128/JB.01802-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barloy-Hubler F, Chéron A, Hellégouarch A, Galibert F. 2004. Smc01944, a secreted peroxidase induced by oxidative stresses in Sinorhizobium meliloti 1021. Microbiology 150:657–664. doi: 10.1099/mic.0.26764-0. [DOI] [PubMed] [Google Scholar]
- 49.Davies BW, Walker GC. 2008. A highly conserved protein of unknown function is required by Sinorhizobium meliloti for symbiosis and environmental stress protection. J Bacteriol 190:1118–1123. doi: 10.1128/JB.01521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies BW, Walker GC. 2007. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J Bacteriol 189:2101–2109. doi: 10.1128/JB.01377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnett MJ, Toman CJ, Fisher RF, Long SR. 2004. A dual-genome Symbiosis Chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc Natl Acad Sci U S A 101:16636–16641. doi: 10.1073/pnas.0407269101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker A, Bergès H, Krol E, Bruand C, Ruberg S. 2004. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol Plant Microbe Interact 17:292–303. doi: 10.1094/MPMI.2004.17.3.292. [DOI] [PubMed] [Google Scholar]
- 53.Martínez-Salazar JM, Sandoval-Calderon M, Guo X, Castillo-Ramirez S, Reyes A, Loza MG, Rivera J, Alvarado-Affantranger X, Sanchez F, González V. 2009. The Rhizobium etli RpoH1 and RpoH2 sigma factors are involved in different stress responses. Microbiology 155:386–397. doi: 10.1099/mic.0.021428-0. [DOI] [PubMed] [Google Scholar]
- 54.Hanahan D. 1985. Techniques for transformation of E. coli, p 109–135. In Glover DM. (ed), DNA cloning: a practical approach. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 55.Finan TM, Kunkel B, De Vos GF, Signer ER. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol 167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cronan GE, Keating DH. 2004. Sinorhizobium meliloti sulfotransferase that modifies lipopolysaccharide. J Bacteriol 186:4168–4176. doi: 10.1128/JB.186.13.4168-4176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehman AP, Long SR. 2013. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J Bacteriol 195:5362–5369. doi: 10.1128/JB.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol 149:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson JA, Mulligan JT, Long SR. 1993. Regulation of syrM and nodD3 in Rhizobium meliloti. Genetics 134:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hulsen T, De Vlieg J, Alkema W. 2008. BioVenn—a Web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics 9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra RM, Shaw SL, Long SR. 2004. Six nonnodulating plant mutants defective for Nod factor-induced transcriptional changes associated with the legume-rhizobia symbiosis. Proc Natl Acad Sci U S A 101:10217–10222. doi: 10.1073/pnas.0402186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.