Abstract
A 2-year-old boy with a history of pica was admitted with vomiting and treated overnight for viral tonsillitis. A week later, he presented with a prolonged afebrile seizure and required intubation and ventilation. Antibiotics and acyclovir were started. Despite extensive investigations including MRI head, no cause was identified. Four days later, he deteriorated with signs of raised intracranial pressure. On day 5, blood lead concentration in the sample collected at admission was reported as grossly elevated, consistent with a diagnosis of severe lead poisoning from ingesting lead-containing paint at the family home. Chelation therapy was started but, unfortunately, he did not make a neurological recovery, and care was withdrawn. A serious case review identified a lack of awareness of lead poisoning and its relation to pica as a root cause. We report this case to share our experience and the importance of considering lead poisoning in children with pica.
Keywords: Poisoning, Paediatrics, Neonatal And Paediatric Intensive Care
Background
Lead is a poison with no toxic threshold. Up until the 1950s, lead compounds were widely used as pigments in domestic paint, and the presence of lead carbonate in some white paints resulted in a lead content of greater than 50% dry weight. Lead pigments were banned from household paints in the USA in 1978, and in 1992 the European Union passed legislation to prevent the addition of lead to domestic paint. Levels of lead in the atmosphere have reduced dramatically since the use of lead in petrol was phased out and ultimately stopped from 1984 to 1999. However, lead poisoning remains a risk to the general population due to the presence of lead in old layers of paint on woodwork inside and outside the home, in contaminated soil and dust and the continued presence of lead water mains in domestic properties.1
This case highlights that lead poisoning remains a significant issue today and one that can be fatal if not recognised early. It is critical that paediatricians and general practitioners are aware of this risk, particularly in children with iron deficiency and exhibiting pica behaviour.
By introducing a simple trigger on our electronic requesting system, we have been able to raise awareness of this risk and facilitated early identification and treatment of children with lead poisoning. Community paediatricians often request blood lead measurement as part of initial investigations for developmental delay. This case illustrates the importance of also considering the possibility of lead poisoning in the acute setting, in children presenting with a non-specific illness and a history of pica. We would like to share our experience to help prevent such a tragic outcome in the future.
Case presentation
A 2-year-old boy was admitted to the ward with a 7-day history of vomiting. He had a 2-month history of pica (eating flakes of painted woodwork at the family home) and a background of speech delay. Initial impression was dehydration due to tonsillitis. He had microcytic anaemia (haemoglobin 8g/dL, ferritin 7 µg/L) consistent with iron deficiency. He had no radiological investigations. He tolerated oral fluids, and his condition improved overnight. The mother self-discharged the patient prior to the consultant ward round.
Seven days later, the patient presented to accident and emergency with a prolonged afebrile seizure and was admitted to the paediatric intensive care unit for intubation and ventilation. He was started on antibiotics and acyclovir for a suspected central nervous system (CNS) infection, and following a normal cranial CT, the patient was extubated. A lumbar puncture was performed and routine blood lead levels requested due to the history of pica.
His sensorium fluctuated over the next 3 days, and a cranial MRI showed diffuse signal abnormality. Following a neuroradiology meeting and in view of the encephalitis, the differential diagnoses considered included CNS infection, metabolic insult to the CNS due to an inborn error of metabolism (particularly L-2-hydroxyglutaric aciduria, 3-hydroxy-methylglutaryl-coenzyme A lyase deficiency, succinic semialdehyde dehydrogenase deficiency or Kearns-Sayre syndrome) or a toxic insult to the CNS from an unidentified poison, including heavy-metal poisoning. After discussions and input from other specialties including neurology, metabolic and biochemist teams, the following laboratory investigations were requested: cerebrospinal fluid culture, cell count, lactate, glycine and serine; blood and urine cultures; renal, liver and thyroid function tests; plasma salicylate, plasma ammonia, plasma and urine amino acids, urine organic acids, urine drugs of abuse; blood-spot acylcarnitines; repeat blood lead; N-methyl-D-aspartate (NMDA) receptor antibodies; voltage gated potassium antibodies; white cell enzymes; very long chain fatty acids; phytanate levels; serum biotinidase and a muscle biopsy.
On day 4 of admission, his condition deteriorated with focal seizures and apnoeas. Following stabilisation and instigation of neuroprotective measures, a repeat CT confirmed signs of raised intracranial pressure. An electroencephalogram showed severe cortical dysfunction. On day 5, blood lead analysis from the sample taken at admission was completed (in our lab blood lead samples are tested only twice a week unless requested as urgent). Blood lead was grossly elevated at 17.59 µmol/L (364 µg/dL) (reference range <0.24 µmol/L (5 µg/dL), concentrations >7.25 µmol/L (150 µg/dL) thought to be fatal). The result was consistent with encephalopathy due to severe lead toxicity, presumed to result from ingesting lead-containing paint. Repeat blood lead from a sample collected on day 4 was 11.47 µmol/L (237 µg/dL), reflecting removal from lead exposure during hospital admission. An abdominal X-ray showed no evidence of metallic opacities in the stomach but a chest X-ray formally reported that there was mild increased density of the metaphyses of the proximal humeri and some anterior rib ends. This would not be diagnostic of lead poisoning but would support such a diagnosis.
Treatment
The paediatric team consulted the National Poisons Unit, and he was started on sodium edetate chelation therapy (75 mg/kg once daily). Blood and urine lead concentrations were monitored during the course of chelation therapy at the request of the UK National Poisons Unit.
Outcome and follow-up
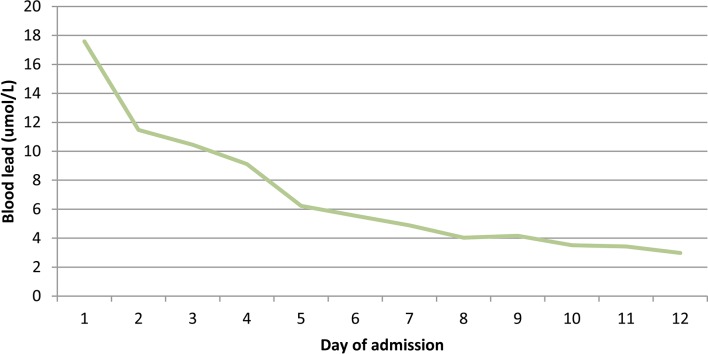
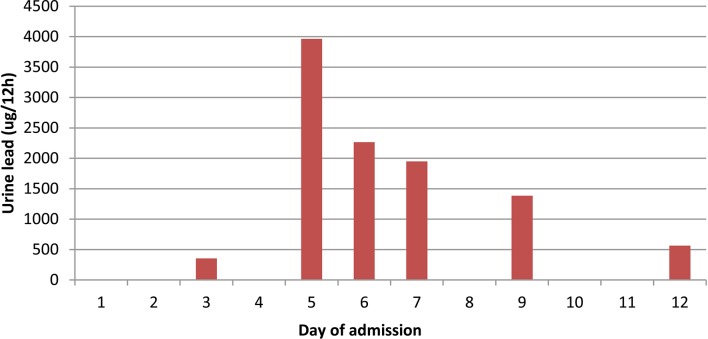
Despite reduction in his lead levels (figures 1 and 2), he did not make a neurological recovery and after discussions with the family, care was finally withdrawn.
Figure 1.
Reduction in blood lead levels over admission.
Figure 2.
Urine lead levels over admission.
Following this fatal lead poisoning, an extensive serious case review was undertaken. It was found that the root cause was multifactorial—a lack of clinician awareness and experience of lead poisoning and lead encephalopathy, and a lack of knowledge that the habit of pica may lead to ingestion of lead. Public Health England was informed, and investigations revealed the presence of lead-containing paint at the family home. To raise awareness, the case was presented at multidisciplinary forums both locally and regionally. In addition, by working closely with laboratory colleagues, a trigger was designed on the electronic pathology requesting system to prompt all clinicians requesting ferritin measurement in a child to check for a history of pica and, if present, to request lead measurement.
Discussion
We searched PubMed and MEDLINE for recent similar cases of lead poisoning from pica. Lead poisoning was first recognised in children in 1924 when a series of cases in Queensland, Australia, found peeling lead-based paint on the verandas of family homes as the source of exposure.2 Further cases have since been reported, and in the last 10 years, we found four cases of children with pica who presented with lead poisoning: two related to paint peelings, one from a battery and one from soil.3–6 In one of the cases, lead toxicity from paint chippings was also initially misdiagnosed as a viral illness until the child presented 2 days later with worsening symptoms and fluctuating consciousness.6
Children absorb up to 50% of ingested lead, in contrast with adults, who typically absorb less than 10%.7 Therefore, children who exhibit pica behaviour and ingest non-food substances such as soil, wood or painted material, are particularly at risk of lead poisoning. Epidemiological studies suggest that iron deficiency may increase the risk of lead poisoning in exposed individuals.5 8 Therefore, children with pica who are also iron deficient are at an increased risk of lead poisoning, and it is important to recognise this.
Lead poisoning in children typically presents with non-specific symptoms such as vomiting and abdominal pains, mimicking a viral illness. Therefore, lead poisoning should be considered a differential diagnosis in all children presenting with these features and a history of pica. The gold standard investigation for lead poisoning is measurement of blood lead concentration. The blood lead reference range in the UK is <0.24 µmol/L. Other useful laboratory investigations include full blood count and ferritin to identify iron deficiency, blood film microscopy to detect basophilic stippling and urinalysis to assess for proteinuria, glycosuria and aminoaciduria to identify renal tubular dysfunction typical of acute poisoning.7 X-rays may show lead lines in the metaphyses of long bones in chronic poisoning and abdominal X-ray imaging may identify lead opacities.5
If lead poisoning is found, the primary treatment is to remove the child from the source of exposure; often this is the only treatment required for children alongside regular blood lead monitoring. The National Poisons Information Service recommends considering chelation therapy in children who are symptomatic or have a blood lead >2.4 µmol/L.
Following investigation into this fatal case, paediatric and laboratory consultants worked together to develop a ‘blood lead trigger’ on the hospitals’ electronic test requesting interface. The trigger works via a pop-up text box which appears when ferritin is requested on a child and acts to alert paediatricians to consider measuring blood lead in any child presenting with possible iron deficiency and pica. In the 10 months following introduction of the electronic trigger, 12 more cases of lead poisoning have been diagnosed locally. Blood lead concentrations ranged from 0.43 µmol/L (8.9 µg/dL) to 2.16 µmol/L (44.7 µg/dL), and old lead paint was the source in the majority of cases. None of these children required chelation therapy, and interventions were made to reduce exposure, resulting in lower lead levels on follow-up. Table 1 summarises these 12 cases.
Table 1.
Summary of lead cases
| Total cases since January 2016 | 12 |
| Gender | Nine boys, three girls |
| Age range | One year 8 months to 8 years 5 months |
| Median age | Three years 5 months |
| Presentation to | General practitioner: 3 Children’s assessment unit: 1 Children’s outpatient services: 8 |
| Clinical details | Pica: 7 Developmental delay/decline: 4 Autism: 2 Anaemia: 2 |
| Blood lead range | 0.43–2.16 µmol/L (8.9–44.7 µg/dL) |
It is not possible to determine whether these cases would have been diagnosed in the absence of the trigger on the electronic requesting system, particularly as community paediatricians within our region screen all children presenting with developmental delay for blood lead poisoning. However, it is likely that this trigger has raised the awareness of general practitioners and hospital paediatricians using the electronic test requesting interface that blood lead poisoning should be considered in children with pica and iron deficiency. This case also illustrates the importance of considering the possibility of lead poisoning in the acute setting, in children presenting with a non-specific illness and a history of pica.
Learning points.
Lead poisoning remains a significant issue today and one that can be fatal if not recognised early.
Children with pica who are also iron deficient are at an increased risk of lead poisoning.
It is important to consider the possibility of lead poisoning in the acute setting, in children presenting with a non-specific illness and a history of pica.
Footnotes
Contributors: All authors have contributed to the writing of this report. ALT wrote the summary, case and organised the final formatting of the report. CL wrote the discussion. AT collected the date in the tables. All authors met regularly and were involved in the editing of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Koller K, Brown T, Spurgeon A, et al. . Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect 2004;112:987–94. 10.1289/ehp.6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruddock JC. Lead poisoning in children with special reference to pica. JAMA 1924;82:1682–4. 10.1001/jama.1924.02650470022010 [DOI] [Google Scholar]
- 3.Jouhadi Z, Bensabbahia D, Chafiq F, et al. . Lead poisoning in children: a case report. Pan Afr Med J 2016;24:316 10.11604/pamj.2016.24.316.10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maji B, Ganguly N. Lead poisoning in an infant. Indian Pediatr 2014;51:319–20. [PubMed] [Google Scholar]
- 5.Howarth D. Pica -- a case report. Aust Fam Physician 2013;42:299–300. [PubMed] [Google Scholar]
- 6.George M, Heeney MM, Woolf AD. Encephalopathy from lead poisoning masquerading as a flu-like syndrome in an autistic child. Pediatr Emerg Care 2010;26:370–3. 10.1097/PEC.0b013e3181db2237 [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Childhood lead poisoning. 2010. ISBN 978 92 4 150033 3 http://www.who.int/ceh/publications/childhoodpoisoning/en/ (accessed 9 Jul 2015).
- 8.Kwong WT, Friello P, Semba RD. Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ 2004;330:21–37. 10.1016/j.scitotenv.2004.03.017 [DOI] [PubMed] [Google Scholar]