Abstract
Cytomegalovirus (CMV) gastrointestinal disease usually arises in patients with immunodeficiency or immunosuppression, being rare in immunocompetent hosts. Although increasing in incidence, few cases of CMV gastrointestinal disease have been described among young healthy patients. Currently, there is uncertainty in approaching these patients, including the need for antiviral therapy that remains to be established. This case report describes a CMV ulcerative oesophagitis in a young healthy immunocompetent patient with good evolution with no need for antiviral therapy, the youngest case being reported in the literature until now.
Keywords: endoscopy, oesophagus, drugs: infectious diseases
Background
Cytomegalovirus (CMV) gastrointestinal disease is common in immunocompromised patients, especially those with HIV/AIDS with CD4 cell count below 50 cells/uL, organ transplantation and active malignancy under chemotherapy.1–10 Following the colon, the oesophagus is the most common site of CMV gastrointestinal disease.1–6 8 11–14 In immunocompetent patients, this condition is rare with few cases reported in the literature, although incidence has increased in last decades.2 6 7 10 13 15–17 Risk factors such as advanced age and comorbidities/critical illnesses contribute to this increased incidence due to immune senescence and impaired host immune response, respectively.1 2 7 15 16 Despite a well-established approach in immunocompromised patients, no clear guidelines have been proposed for immunocompetent patients, including when to institute antiviral therapy.1 10 15 16
In this paper, we present a CMV ulcerative oesophagitis in a young healthy immunocompetent patient with good evolution without antiviral therapy, the youngest case being reported in the literature. Additionally, a review of all reported cases of CMV oesophagitis in immunocompetent patients is carried out.
Case presentation
Authors present a case of a 25-year-old Caucasian female with medical history of hypothyroidism, former smoker (six pack-years), infectious mononucleosis and left thoracic zoster at age of 16 and 7 years, respectively. She was also exposed to a coworker with recent diagnosis of pulmonary tuberculosis, but she did not receive preventive therapy. Currently, she is under levothyroxine, with no other medications presently or previously, including steroids or immunosuppressive therapy. The patient was sent to a gastroenterology consultation for 1 month of odynophagia, with no fever, nausea/vomiting or abdominal pain. Treatment with sucralfate and double-dose proton pump inhibitor was attempted with partial clinical improvement. The physical examination was unremarkable.
Investigations and differential diagnosis
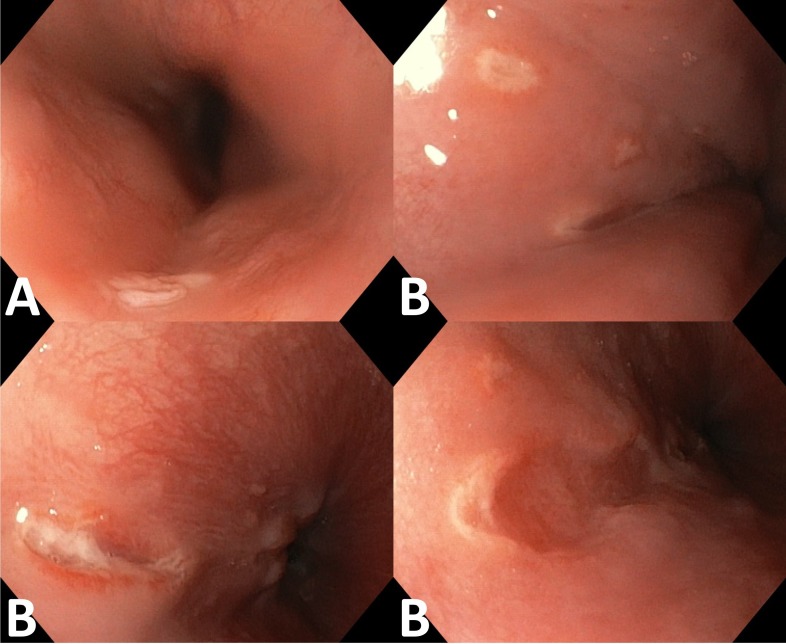
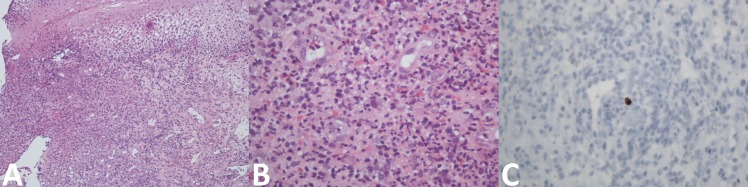
Laboratory analysis showed a C reactive protein of 2.63 mg/dL (n<0.5), with no other abnormalities. An oesophagogastroduodenoscopy was performed, showing a solitary 6 mm sessile polyp in the proximal oesophagus and multiple superficial and deep well-circumscribed ulcers with 4–10 mm in diameter in the distal oesophagus and diffuse erythematous gastropathy of the antrum (figure 1). Oesophageal polypectomy was compatible with squamous cell papilloma negative for high-risk human papillomavirus DNA. Biopsies of the distal oesophagus showed an intense inflammatory infiltrate of eosinophils and neutrophils and some cells with amphophilic nucleus and undefined limit cytoplasm, with no diagnostic criteria for eosinophilic oesophagitis. Immunohistochemical staining using the anti-CMV monoclonal antibody was positive (figure 2). Gastric biopsies showed chronic non-atrophic pangastritis with mononuclear cell infiltrate and mild colonisation by Helicobacter pylori. Complementary study with immunoglobulins, serum protein electrophoresis, systemic autoimmunity, gamma-interferon assay, syphilis screening, lymphocyte subpopulations study and serology for HIV-1/2 and hepatitis A, B and C viruses were negative. Acid-fast staining and culture of oesophageal biopsy tissue for mycobacteria were also negative. Serology and DNA viral load for Epstein-Barr virus revealed previous infection (IgM-, IgG+) and herpes simplex virus 1 serology was negative. CMV serology was compatible with reactivation (IgM+, IgG+) despite negative serum DNA viral load. The diagnosis of symptomatic CMV ulcerative oesophagitis by latent CMV reactivation was made.
Figure 1.
Upper gastrointestinal endoscopy showing a solitary 6 mm sessile polyp in the proximal oesophagus (A) and multiple well-delimited, superficial and deep longitudinal ulcers, ranging in size from 4 to 10 mm, located in the lower third of the oesophagus (B).
Figure 2.
Histopathology of the lower third of the oesophagus with basal hyperplasia and vacuolisation of stratified squamous epithelium, numerous neovases with prominent endothelium, an intense inflammatory infiltrate of eosinophils and neutrophils and some cells with amphophilic nucleus and undefined limit cytoplasm. No granulomas were observed (A: H&E, 100×; B: H&E, 400×). Immunohistochemical staining using anti-CMV monoclonal antibody was positive (C: anti-CMV, 400×).
Treatment, outcome and follow-up
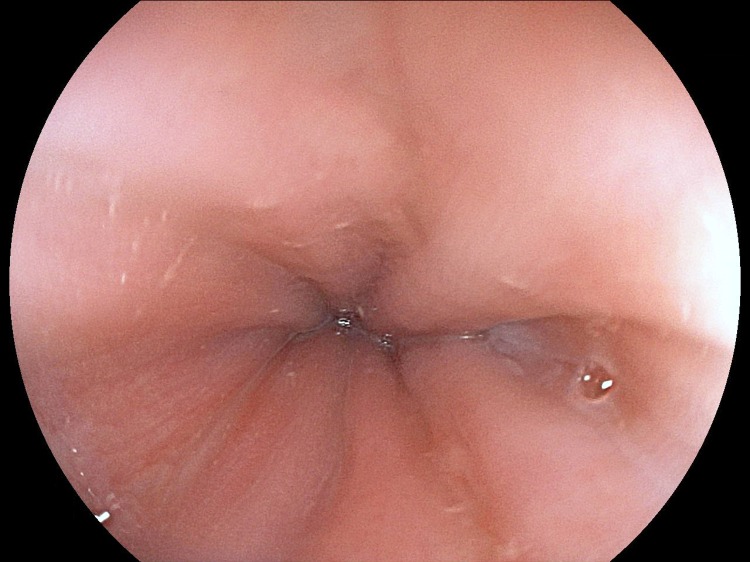
At the multidisciplinary decision meeting involving gastroenterologists and infeciologists, it was decided to maintain supportive therapy with proton pump inhibitor and clinical surveillance without starting antiviral therapy. The patient gradually improved and became completely asymptomatic 2 months after the onset of symptoms. Two months after the diagnosis, CMV IgM became negative and a revision endoscopy showed complete healing of oesophageal ulcers (Figure 3). An exhaustive evaluation was carried out in a consultation of infectious disease without identifiable immune system defects.
Figure 3.
Revision of upper gastrointestinal endoscopy showing complete healing of oesophageal ulcers.
Discussion
Authors report a case of histologically proven CMV ulcerative oesophagitis in a young healthy immunocompetent patient with good evolution without antiviral therapy.
Clinical features of all 25 published cases of histologically proven CMV oesophagitis in immunocompetent patients were reviewed and summarised in tables 1 and 2.2–9 11–20
Table 1.
Literature review summary of all cases of CMV oesophagitis in immunocompetent patients
| Characteristics | Number of patients (n=25) |
| Age (mean±σ; minimum–maximum) | 65.2±15.8 (32–91) |
| >55 years old, n (%) | 16 (64.0) |
| Male gender, n (%) | 13 (52.0) |
| Presenting symptoms, n (%) | |
| Upper gastrointestinal bleeding | 11 (52.4) |
| Epigastric pain | 7 (33.3) |
| Odynophagia/Dysphagia/Chest pain | 6 (28.6) |
| Nausea/Vomiting | 2 (9.5) |
| Fever | 3 (14.3) |
| NA | 4 (16.0) |
| Disseminated CMV disease, n(%) | 5 (20.0) |
| Larynx | 1 (20.0) |
| Small bowel | 3 (60.0) (Duodenum: 2; entire small bowel: 1) |
| Colon | 2 (40.0) |
| Endoscopic findings, n (%) | |
| Location of lesions | |
| Upper third | 2 (8.0) |
| Middle third | 6 (24.0) |
| Distal third | 18 (72.0) |
| Entire oesophagus | 3 (12.0) |
| Type of lesions | |
| Necrotising oesophagitis | 2 (8.0) |
| Ulcers | 20 (80.0) (Deep: 4, superficial: 6, NA: 10) (Few: 7, multiple: 9, NA: 4) |
| Erosions | 3 (12.0) |
| Histopathological findings, n (%) | |
| CMV inclusions | 24 (96.0) |
| Immunohistochemical staining | 14 (93.3) |
| NA | 10 (40.0) |
| CMV serology, n(%) | |
| CMV IgG/IgM | 8 (57.1)/8 (57.1) |
| NA | 11 (44.0) |
| CMV DNA (IU/mL), (mean±σ) | 8121±2576 |
| NA | 23 (92.0) |
| Treatment, n(%) | |
| Supportive care | 6 (30.0) |
| Antiviral drugs | 14 (70.0) |
| Intravenous ganciclovir | 12 (85.7) |
| Oral valganciclovir | 1 (7.1) |
| NA | 1 (7.1) |
| NA | 5 (20.0) |
| Comorbidities/Critical illnesses, n(%) | 21 (84.0) |
| Critical illnesses | 10 (47.6) |
| Immunomodulatory comorbidities | 16 (76.2) |
| Outcome, n(%) | |
| Recovery | 17 (85.0) |
| Death* | 3 (15.0) |
| NA | 5 (20.0) |
Few ulcers: <3; multiple ulcers:≥3.
*Died while awaiting CMV histopathological diagnosis without initiation antiviral therapy (n=1); died with multiple organ failure, with postmortem diagnosis of CMV oesophagitis (n=1); died due to progressive complications of post-ERCP pancreatitis (n=1).
CMV, cytomegalovirus; ERCP, endoscopic retrograde cholangiopancreatography; NA, not available.
Table 2.
Clinical features of literature review of all cases of CMV oesophagitis in immunocompetent patients
| No./ (Reference) |
Age/ Gender |
Comorbidities/ Critical illnesses |
Clinical presentation | Oesophagus CMV location | Endoscopic findings | Disseminated CMV disease | Antiviral therapy (dose, duration in days) |
Mortality (time in months after diagnosis) |
| 12 | 80 F | Diabetes mellitus; arterial hypertension; 4 years poststenting status due to CAD | UGIB Epigastric pain Dysphagia |
Middle and distal thirds | Erosions (LA classification grade D) | Yes (Duodenum) | Intravenous ganciclovir (NA) |
No |
| 23 | 86 F | Cerebral vascular disease in the past | Nausea/Vomiting | Distal third | Ulcers (superficial) | No | No | Yes (1 month) |
| 34 | 84 F | Diabetes mellitus; COPD | Epigastric pain | Middle and distal thirds | Ulcers (multiple) | No | NA | NA |
| 44 | 73 F | Diabetes mellitus; Stage V CKD | UGIB | Upper third | Ulcers (multiple) | No | NA | NA |
| 54 | 72 M | COPD | UGIB | Distal third | Ulcers | No | NA | NA |
| 64 | 53 F | Squamous cell carcinoma of middle-third of oesophagus | Dysphagia/ Odynophagia |
Middle and distal thirds | Ulcers | No | NA | NA |
| 74 | 75 F | Diabetes mellitus, DLBCL | Epigastric pain | Distal third | Ulcers | No | NA | NA |
| 85 | 54 M | Ongoing alcoholism | UGIB | Middle third | Necrotising oesophagitis | No | No | No |
| 96 | 53 M | Status postcraniotomy due to traumatic intracerebral bleeding and subdural haematoma under mechanical ventilation; arterial hypertension | UGIB | Upper third | Ulcers (few; deep) | Yes (duodenum) | Intravenous ganciclovir (5 mg/kg 12/12 hour, 17 days) | No |
| 107 | 58 M | Status postleft lobectomy and cholecystectomy due to intrahepatic duct stones and gallstones; ongoing alcoholism | UGIB Epigastric pain Fever |
Distal third | Ulcers (multiple; superficial) | No | Yes (NA) |
No |
| 118 | 76 M | Klatskin tumour (bismuth 1) | Epigastric pain | Distal third | Ulcers (few; superficial) | No | No | No |
| 129 | 69 F | Severe post-ERCP pancreatitis due to suspected choledocholithiasis; blood transfusion | UGIB Odynophagia |
Middle and distal thirds | Ulcers (multiple; deep) | No | Intravenous ganciclovir (5 mg/kg 12/12 hours, 14 days) | Yes (3 months) |
| 1311 | 53 M | Diabetes mellitus; 10 days post-toes amputation due to left calf cellulitis | UGIB | Distal third | Ulcers (multiple; deep) | No | Oral valganciclovir (900 mg/day, 21 days) | No |
| 1412 | 82 F | Aspiration pneumonitis; Parkinson’s disease | UGIB Vomiting |
Entire oesophagus | Erosions | No | Intravenous ganciclovir (5 mg/kg 12/12 hours, 14 days) | No |
| 1513 | 71 F | Diabetes mellitus; arterial hypertension; depression | Fever | Distal third | Ulcers (few; superficial) | Yes (small bowel, colon) | Intravenous ganciclovir (5 mg/kg 12/12 hour, 21 days >5 mg/kg/day, 14 days) | No |
| 1614 | 47 M | Status postaneurysm ligation with subarachnoid bleeding under short-term treatment with corticosteroids; ongoing tobacco smoking | UGIB | Middle and distal thirds | Ulcers (multiple; superficial) Erosions |
Yes (colon) | No | No |
| 1715 | 67 M | Status post-traffic accident with 2 days postsurgery due to burst fracture of the 12th thoracic vertebra and left orbital bone fracture, complicated by ileus and aspiration pneumonitis; diabetes mellitus; COPD; arterial hypertension | Dysphagia Chest pain |
Entire oesophagus | Ulcers (multiple; superficial) | Yes (larynx) | Intravenous ganciclovir (5 mg/kg 12/12 hours, 14 days) | No |
| 1816 | 63 M | Decompensated COPD under short-term treatment with corticosteroids; ongoing tobacco smoking | Odynophagia Chest pain |
Entire oesophagus | Ulcers (multiple; superficial) | No | Intravenous ganciclovir (14 days) | No |
| 1917 | 32 M | Status post-traffic accident with splenectomy by abdominal trauma and multiple fractures with spleen rupture; blood transfusion | Epigastric pain Chest pain Fever |
Distal third | Erosions | No | No | No |
| 2018 | 91 M | NA | Distal third | Ulcers (few) | No | Intravenous ganciclovir (21 days) | No | |
| 2118 | 56 M | NA | Distal third | Ulcers (multiple) | No | Intravenous ganciclovir (21 days) | No | |
| 2218 | 46 F | NA | Distal third | Ulcers (few) | No | Intravenous ganciclovir (21 days) | No | |
| 2318 | 90 F | NA | Distal third | Ulcers (few) | No | Intravenous ganciclovir (21 days) | No | |
| 2419 | 50 M | Obstructive acute renal failure | UGIB | Distal third | Necrotising oesophagitis | No | No | Yes (<1 month) |
| 2520 | 48 F | Ongoing alcoholism and tobacco smoking | Epigastric pain | Distal third | Ulcers (few; deep) | No | Intravenous ganciclovir 5 mg/kg/day, 15 days) | No |
Few ulcers: <3; Multiple ulcers: ≥3.
CAD, coronary artery disease; CKD, chronic kidney disease; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; DLBCL, diffuse large B-cell lymphoma; ERCP, endoscopic retrograde cholangiopancreatography; F, female; LA classification, Los Angeles classification; M, male; NA, not available; UGIB, upper gastrointestinal bleeding.
In immunocompetent hosts, CMV oesophagitis occurred more frequently in male gender (52.0%), with a mean age of 65.2 years and 64.0% patients over 55 years. The youngest reported case was 32 years old. Symptomatology of CMV oesophagitis was variable and non-specific, indistinguishable from other oesophageal conditions, denoting the importance of a high index of clinical suspicion.1 3 8 10 11 13–16 Upper gastrointestinal bleeding was the main presenting symptom of CMV infection (52.4%), followed by epigastric pain (33.3%). At presentation, CMV disease was disseminated to other organs besides the oesophagus in 20.0% of cases with the small bowel being the most involved (60.0%). Endoscopic features were also non-specific, including erosions and ulcers of the oesophagus, but rare complications may occur such as stenosis, fistula, perforation and gastrointestinal bleeding.1 3 4 6–8 11 14 16 18 In this literature review, oesophageal ulceration was the predominant appearance of CMV disease (80.0%), with necrotising oesophagitis in 8.0% of patients. Concerning lesions location, the distal third of the oesophagus was the most involved site (72.0%), with involvement of all oesophageal mucosa in 12.0%.3 4 Diagnosis gold standard includes the collection of biopsies with identification of typical CMV inclusion bodies and/or positive immunohistochemical staining using anti-CMV monoclonal antibody, reaching a sensitivity of 78%–93%.1 3 4 7 11 13 14 CMV diagnosis was histologically proven in all cases. Antiviral therapy with intravenous ganciclovir or oral valganciclovir represents the first-line therapy during at least 2–3 weeks, according to CMV viral load and clinical/endoscopic response.1 6 10 11 14 The majority of patients had critical illness or immunomodulatory comorbidities (84.0%). Only four patients were healthy immunocompetent hosts, as our reported case. Antiviral therapy was started in 70.0% of patients with 90-day mortality rate of 15.0% (3/20). All patients who died had critical illnesses or advanced age. Of the six patients who did not receive antiviral therapy, 66.7% had a full recovery.2–9 11–20
In immunocompetent patients, CMV infection has been considered benign and self-limiting, but severe and life-threatening cases were reported, remaining to be established when to initiate antiviral therapy.1 5 9 10 13–15 17 20 This difficulty is related to the establishment of the potential role of CMV as a pathogenic agent or a mere opportunistic bystander.5 9 15 17 The literature review is in line with the fact that advanced age and comorbidities/critical illnesses are potential risk factors for adverse clinical outcome of CMV oesophagitis in immunocompetent patients.1 6 7 13 15–17
In conclusion, we present a rare case of CMV ulcerative oesophagitis in a young healthy immunocompetent patient with uneventful course without antiviral therapy. This case represents the youngest case being reported in the literature in an immunocompetent patient without critical illnesses or significant comorbidities. Additionally, we reviewed all previously reported cases. This case report and those reviewed highlight the need of a high index of clinical suspicion, particularly in immunocompetent patients. The establishment of antiviral therapy may be reserved for severe CMV disease, advanced age and critical illnesses or immunomodulatory comorbidities.
Learning points.
Cytomegalovirus (CMV) oesophagitis often arises in patients with immunodeficiency or immunosuppression, requiring a high index of clinical suspicion in immunocompetent hosts.
Even in young healthy patients, CMV oesophagitis should be considered in the differential diagnosis of odynophagia without improvement with antacid therapy.
Histopathology of oesophageal ulcers is crucial for the diagnosis of CMV oesophagitis.
Establishment of antiviral therapy in immunocompetent hosts should be considered on a case-by-case basis, being often reserved for severe CMV disease, advanced age and critical illnesses or immunomodulatory comorbidities.
CMV oesophagitis represents a rare condition in young healthy immunocompetent hosts, and an exhaustive investigation to rule out latent immune deficiency should be performed.
Footnotes
Contributors: EG-S and MG-S contributed equally, writing the manuscript and reviewing the literature. EG-S is the article guarantor. EC and LT critically reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gravito-Soares E, Almeida N. Cytomegalovirus disease of the upper gastrointestinal tract: an emerging infection in immunocompetent hosts. GE Port J Gastroenterol 2017;24:259–61. 10.1159/000479974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subedi R, Dean R, Subedi D. Cytomegalovirus esophagitis and duodenitis in immunocompetent patient. J Hosp Med 2017;12(suppl 2) http://www.shmabstracts.com/abstract/cytomegalovirus-esophagitis-and-duodenitis-in-immunocompetent-patient/. [Google Scholar]
- 3.Marques S, Carmo J, Pinto D, et al. . Cytomegalovirus disease of the upper gastrointestinal tract: a 10-year retrospective study. GE Port J Gastroenterol 2017;24:262–8. 10.1159/000479232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HW, Kuo CJ, Lin WR, et al. . The clinical characteristics and manifestations of cytomegalovirus esophagitis. Dis Esophagus 2016;29:392–9. 10.1111/dote.12340 [DOI] [PubMed] [Google Scholar]
- 5.Yagain K, Rao L, Pai K, et al. . Cytomegalovirus esophagitis in nonimmunocompromised patient:presenting as an acute necrotic (black) esophagitis. Indian J Pathol Microbiol 2011;54:852–3. 10.4103/0377-4929.91526 [DOI] [PubMed] [Google Scholar]
- 6.Lee S-Y, Kim YS, Lee J-H, et al. . A case of cytomegalovirus-associated esophageal and duodenal ulcers in a critically ill immunocompetent patient. Korean J Gastrointest Endosc 2009;39:217–21. [Google Scholar]
- 7.Lee KH, Jang BI, Kim KO, et al. . A case of cytomegalovirus esophagitis associated with upper gastrointestinal bleeding. Korean J Med 2009;76:40–3. [Google Scholar]
- 8.Maiorana A, Baccarini P, Foroni M, et al. . Human cytomegalovirus infection of the gastrointestinal tract in apparently immunocompetent patients. Hum Pathol 2003;34:1331–6. 10.1016/j.humpath.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Venkataramani A, Schlueter AJ, Spech TJ, et al. . Cytomegalovirus esophagitis in an immunocompetent host. Gastrointest Endosc 1994;40:392–3. 10.1016/S0016-5107(94)70094-X [DOI] [PubMed] [Google Scholar]
- 10.Lancini D, Faddy HM, Flower R, et al. . Cytomegalovirus disease in immunocompetent adults. Med J Aust 2014;201:578–80. 10.5694/mja14.00183 [DOI] [PubMed] [Google Scholar]
- 11.Tabacelia D, Ilie M, Constantinescu G, et al. . Upper GI bleeding with hemorrhagic shock caused by infectious esophagitis. Modern Medicine 2016;23:314–7. [Google Scholar]
- 12.Hashimoto R, Chonan A. Esophagitis caused by cytomegalovirus infection in an immune-competent patient. Clin Gastroenterol Hepatol 2016;14:e143–e144. 10.1016/j.cgh.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 13.Telakis E, Tsironi E, Papatheodorou K, et al. . Debilitating chronic diarrhea caused by generalized gastrointestinal cytomegalovirus infection in an immunocompetent adult. Case Rep Gastrointest Med 2014;2014:1–4. 10.1155/2014/260120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KY, Cho KB, Hwang JY, et al. . A case of CMV infection associated with simultaneous esophageal and colonic ulcers. Korean J Gastrointest Endosc 2003;26:26–30. [Google Scholar]
- 15.Lim DS, Lee TH, Jin SY, et al. . Cytomegalovirus esophagitis in an immunocompetent patient: case report. Turk J Gastroenterol 2014;25:571–4. 10.5152/tjg.2014.4073 [DOI] [PubMed] [Google Scholar]
- 16.Weile J, Streeck B, Muck J, et al. . Severe cytomegalovirus-associated esophagitis in an immunocompetent patient after short-term steroid therapy. J Clin Microbiol 2009;47:3031–3. 10.1128/JCM.00143-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villar LA, Massanari RM, Mitros FA. Cytomegalovirus infection with acute erosive esophagitis. Am J Med 1984;76:924–8. 10.1016/0002-9343(84)91011-8 [DOI] [PubMed] [Google Scholar]
- 18.Reggiani Bonetti L, Losi L, Di Gregorio C, et al. . Cytomegalovirus infection of the upper gastrointestinal tract: a clinical and pathological study of 30 cases. Scand J Gastroenterol 2011;46:1228–35. 10.3109/00365521.2011.594083 [DOI] [PubMed] [Google Scholar]
- 19.Barjas E, Pires S, Lopes J, et al. . Cytomegalovirus acute necrotizing esophagitis. Endoscopy 2001;33:735 10.1055/s-2001-16215 [DOI] [PubMed] [Google Scholar]
- 20.Altman C, Bedossa P, Dussaix E, et al. . Cytomegalovirus infection of esophagus in immunocompetent adult. Dig Dis Sci 1995;40:606–8. 10.1007/BF02064378 [DOI] [PubMed] [Google Scholar]