Abstract
Diabetic ketoacidosis (DKA) during pregnancy is a serious metabolic complication of diabetes with high mortality and morbidity if not detected and treated immediately. We report a case of a woman with type 1 diabetes mellitus who had poorly controlled diabetes in the first half of pregnancy and developed DKA at 29 weeks gestation. At presentation, she had a pathological fetal heart tracing but delivery was delayed for maternal stabilisation and reversal of acidosis. Once hyperglycaemia, acidosis and maternal stabilisation were achieved, fetal compromise resolved and delivery was no longer indicated. The patient was subsequently discharged home. She delivered vaginally a 2400 g baby at 34 weeks gestation after presenting with spontaneous rupture of membranes.
Keywords: obstetrics, gynaecology and fertility; diabetes
Background
Diabetic ketoacidosis (DKA) is a medical emergency. It demands prompt and vigorous treatment. It occurs in about 3%1 2 of diabetic pregnant women and can be complicated with up to 35% fetal loss.1 In pregnancy, it can manifest at lower blood glucose levels and develop faster than in non-pregnant women, which can cause delay in diagnosis and treatment.3 Often the presenting fetal heart rate monitoring can show non-reassuring fetal status and this can be mistaken for a diagnosis of abruption leading to premature delivery and negative findings for abruption. Subjecting a woman in DKA to an emergency caesarean section could also cause further maternal deterioration with minimal benefit to the baby. In this case report, we showed that a pathological fetal tracing was not necessarily an indication for immediate delivery in a woman with DKA. This was proven with the resolution of fetal compromise and good outcomes for both the mother and baby.
Case presentation
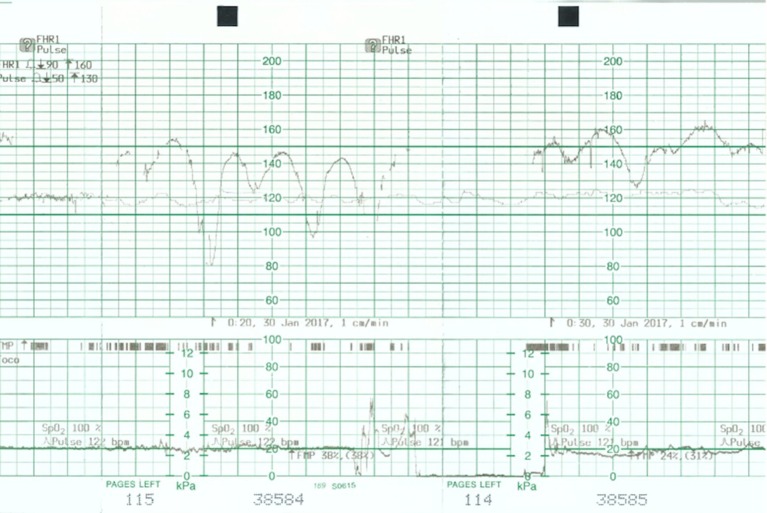
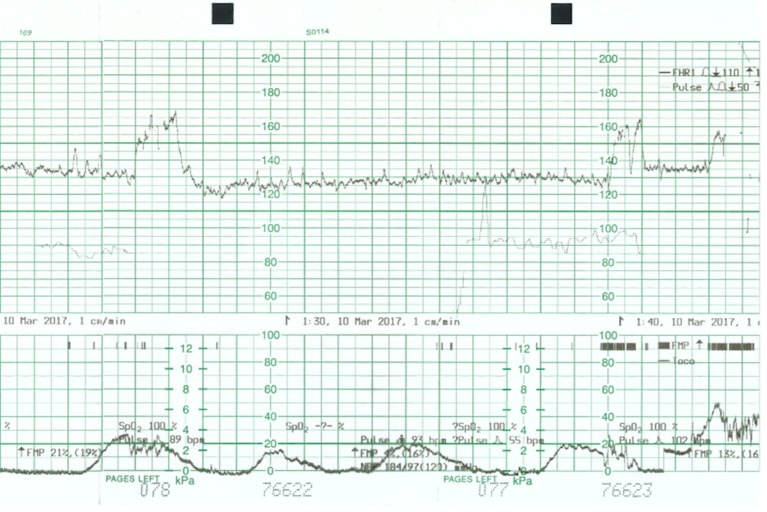
A 34-year-old woman, gravida 5, para 2, with type 1 diabetes of 16 years duration, and with no evidence of diabetic vascular complications, presented at 29 weeks gestation with 1 day history of persistent abdominal pain, diarrhoea, vomiting and decreased fetal movements. On questioning she admitted to have omitted her insulin for a day because of vomiting and decreased oral intake. She never had any previous admissions for diabetes and regularly attended the diabetes clinic. During this pregnancy, she was seeing her own diabetes specialist for blood glucose control. Her glycaemic control as measured by glycated haemoglobin (HbA1c) was 8.5% a week before presentation. On examination she was tachypnoeic with Kussmaul breathing, tachycardic and dehydrated. Her abdomen was soft, non-tender and the uterus was relaxed. Systemic examination showed no sign of infection. Continuous fetal monitoring showed a pathological fetal heart tracing with repetitive unprovoked decelerations and reduced variability (figure 1). There was no uterine activity on the tocograph. A bedside ultrasound done showed a fetus of estimated weight 1.7 kg, cephalic presentation, upper anterior placenta and normal amniotic fluid index. Based on these clinical signs, a differential diagnosis of DKA versus DKA and concurrent placental abruption was made. The absence of abdominal pain or per vaginal bleeding or abnormal hyperechoic placenta on ultrasound made the latter less likely. Delivery was initially proposed because of severe fetal distress. However, delivery was held off after the discovery of the maternal DKA status and the suspicion that this was the underlying cause of the pathological fetal heart pattern. The decision was then made for aggressive resuscitation of the mother and prompt correction of her DKA while maintaining close observation for improvement in the fetal heart tracing.
Figure 1.
Cardiotocograph shows poor variability and persistent unprovoked decelerations.
Investigations
Biochemistry: Venous plasma glucose 20 mmol/L, sodium 132 mmol/L, potassium 6.1 mmol/L, bicarbonate 2.9 mmol/L, chloride 103.5 mmol/L, creatinine 55 mmol/L, hydroxybutyrate 5.6 mmol/L, CRP 64.
Haematology: Haemoglobin 15.2 g/dL, white cell count 14.54×109/L.
Arterial blood gas: pH 7.15, PO2 138 mm Hg, PCO2 11.7 mm Hg. Urine dipstick showed 4+ ketones, 4+ glucose, no white cell count, no red cell count and no nitrates. Urine, stool and blood cultures were negative 72 hours later.
Treatment
She was immediately admitted to the labour ward and resuscitated with intravenous fluids, started on empiric intravenous ceftriaxone and an insulin infusion for DKA. An endocrinologist was involved in her care, blood capillary glucose was monitored hourly and blood tests repeated every 2 to 6 hours (table 1); per rectal resonium 15 g and 10% calcium gluconate were recommended for hyperkalaemia. She was placed in left lateral position and given supplementary oxygen to decrease aortocaval compression and increase fetal oxygenation. She was given a total of 3 L of normal saline intravenously. Initial ECG showed a heart rate of 120 beats per minute. Subsequent blood tests done 4 hours later showed serum ketones 2.6 mmol/L, glucose 11 mmol/L and potassium 4.8 mmol/L. The shifting mechanism of metabolic acidosis was kept in mind, and potassium was added into her intravenous hydration regime. As hyperglycaemia and acidosis were reversed, the electronic continuous fetal monitoring showed improvement in the trace with resolution of the decelerations and improvement in variability (figures 2–4) over the subsequent 20 hours. A fetal ultrasound scan 8 hours after presentation showed a well-grown baby with an estimated fetal weight of 1309 g, normal amniotic fluid index and normal Dopplers. Fetal compromise was no longer evident and a reactive trace was seen, 24 hours after initial presentation, while maternal ketoacidosis resolved. Intravenous insulin was gradually withdrawn and replaced with regular subcutaneous insulin.
Table 1.
Laboratory data on presentation and after starting treatment
| At presentation | 2 hours later | 4 hours later | 8 hours later | 16 hours later | Normal range | |
| Analysis on air | ABG | VBG | – | VBG | VBG | |
| pH | 7.15 | 7.23 | 7.26 | 7.33 | 7.35–7.45 | |
| pCO2 (mm Hg) | 11.7 | 22.3 | 26.2 | 27.7 | 35–40 | |
| pO2 (mm Hg) | 138.0 | 69.5 | 40.4 | 62.7 | 75–100 | |
| HCO3 (mmol/L) | 7.4 | 13.8 | 16.4 | 21–27 | ||
| Glucose (mmol/L) | 20 | 12.9 | 11.9 | 11.3 | 14.3 | 3.9–11 |
| Sodium (mmol/L) | 132 | 134 | 134 | 133 | 137 | 136–146 |
| Potassium (mmol/L) | 6.1 | 4.8 | 4.8 | 4.5 | 3.7 | 3.6–5.0 |
| Bicarbonate (mmol/L) | 2.9 | 4.6 | 8.7 | 11.3 | 15.3 | 19–29 |
| Ketones (mmol/L) | 5.6 | – | 2.6 | – | – | 0–0.6 |
| Creatinine (mmol/L) | 55 | 57 | 44 | 34 | 37–75 | |
| CRP | 64 | <5 | ||||
| Haemoglobin (g/dL) | 15.2 | 12–16 | ||||
| WBC count (109/L) | 14.54 | 4–10 |
ABG, arterial blood gas; CRP, C reactive protein; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; VBG, venous blood gas; WBC, white blood cell.
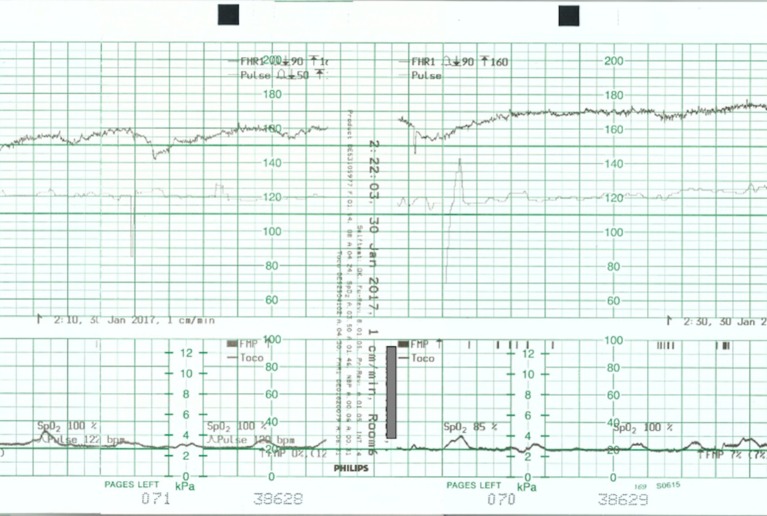
Figure 2.
Cardiotocograph shows a baseline of 160 beats per minute and improved variability.
Figure 3.
Cardiotocograph shows a baseline of 170 beats per minute, improved variability and occasional decelerations with recovery to baseline.
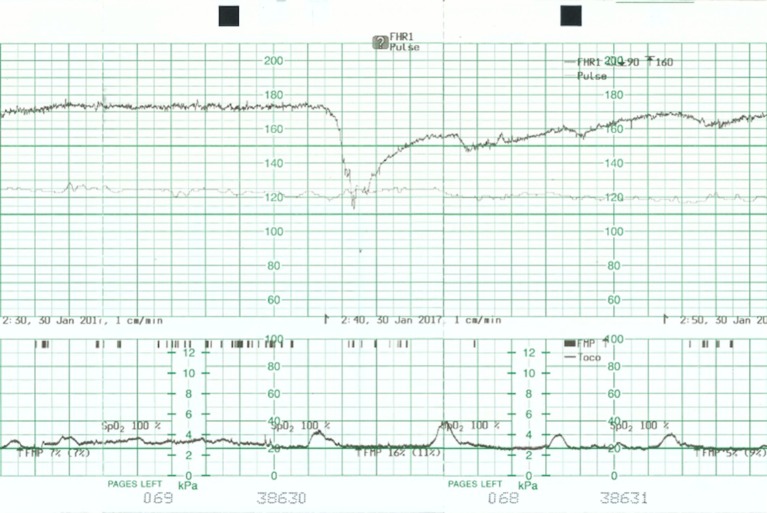
Figure 4.
Cardiotocograph shows a baseline of 150 beats per minute and normal variability.
Outcome and follow-up
She was subsequently discharged from hospital after 6 days with endocrinologist and obstetrician outpatient follow-up. At 34 weeks gestation, she presented with spontaneous rupture of membranes and had a precipitous normal vaginal delivery. Cardiotocograph (figure 5) at presentation was normal. A female baby was born weighing 2400 g, with Apgar scores of 8 and 9.
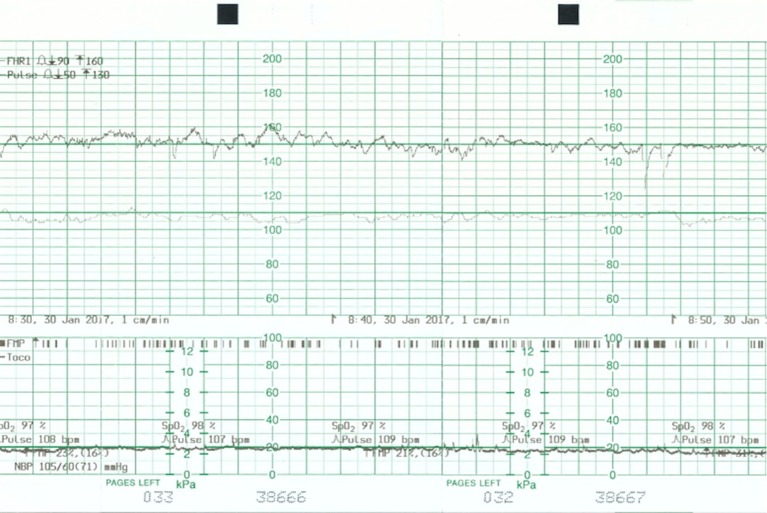
Figure 5.
Cardiotocograph shows a baseline 130 beats per minute, normal variability and accelerations.
Discussion
Diabetic ketoacidosis (DKA) in pregnancy is a medical emergency that warrants immediate treatment and resuscitation in a high dependency unit with combined obstetric and medical care to minimise maternal and fetal mortality and morbidity.2 Conditions and risk factors that can contribute to the development of DKA include hyperemesis, gastroenteritis, infection, non-compliance with insulin regime, use of steroids and insulin pump failure. During pregnancy, a woman is in a state of relative insulin resistance, respiratory alkalosis and has changes in the levels of progesterone, cortisol and human placental lactogen.4 These characteristics unique to pregnancy predispose them to DKA. Fetal distress results from a compromised uteroplacental perfusion secondary to maternal volume deletion, acidaemia and hypoxaemia. These changes have proven to resolve if the maternal metabolic acidotic state reverses.4
This case illustrates that fetal heart tracing performed during DKA can demonstrate fetal acidotic changes, representing the effect of maternal acidosis on the fetus. If the acidosis is not corrected, fetal mortality is likely to follow. Correction of these changes occurs after maternal hydration and treatment of maternal acidosis. This may take 4 to 24 hours as seen in this case.
We reviewed seven case reports5–10 of pregnant women with type 1 diabetes who presented unwell and diagnosed with DKA. This review showed that six out of seven women responded to prompt resuscitation with intravenous fluids and insulin. However, out of these seven cases, four women had intrauterine fetal demise at presentation. One woman was aggressively treated at presentation and the reversal of maternal condition and fetal distress was seen. Another woman was misdiagnosed and this caused delayed therapy resulting in fetal death. These cases show the importance of timely diagnosis and treatment of pregnant women with DKA.
The high fetal mortality in untreated maternal DKA proves that it creates a harsh intrauterine environment for the fetus. The exact pathophysiology resulting in fetal demise is unclear; however, it may be due to several factors. Fetal distress from a decrease in uteroplacental perfusion secondary to maternal dehydration, acidosis and increased catecholamines,2 11 fetal hyperinsulinaemia from hyperglycaemia can result in hypokalaemia leading to fetal arrhythmia and myocardial suppression.12 The resultant fetal acidosis and changes in electrolyte balance hampering oxygen delivery to the fetus by decreasing 2,3-diphosphoglycerate secondary to maternal hypophosphatemia13 and fetal hyperinsulinaemia, thereby stimulating the oxidative metabolic pathway and resulting in increased fetal oxygen needs.14
In another case report,15 a woman presented at 33 weeks gestation with DKA after her insulin pump malfunctioned. The fetal heart trace showed poor variability with late decelerations. These changes resolved after she was given intravenous fluids, insulin and potassium replacement and she delivered uneventfully at term. Immediate delivery in response to the presence of a pathological fetal heart trace is not indicated until the metabolic condition is corrected because an emergency caesarean delivery could further worsen the maternal condition with minimal benefit to the fetus.3
When a clinician is faced with a situation in which the mother has a serious medical disorder such as DKA and the fetus shows a pathological fetal heart pattern, the intuitive response is to deliver the fetus immediately. It is prudent to remember that the initial response to a pathological fetal heart trace is to correct an underlying cause, if possible. Delivery of a severely premature and acidotic fetus in an ill mother is likely to result in poor outcome for both. We would recommend an approach that includes aggressive in utero resuscitation to stabilise the woman with replacement of fluid deficit and electrolytes, concurrent with reversal of metabolic acidosis under the supervision of a multidisciplinary team. Frequent re-evaluations of the clinical situation at close intervals are also paramount to ensure the safety of the mother and baby. If fetal compromise persists despite adequate maternal resuscitation, fetal delivery should be considered. The individual clinical context must of course be taken into consideration for the timing of delivery, for example if the suspicion of other causes of fetal compromise such as a placental abruption is high, there may not be time to wait for the reversal of DKA before delivery.
Patient’s perspective.
Initially I thought I had stomach influenza or food poisoning since it happened on the second day of Chinese New Year. I did not realise the seriousness until the doctor told me I needed to deliver soon with an emergency caesarean section.
I contacted my trusted endocrinologist doctor who assured me that the obstetric doctors had to do what they needed to do. Still, when I heard ultimately the senior consultant decided to reverse the DKA and monitor the situation, I heaved a sigh of relief.
Things got better but there were still some decelerations of my baby’s heartbeat intermittently but beside the cardiotocograph done every 8 hours there was nothing for me to do and I could not sleep and rest well in the ward.
As I also have two older kids to look after, after much consideration I decided to discharge at own risk. It was an awful feeling but I needed to logistically be around for my older kids.
Learning points.
Diabetic ketoacidosis (DKA) is an emergency that requires prompt and rigorous resuscitation with intravenous fluids and insulin in addition to a multidisciplinary involvement of the endocrinologist, anaesthetist and obstetrician.
A pathological fetal heart tracing, with recurrent decelerations and low variability in a pregnant woman with DKA, is not necessarily an indication for immediate delivery.
An emergency caesarean section in a woman with DKA can cause further maternal deterioration.
Reversal of hyperglycaemia, metabolic acidosis and maternal stabilisation can reverse the fetal compromise seen on fetal heart tracing.
Footnotes
Contributors: YHGN wrote the case report and obtained patient consent. TXE edited the case report. DK made a substantial involvement in the case and revision of the case report. HKT served as the primary doctor of the patient and played a substantial role in conceptualising the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chauhan SP, Perry KG, McLaughlin BN, et al. Diabetic ketoacidosis complicating pregnancy. J Perinatol 1996;16:173–5. [PubMed] [Google Scholar]
- 2.Sibai BM, Viteri OA. Diabetic ketoacidosis in pregnancy. Obstet Gynecol 2014;123:167–78. 10.1097/AOG.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 3.Chauhan SP, Perry KG. Management of diabetic ketoacidosis in the obstetric patient. Obstet Gynecol Clin North Am 1995;22:143–55. [PubMed] [Google Scholar]
- 4.Mohan M, Baagar KAM, Lindow S. Management of diabetic ketoacidosis in pregnancy. Obstet Gynecol 2017;19:55–62. 10.1111/tog.12344 [DOI] [Google Scholar]
- 5.Himuro H, Sugiyama T, Nishigori H, et al. A case of a woman with late-pregnancy-onset DKA who had normal glucose tolerance in the first trimester. Endocrinol Diabetes Metab Case Rep 2014;2014:130085 10.1530/EDM-13-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamalakannan D, Baskar V, Barton DM, et al. Diabetic ketoacidosis in pregnancy. Postgrad Med J 2003;79:454–7. 10.1136/pmj.79.934.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MY, Lin KD, Chang YH, et al. Fulminant type 1 diabetes mellitus leading to fetal loss in a pregnant Chinese woman. Kaohsiung J Med Sci 2010;26:316–20. 10.1016/S1607-551X(10)70045-0 [DOI] [PubMed] [Google Scholar]
- 8.Yan JH, Zhang GC, Zhu YH, et al. Fulminant type 1 diabetes during pregnancy in Chinese patients. Kaohsiung J Med Sci 2014;30:161–2. 10.1016/j.kjms.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 9.Carroll MA, Yeomans ER. Diabetic ketoacidosis in pregnancy. Crit Care Med 2005;33:S347–S353. 10.1097/01.CCM.0000183164.69315.13 [DOI] [PubMed] [Google Scholar]
- 10.Hagay ZJ, Weissman A, Lurie S, et al. Reversal of fetal distress following intensive treatment of maternal diabetic ketoacidosis. Am J Perinatol 1994;11:430–2. 10.1055/s-2007-994613 [DOI] [PubMed] [Google Scholar]
- 11.Blechner JN, Stenger VG, Prystowsky H. Blood flow to the human uterus during maternal metabolic acidosis. Am J Obstet Gynecol 1975;121:789–94. [PubMed] [Google Scholar]
- 12.Kitzmiller J. Diabetic ketoacidosis and pregnancy. Contemp Obstet Gynecol 1982;20:141–7. [Google Scholar]
- 13.Ditzel J, Standl E. The oxygen transport system of red blood cells during diabetic ketoacidosis and recovery. Diabetologia 1975;11:255–60. 10.1007/BF00422388 [DOI] [PubMed] [Google Scholar]
- 14.Philipps AF, Rosenkrantz TS, Raye J. Consequences of perturbations of fetal fuels in ovine pregnancy. Diabetes 1985;34(Suppl 2):32–5. 10.2337/diab.34.2.S32 [DOI] [PubMed] [Google Scholar]
- 15.Má GR, D GR, Mj P, et al. Managing diabetic ketoacidosis in pregnancy. Saudi J Anaesth 2016;10:238–9. 10.4103/1658-354X.168829 [DOI] [PMC free article] [PubMed] [Google Scholar]