Summary
A serum biomarker of biological versus chronological age would have significant impact on clinical care. It could be used to identify individuals at risk of early‐onset frailty or the multimorbidities associated with old age. It may also serve as a surrogate endpoint in clinical trials targeting mechanisms of aging. Here, we identified MCP‐1/CCL2, a chemokine responsible for recruiting monocytes, as a potential biomarker of biological age. Circulating monocyte chemoattractant protein‐1 (MCP‐1) levels increased in an age‐dependent manner in wild‐type (WT) mice. That age‐dependent increase was accelerated in Ercc1 −/Δ and Bubr1 H/H mouse models of progeria. Genetic and pharmacologic interventions that slow aging of Ercc1 −/Δ and WT mice lowered serum MCP‐1 levels significantly. Finally, in elderly humans with aortic stenosis, MCP‐1 levels were significantly higher in frail individuals compared to nonfrail. These data support the conclusion that MCP‐1 can be used as a measure of mammalian biological age that is responsive to interventions that extend healthy aging.
Keywords: biological age, biomarkers of aging, CCL2, chemokine, geropathology, monocyte chemoattractant protein‐1
1. INTRODUCTION
Aging is the major risk factor for numerous chronic diseases and is responsible for the bulk of healthcare costs (Goldman et al., 2013). The fastest growing segment of the world population is the elderly, causing an exponential rise in the incidence of chronic diseases. To address this healthcare crisis, there is a growing interest in identifying ways to therapeutically target aging in order to prevent, delay or attenuate multiple age‐related diseases simultaneously (Burd et al., 2016). A number of therapeutic strategies have emerged (Barzilai, Crandall, Kritchevsky & Espeland, 2016; Harrison et al., 2009; Zhu et al., 2015). However, a major barrier to clinical trials targeting aging is the prolonged time between intervention and clinical outcomes (e.g., incidence of age‐related morbidities) and surrogate endpoints are desperately needed. The first clinical trial aimed at delaying the processes that cause aging (TAME: Targeting Aging with Metformin) will soon begin (Barzilai et al., 2016). If this trial is successful, new clinical trials will quickly follow. For these studies, surrogate endpoints will dramatically improve the economy and timescale in which we can measure the effects of interventions on biological age (Niedernhofer, Kirkland & Ladiges, 2016).
Biological age is defined by the health or fitness of an individual, and lack of age‐related diseases, irrespective of their chronological age (Liang et al., 2016). Biological age can be quite distinct from chronological age. For example, cancer survivors are biologically older than their chronological age due to exposure to genotoxic agents, while centenarians are frequently biologically younger than their chronological age (Govindaraju, Atzmon & Barzilai, 2015; Ness et al., 2013). A biomarker of biological age in accessible bodily fluids or tissues would be extremely valuable for clinical trials testing antigeronic factors, but also potentially for triaging patients facing onerous therapeutic procedures. Hundreds of studies have aimed to discover age‐related changes in circulating factors including metabolites, advanced glycation end‐products, exosome content, miRNA, and inflammatory molecules, with varying success. The most successful example of measuring biological age to date is detection of DNA methylation at a subset of CpG islands (Horvath, 2013).
2. RESULTS
In hopes of identifying a factor in peripheral blood that correlates with biological age, multiple serum cytokines and chemokines were measured in young and old WT mice using a Luminex platform designed to detect 14 circulating peptides in mouse plasma (Figure S1 and Appendix S1.). Notably, neither TNF‐α nor IL‐6 was increased in aged mice compared to young. In contrast, in this targeted analysis, MCP‐1 was the only peptide that increased significantly and reproducibly with chronological age (Figure 1a). Monocyte chemoattractant protein‐1 (MCP‐1/CCL2) is a chemokine produced by a number of cell types including endothelial, epithelial, mesangial, myocytes, monocytes, and microglial cells, either in a constitutive manner or in response to various stimulants, such as oxidative stress, cytokines, and growth factors (Deshmane, Kremlev, Amini & Sawaya, 2009). Monocyte chemoattractant protein‐1 is a potent monocyte chemoattractant that binds the CCR2 receptor and induces monocytes to exit the bloodstream to become tissue macrophages in response to inflammatory signals (Deshmane et al., 2009).
Figure 1.
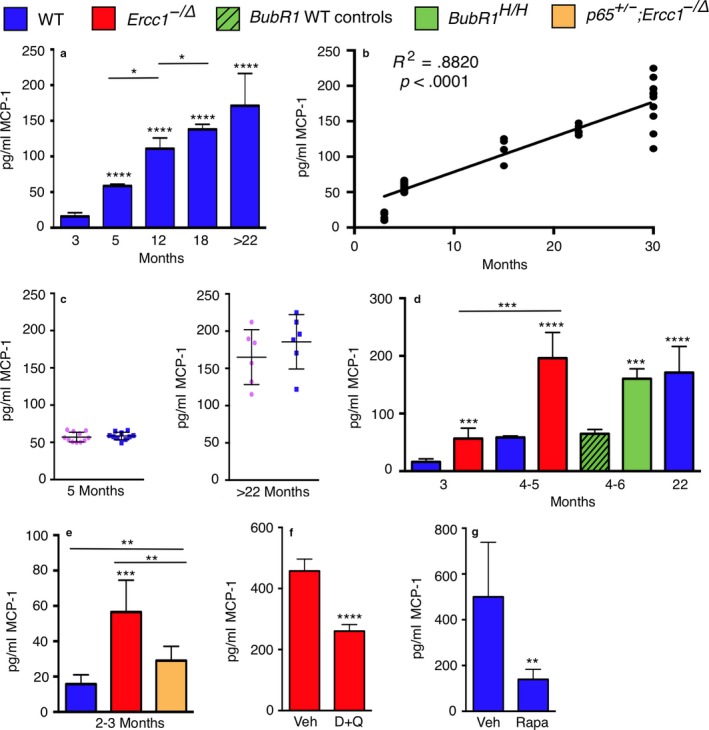
Circulating MCP‐1 levels correlate with biological age. (a) Detection of MCP‐1 in the serum of mice by ELISA. All mice were WT f1 of varying ages and gender. (b) Linear regression analysis of the same data showing a highly significant correlation between serum MCP‐1 and chronological age. (c) Graphing of the same date by gender (pink females; blue male mice). (d) MCP‐1 serum concentrations were quantified by ELISA in progeroid Ercc1 −/Δ and Bubr1 H/H mice and WT littermate controls. WT (blue), Ercc1 −/Δ (red), Bubr1 H/H (green) and WT controls (green with black slashes). (e) Genetic depletion of NF‐κB in p65 +/− ;Ercc1 −/Δ mice (yellow), which extends the healthspan of the progeroid mice, reduces MCP‐1 levels relative to Ercc1 −/Δ. Five to six mice were used per group except for Bubr1 and their respective wild‐type controls (n = 3). (f) 16‐week‐old Ercc1 −/Δ mice (5–6 per group) treated with vehicle (Veh) or a combination of the senolytic drugs dasatinib and quercetin (D+Q) weekly starting at 4–6 weeks, and (g) 26‐month‐old WT mice (6 per group) that were placed on a rapamycin (Rapa) or control (Ctrl) diet for 8 weeks prior to analysis of serum MCP‐1 by ELISA. Values represent the mean ± SD, two‐tailed t test. p < .05*, p < .01**, p < .001***, p < .0001****, p < .00001*****
Numerous studies previously demonstrated that plasma levels of MCP‐1 correlate with chronologic age in humans (Brouwers et al., 2015; Deo et al., 2004; Inadera, Egashira, Takemoto, Ouchi & Matsushima, 1999; Mansfield et al., 2012; Pinke et al., 2013; Scully et al., 2016) and mice (Chiao et al., 2011). Monocyte chemoattractant protein‐1 is a senescence‐associated secretory phenotype (SASP) factor secreted by senescent cells (Jin et al., 2016). Senescence‐associated secretory phenotype can promote secondary senescence in healthy cells (Coppe, Desprez, Krtolica & Campisi, 2010), and senescent cells have been demonstrated to promote aging and age‐related disease (Baker et al., 2011, 2016; Zhu et al., 2015). Circulating levels of MCP‐1 are increased in patients with renal disease (Akdogan et al., 2015), cognitive impairment and Alzheimer's disease (Bettcher et al., 2016), atherosclerosis and cardiovascular disease (Deo et al., 2004). Monocyte chemoattractant protein‐1 is considered to be a marker of “inflammaging,” defined as chronic sterile inflammation that is associated with numerous age‐related diseases (Franceschi & Campisi, 2014). Therefore, we focused on MCP‐1 as a potential biomarker of biological age because it is readily measured in humans, with a relatively small coefficient of variation compared to other inflammatory markers (Figure S2), and there is a rationale for it potentially correlating with aging rather than merely inflammation.
As previously shown in inbred C57BL/6 mice (Chiao et al., 2011), MCP‐1 levels increased linearly with the chronological age of WT f1 mice (FVB/n;C57BL/6; Figure 1b). It is interesting to note that the interindividual variation in MCP‐1 levels increased dramatically in older mice (Figure 1a,b). This is consistent with aging being incredibly heterogeneous at the physiological and molecular level (Burd et al., 2013; Lowsky, Olshansky, Bhattacharya & Goldman, 2014). Also of note, no sex‐based differences in MCP‐1 levels were detected in mice (Figure 1c).
To determine whether MCP‐1 levels corresponded with biological rather than chronological age, we measured serum MCP‐1 in two unrelated models of accelerated aging. Ercc1 −/Δ mice model a human progeroid syndrome caused by defective DNA repair (Niedernhofer et al., 2006), have a median lifespan of 5 months (Dolle et al., 2011) and spontaneously develop numerous diseases and pathologies associated with old age in humans (Table S1). BubR1 H/H mice age rapidly due to defective mitotic spindle assembly checkpoint and have a median lifespan of 6 months (Table S2). In both progeroid strains, serum MCP‐1 levels were significantly increased compared to age‐matched WT mice (Figure 1d). To validate these ELISA data, we used Luminex to measure MCP‐1 in Ercc1 −/Δ mouse serum and observed a significant increase in MCP‐1 compared to age‐matched controls (Figure S3). Notably, at an age equivalent to the median lifespan of Ercc1 −/Δ and BubR1 H/H mice, serum MCP‐1 levels were equivalent to that of 22‐month‐old WT mice, an age when WT mice begin to display age‐related pathologies (Fox, 2007). The data are not strain dependent as the Ercc1 −/Δ and naturally aged mice were in an f1 (C57BL/6;FVB) genetic background, while the BubR1 H/H mice were C57BL/6.
To determine whether MCP‐1 levels can detect reduced biological age, serum chemokine levels were measured in p65 +/−;Ercc1 −/Δ mice. We previously established that genetic depletion of the RelA/p65 subunit of NF‐κB significantly extends the healthspan of Ercc1 −/Δ mice (Tilstra et al., 2012). Indeed, p65 +/−;Ercc1 −/Δ mice had significantly reduced circulating levels of MCP‐1 compared to age‐matched Ercc1 −/Δ mice (Figure 1e). Together, these data support the conclusion that MCP‐1 is a better marker of biological than chronological age.
Monocyte chemoattractant protein‐1 expression is increased in fibroblasts from Hutchinson–Gilford progeria syndrome patients compared to control cell lines (Csoka et al., 2004). This was recapitulated in mouse embryonic fibroblasts derived from Ercc1‐deficient mice. Monocyte chemoattractant protein‐1 expression was elevated in Ercc1 −/− MEFs compared to WT as early as passage 2 and levels increased significantly in both WT and Ercc1 −/− cells with passaging (Figure S4a and Table S3 for primers). Similarly, MCP‐1 protein abundance was higher in the media of p7 cells compared to p2, and significantly greater in Ercc1 −/− MEFs compared to WT (Figure S4b). The MCP‐1 data corresponded with a significant increase in the expression of other markers of cellular senescence in the Ercc1 −/− cells relative to WT (p16 and p21; Figure S4c‐d). Thus, MCP‐1 expression, at both the RNA and protein level, may serve as an indicator of the burden of senescent cells, which drive aging.
By definition, a biomarker of biological age should respond to therapeutic interventions proven to significantly improve healthspan or lifespan. Here, we measured serum MCP‐1 in two distinct, established intervention paradigms. Genetic or pharmacologic ablation of senescent cells extends healthspan of mice (Baker et al., 2016; Zhu et al., 2015). A combination of two senolytic drugs (dasatinib and quercetin) extends the healthspan of Ercc1 −/Δ mice and delays multiple age‐related pathologies (Zhu et al., 2015). In that study, Ercc1 −/Δ mice were treated weekly with a combination of dasatinib (5 mg/kg) and quercetin (50 mg/kg) for 10 weeks, starting at 6 weeks of age. Here, we analyzed serum from these mice for circulating levels of MCP‐1. Ercc1 −/Δ mice treated with D+Q had significantly lower circulating concentrations of MCP‐1 than vehicle‐treated controls (Figure 1f). Of note, serum MCP‐1 levels in the vehicle only group of Ercc1 −/Δ mice in this study are higher than those of untreated animals Ercc1 −/Δ mice (4–6 months Ercc1 −/Δ mice in Figure 1d was ~175 pg/mL vs. ~400 pg/ml in 4‐month‐old mice in Figure 1f). We attribute this to the repeated i.p. injections and frequent handling of the Ercc1 −/Δ mice in the latter study, which exacerbates their frailty.
Rapamycin, an inhibitor of the mTOR kinase, causes a significant extension in the lifespan of WT mice (Harrison et al., 2009). Furthermore, late‐life intervention with rapamycin is sufficient to reduce multiple characteristics of cardiac aging (Dai et al., 2014). Two‐year‐old C57BL/6J mice were fed a diet containing rapamycin (14 ppm for females or 42 ppm for males) or a control diet for 2 months. Longitudinal echocardiography demonstrated that rapamycin significantly reversed aging‐related decline in cardiac performance and substantially attenuated cardiac hypertrophy, as previously described (Dai et al., 2014). In addition, rapamycin attenuated composite lesion scores in kidneys (Figure S5), liver, and lungs of these mice by an average of 40%, 41%, and 29%, respectively. Composite lesion scores generated by a geropathology grading platform have been shown to increase in mice in an age‐dependent manner and align with biological age (Ladiges et al., 2017). Serum levels of MCP‐1 were significantly decreased in 26‐month‐old WT mice after treatment with rapamycin compared to controls (Figure 1g). These data provide strong experimental evidence that in preclinical models, circulating MCP‐1 levels serve as a surrogate endpoint; that is, it responds to interventions that improve clinical endpoints of healthy aging, irrespective of the chronological age of the animals.
Interestingly, MCP‐1 levels were greater in inbred C57BL/6NJ mice compared to age‐matched f1 mice (~500 pg/ml for vehicle‐treated 26‐month‐old C57BL/6NJ mice in Figure 1f compared to ~175 pg/ml for f1 C57BL/6J:FVB/NJ mice >22 months of age in Figure 1a). This suggests that f1 mice are biologically younger than chronologically age‐matched inbred mice. In fact, f1 mice are healthier and longer‐lived than inbred mice (Flurkey, Currer & Harrison, 2006). In addition, inbred mice accumulate numerous age‐related histopathological lesions in multiple organs at an earlier age than f1 mice (Ladiges et al., 2017) (Figure S6). The fact that rapamycin lowers serum MCP‐1 levels to a range consistent with f1 mice suggests that rapamycin reverses aging.
Our findings demonstrate striking associations between circulating MCP‐1 concentrations and biological age in multiple mouse strains. However, establishing whether a comparable relationship exists in humans is necessary for determining translational utility. Accordingly, we measured plasma MCP‐1 levels in a cohort of older adults undergoing valve replacement surgery for severe aortic stenosis (Table S4). Cardiovascular health study (CHS) frailty testing was conducted as a surrogate measure of biological age, using the presence of three or more frailty criteria (slow gait, weak grip, reduced physical activity, low endurance, and unintentional weight loss) as an operational frailty definition (Fried et al., 2001). Within this sample of 27 women and 36 men, mean age of 81 years, circulating MCP‐1 levels were 54% higher in frail participants (Figure 2). As frailty status was associated with age and sex (Table S4), we also applied linear regression analyses to control for these factors. A one unit increase in the natural log of MCP‐1 levels was associated with a 0.86 unit increase in frailty score, and the strength and significance of this relationship did not meaningfully change after adjusting for age, sex, or combined age and sex (Table S5). To further explore potential sex differences, we split our sample into male and female groups and applied univariate linear regression. A one unit increase in natural log MCP‐1 levels corresponded to a 0.74 and 1.45 unit increase in frailty score in women (p = .004) and men (p = .002), respectively (Table S6; Figure S7). Thus, we conclude that circulating MCP‐1 concentrations are a robust indicator of biological age in humans, regardless of sex.
Figure 2.
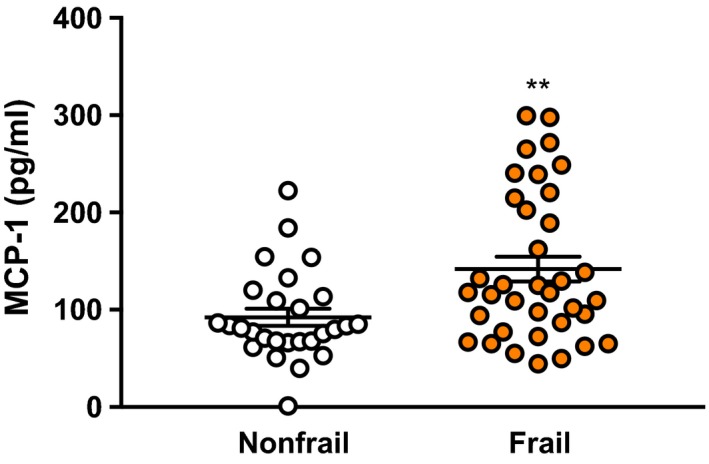
Circulating MCP‐1 levels are elevated in frail older adults. Plasma MCP‐1 concentrations were quantified by a Luminex platform. Frail individuals possessed three or more of the following criteria: slow gait, weak grip, reduced physical activity, low endurance, and unintentional weight loss. Graphed are individual values. The black bars represent the mean ± SEM (nonfrail n = 27, frail n = 36, Mann–Whitney test, **p = .009)
3. DISCUSSION
There are many prior studies correlating inflammatory biomarkers with chronological age, age‐related disease or functional decline (Charlton et al., 2017; Collerton et al., 2012; Figueroa‐Vega, Moreno‐Frias & Malacara, 2015; Franceschi, Monti, Sansoni & Cossarizza, 1995; Julian et al., 2015; Kleinschmidt et al., 2016; Lippi, Sanchis‐Gomar & Montagnana, 2014; Lu et al., 2016; Matsushima et al., 2015; Nadrowski et al., 2016; Noren Hooten, Ejiogu, Zonderman & Evans, 2012; Sesso et al., 2015). A very comprehensive study by Collerton et al. identified low IL‐6 or TNF‐α as negatively correlating with risk of frailty, while high C‐reactive protein and low albumin correlated with a high risk of frailty (Collerton et al., 2012). MCP‐1 was not measured in this study. In contrast to our study, Lu et al., in a small study, found MCP‐1 to be negatively associated with frailty, as was IL‐6R (Lu et al., 2016). Other studies found a lack of correlation between inflammatory biomarkers and a decline in cognitive function (Julian et al., 2015; Matsushima et al., 2015) or risk of cardiovascular disease (Sesso et al., 2015). In these studies, MCP‐1/CCL2 was not measured.
In summary, we report for the first time that circulating levels of MCP‐1 correlate with biological age of mammals. This is supported by data in both mice and humans. MCP‐1 is a SASP factor secreted by senescent cells (Jin et al., 2016). Senescent cells and the pro‐inflammatory cytokines that they secrete negatively affect tissue homeostasis and repair, leading to organ dysfunction and aging (van Deursen, 2014). Thus, elevated MCP‐1 levels could correlate with increased biological age because it reflects a greater burden of senescent cells and/or a state of sterile inflammation that is known to promote aging and age‐related disease (Tchkonia, Zhu, van Deursen, Campisi & Kirkland, 2013). Because of the urgent need for measures of biological age, further studies are needed to reproduce this study, validate MCP‐1 in other systems, and determine its power to predict morbidity and mortality in prospective studies.
AUTHOR CONTRIBUTIONS
MJY did the experiments with mouse serum. MJS, EJA and NKL contributed data from the human frailty study. NNH and MKE contributed data from the HANDLS cohort. EKQ provided rapamycin‐treated mouse tissues. DJB contributed BubR1 H/H mouse tissues. WCL contributed geropathology assessment. PDR contributed to D+Q and p65 studies. MJY, MJS, NKL, NNH, MKE and LJN did the data analysis and prepared the manuscript.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGMENTS
This work was supported by NIH/NIA P01 AG043376 (LJN & PDR), NIH/NIA R24 AG047115 (WCL), NIH/NIA R01 AG038550 (EKQ, PI Rabinovitch), NIH/NIA T32 AG000057 (EKQ, PI Horton), NIH/NIA AG052958 and AG053832 (NKL). NNH and MKE are supported by the National Institute of Aging Intramural Research Program, National Institutes of Health (AG000519). We are grateful to Sara McGowan, Luise Angelini, Alexshiandria Ingle, and the Scripps Florida Animal Research Center for help with animal care. We gratefully acknowledge the contributions of the Geropathology Grading Committee for validating the composite lesion scores. We thank Nathan Foster for statistical consultation.
Yousefzadeh MJ, Schafer MJ, Noren Hooten N, et al. Circulating levels of Monocyte chemoattractant protein‐1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2018;17:e12706 https://doi.org/10.1111/acel.12706
REFERENCES
- Akdogan M. F., Azak A., Denizli N., Huddam B., Kocak G., Gucun M., … Duranay M. (2015) MCP‐1 and soluble TWEAK levels are independently associated with coronary artery disease severity in patients with chronic kidney disease. Renal Failure 37, 1297–1302. https://doi.org/10.3109/0886022X.2015.1065428 [DOI] [PubMed] [Google Scholar]
- Baker D. J., Childs B. G., Durik M., Wijers M. E., Sieben C. J., Zhong J., … van Deursen J. M. (2016) Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature 530, 184–189. https://doi.org/10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., van de Sluis B., … van Deursen J. M. (2011) Clearance of p16Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature 479, 232–236. https://doi.org/10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Crandall J. P., Kritchevsky S. B., Espeland M. A. (2016) Metformin as a Tool to Target Aging. Cell Metabolism 23, 1060–1065. https://doi.org/10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher B. M., Fitch R., Wynn M. J., Lalli M. A., Elofson J., Jastrzab L., … Kramer J. H. (2016) MCP‐1 and eotaxin‐1 selectively and negatively associate with memory in MCI and Alzheimer's disease dementia phenotypes. Alzheimer's & Dementia (Amsterdam, Netherlands) 3, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers B., Dalmasso B., Hatse S., Laenen A., Kenis C., Swerts E., … Wildiers H. (2015) Biological ageing and frailty markers in breast cancer patients. Aging 7, 319–333. https://doi.org/10.18632/aging.v7i5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. E., Gill M. S., Niedernhofer L. J., Robbins P. D., Austad S. N., Barzilai N., Kirkland J. L. (2016) Barriers to the Preclinical Development of Therapeutics that Target Aging Mechanisms. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71, 1388–1394. https://doi.org/10.1093/gerona/glw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. E., Sorrentino J. A., Clark K. S., Darr D. B., Krishnamurthy J., Deal A. M., … Sharpless N. E. (2013) Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)‐luciferase model. Cell 152, 340–351. https://doi.org/10.1016/j.cell.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton, R. A. , Lamar, M. , Zhang, A. , Ren, X. , Ajilore, O. , Pandey, G. N. , Kumar, A. (2017). Associations between pro‐inflammatory cytokines, learning, and memory in late‐life depression and healthy aging. International Journal of Geriatric Psychiatry, 32, 237–246. https://doi.org/10.1002/gps.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao Y. A., Dai Q., Zhang J., Lin J., Lopez E. F., Ahuja S. S., … Jin Y. F. (2011) Multi‐analyte profiling reveals matrix metalloproteinase‐9 and monocyte chemotactic protein‐1 as plasma biomarkers of cardiac aging. Circulation: Cardiovascular Genetics 4, 455–462. https://doi.org/10.1161/CIRCGENETICS.111.959981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton J., Martin‐Ruiz C., Davies K., Hilkens C. M., Isaacs J., Kolenda C., … Kirkwood T. B. (2012) Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross‐sectional findings from the Newcastle 85 + Study. Mechanisms of Ageing and Development 133, 456–466. https://doi.org/10.1016/j.mad.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Coppe J. P., Desprez P. Y., Krtolica A., Campisi J. (2010) The senescence‐associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology: Mechanisms of Disease 5, 99–118. https://doi.org/10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka A. B., English S. B., Simkevich C. P., Ginzinger D. G., Butte A. J., Schatten G. P., … Sedivy J. M. (2004) Genome‐scale expression profiling of Hutchinson‐Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell 3, 235–243. https://doi.org/10.1111/ace.2004.3.issue-4 [DOI] [PubMed] [Google Scholar]
- Dai D. F., Karunadharma P. P., Chiao Y. A., Basisty N., Crispin D., Hsieh E. J., … Rabinovitch P. S. (2014) Altered proteome turnover and remodeling by short‐term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539. https://doi.org/10.1111/acel.2014.13.issue-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo R., Khera A., McGuire D. K., Murphy S. A., Meo Neto Jde P., Morrow D. A., de Lemos J. A. (2004) Association among plasma levels of monocyte chemoattractant protein‐1, traditional cardiovascular risk factors, and subclinical atherosclerosis. Journal of the American College of Cardiology 44, 1812–1818. https://doi.org/10.1016/j.jacc.2004.07.047 [DOI] [PubMed] [Google Scholar]
- Deshmane S. L., Kremlev S., Amini S., Sawaya B. E. (2009) Monocyte chemoattractant protein‐1 (MCP‐1): An overview. Journal of Interferon and Cytokine Research 29, 313–326. https://doi.org/10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J. M. (2014) The role of senescent cells in ageing. Nature 509, 439–446. https://doi.org/10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle M. E., Kuiper R. V., Roodbergen M., Robinson J., de Vlugt S., Wijnhoven S. W., … van Steeg, H. (2011). Broad segmental progeroid changes in short‐lived Ercc1(‐/Delta7) mice. Pathobiol Aging & Age Related Diseases, 1, 7219 https://doi.org/10.3402/pba.v1i0.7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa‐Vega N., Moreno‐Frias C., Malacara J. M. (2015) Alterations in adhesion molecules, pro‐inflammatory cytokines and cell‐derived microparticles contribute to intima‐media thickness and symptoms in postmenopausal women. PLoS One 10, e0120990 https://doi.org/10.1371/journal.pone.0120990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey, K. , Currer J. M., Harrison D. E. (2006). Mouse models in aging research In Fox J. G., Davisson M. T., Quimby F. W., Barthold S. W., Newcomer C. E., Smith A. L. (eds), The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models (pp. 637–672). Burlington, MA: Academic Press. [Google Scholar]
- Fox J. G. (2007) The mouse in biomedical research. Amsterdam; Boston: Elsevier, AP. [Google Scholar]
- Franceschi C., Campisi J. (2014) Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 69(Suppl 1), S4–S9. https://doi.org/10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Franceschi C., Monti D., Sansoni P., Cossarizza A. (1995) The immunology of exceptional individuals: The lesson of centenarians. Immunology Today 16, 12–16. https://doi.org/10.1016/0167-5699(95)80064-6 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., … Cardiovascular Health Study Collaborative Research G (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56, M146–M156. [DOI] [PubMed] [Google Scholar]
- Goldman D. P., Cutler D., Rowe J. W., Michaud P. C., Sullivan J., Peneva D., Olshansky S. J. (2013) Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Affairs (Project Hope) 32, 1698–1705. https://doi.org/10.1377/hlthaff.2013.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju D., Atzmon G., Barzilai N. (2015) Genetics, lifestyle and longevity: Lessons from centenarians. Applied and Translational Genomics 4, 23–32. https://doi.org/10.1016/j.atg.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., … Miller R. A. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. (2013) DNA methylation age of human tissues and cell types. Genome Biology 14, R115 https://doi.org/10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inadera H., Egashira K., Takemoto M., Ouchi Y., Matsushima K. (1999) Increase in circulating levels of monocyte chemoattractant protein‐1 with aging. Journal of Interferon and Cytokine Research 19, 1179–1182. https://doi.org/10.1089/107999099313127 [DOI] [PubMed] [Google Scholar]
- Jin H. J., Lee H. J., Heo J., Lim J., Kim M., Kim M. K., … Kim S. W. (2016) Senescence‐Associated MCP‐1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxidants & Redox Signaling 24, 471–485. https://doi.org/10.1089/ars.2015.6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian A., Dugast E., Ragot S., Krolak‐Salmon P., Berrut G., Dantoine T., … Paccalin M. (2015) There is no correlation between peripheral inflammation and cognitive status at diagnosis in Alzheimer's disease. Aging Clinical and Experimental Research 27, 589–594. https://doi.org/10.1007/s40520-015-0332-5 [DOI] [PubMed] [Google Scholar]
- Kleinschmidt M., Schoenfeld R., Gottlich C., Bittner D., Metzner J. E., Leplow B., Demuth H. U. (2016) Characterizing Aging, Mild Cognitive Impairment, and Dementia with Blood‐Based Biomarkers and Neuropsychology. Journal of Alzheimer's Disease 50, 111–126. [DOI] [PubMed] [Google Scholar]
- Ladiges W., Snyder J. M., Wilkinson E., Imai D. M., Snider T., Ge X., … Liggitt D. (2017) A New Preclinical Paradigm for Testing Anti‐Aging Therapeutics. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 72, 760–762. https://doi.org/10.1093/gerona/glx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Fratiglioni L., Wang R., Santoni G., Welmer A. K., Qiu C. (2016) Effects of biological age on the associations of blood pressure with cardiovascular and non‐cardiovascular mortality in old age: A population‐based study. International Journal of Cardiology 220, 508–513. https://doi.org/10.1016/j.ijcard.2016.06.118 [DOI] [PubMed] [Google Scholar]
- Lippi G., Sanchis‐Gomar F., Montagnana M. (2014) Biological markers in older people at risk of mobility limitations. Current Pharmaceutical Design 20, 3222–3244. https://doi.org/10.2174/13816128113196660697 [DOI] [PubMed] [Google Scholar]
- Lowsky D. J., Olshansky S. J., Bhattacharya J., Goldman D. P. (2014) Heterogeneity in healthy aging. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 69, 640–649. https://doi.org/10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Tan C. T., Nyunt M. S., Mok E. W., Camous X., Kared H., … Larbi A. (2016) Inflammatory and immune markers associated with physical frailty syndrome: Findings from Singapore longitudinal aging studies. Oncotarget. 7, 28783–28795. https://doi.org/10.18632/oncotarget.v7i20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A. S., Nevala W. K., Dronca R. S., Leontovich A. A., Shuster L., Markovic S. N. (2012) Normal ageing is associated with an increase in Th2 cells, MCP‐1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF‐AA between sexes. Clinical and Experimental Immunology 170, 186–193. https://doi.org/10.1111/cei.2012.170.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima J., Kawashima T., Nabeta H., Imamura Y., Watanabe I., Mizoguchi Y., … Monji A. (2015) Association of inflammatory biomarkers with depressive symptoms and cognitive decline in a community‐dwelling healthy older sample: A 3‐year follow‐up study. Journal of Affective Disorders 173, 9–14. https://doi.org/10.1016/j.jad.2014.10.030 [DOI] [PubMed] [Google Scholar]
- Nadrowski P., Chudek J., Skrzypek M., Puzianowska‐Kuznicka M., Mossakowska M., Wiecek A., … Kozakiewicz K. (2016) Associations between cardiovascular disease risk factors and IL‐6 and hsCRP levels in the elderly. Experimental Gerontology 85, 112–117. https://doi.org/10.1016/j.exger.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Ness K. K., Krull K. R., Jones K. E., Mulrooney D. A., Armstrong G. T., Green D. M., … Hudson M. M. (2013) Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. Journal of Clinical Oncology 31, 4496–4503. https://doi.org/10.1200/JCO.2013.52.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer L. J., Garinis G. A., Raams A., Lalai A. S., Robinson A. R., Appeldoorn E., … Hoeijmakers J. H. (2006) A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043. https://doi.org/10.1038/nature05456 [DOI] [PubMed] [Google Scholar]
- Niedernhofer, L. J. , Kirkland, J. L. , Ladiges, W. (2016). Molecular pathology endpoints useful for aging studies. Ageing Research Reviews, 35, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N., Ejiogu N., Zonderman A. B., Evans M. K. (2012) Association of oxidative DNA damage and C‐reactive protein in women at risk for cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 32, 2776–2784. https://doi.org/10.1161/ATVBAHA.112.300276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinke, K. H. , Calzavara, B. , Faria, P. F. , do Nascimento, M. P. , Venturini, J. , Lara, V. S. (2013) Proinflammatory profile of in vitro monocytes in the ageing is affected by lymphocytes presence. Immunity & Ageing 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully E., Lockhart A., Huang L., Robles Y., Becerril C., Romero‐Tejeda M., … Lin N. H. (2016) Elevated Levels of Microbial Translocation Markers and CCL2 Among Older HIV‐1‐Infected Men. Journal of Infectious Diseases 213, 771–775. https://doi.org/10.1093/infdis/jiv501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso H. D., Jimenez M. C., Wang L., Ridker P. M., Buring J. E., Gaziano J. M. (2015) Plasma Inflammatory Markers and the Risk of Developing Hypertension in Men. Journal of the American Heart Association 4, e001802 https://doi.org/10.1161/JAHA.115.001802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J. L. (2013) Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. The Journal of Clinical Investigation 123, 966–972. https://doi.org/10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra J. S., Robinson A. R., Wang J., Gregg S. Q., Clauson C. L., Reay D. P., … Robbins P. D. (2012) NF‐kappaB inhibition delays DNA damage‐induced senescence and aging in mice. The Journal of Clinical Investigation 122, 2601–2612. https://doi.org/10.1172/JCI45785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Tchkonia T., Pirtskhalava T., Gower A. C., Ding H., Giorgadze N., … Kirkland J. L. (2015) The Achilles' heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 14, 644–658. https://doi.org/10.1111/acel.2015.14.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


