Abstract
Streptococcus anginosus is a clinically important pathogen that is emerging globally but remains poorly investigated. Here, we report the first case of acute glomerulonephritis resulting from infection with S. anginosus. Glomerulonephritis is typically caused by S. pyogenes and reports secondary to other strains including S. zooepidemicus and S. constellatus exist. Infection with S. anginosus in this patient was associated with acute nephritis (haematuria, oedema and hypertension), nephrotic syndrome and progressive azotemia. There was activation of the complement system. The presence of low C1q and elevated anti-C1q binding complexes points to a potential pathogenic role. Testing for streptococcal antigens was strongly positive. Emerging nephritogenic strains of S. anginosus present a significant health concern for both developed and developing countries.
Keywords: pneumonia (infectious disease), nephrotic syndrome, acute renal failure
Background
Streptococcus anginosus is a clinically important pathogen that is emerging globally but remains poorly investigated. Here, we report the first case of acute glomerulonephritis resulting from infection with S. anginosus. Acute glomerulonephritis is well known to be induced by nephritogenic strains of group A beta-haemolytic streptococcus, typically caused by S. pyogenes, but reports secondary to other strains including S. zooepidemicus and S. constellatus exist. Overt glomerulonephritis in the setting of infection with only S. anginosus has never been reported before, to the best of our knowledge.
Case presentation
A 55-year-old African-American woman presented to the emergency room with a chief complaint of haemoptysis. Physical examination revealed an ill-appearing patient with poor dentition, decreased air entry, dullness to percussion and crackles in the right lung fields, and costovertebral tenderness in the left flank. Initial laboratory testing was significant for a leucocytosis, elevated creatinine and elevated C reactive protein (table 1). Urinalysis, obtained prior to medication administration, revealed microscopic haematuria, red blood cell casts and leucocyturia. Thoracic CT imaging revealed a right upper lobe necrotising pneumonia (figure 1). Intravenous vancomycin, piperacillin–tazobactam and clindamycin were promptly started. Testing for eosinophiluria was negative. Blood cultures were drawn, and S. anginosus was initially identified using the Luminex Verigene Gram-positive blood culture assay. This molecular assay was performed directly on the positive blood cultures. The subsequent isolated colonies from the blood cultures as well as the fluid culture were identified using conventional methods—catalase, colony size, colony odour, haemolysis and PYR (pyrrolidonyl arylamidase) testing. Testing for coinfection with HIV and hepatitis B and C were negative.
Table 1.
Laboratory investigations on admission
| Test | Result (normal range) |
| Leucocyte count | 19.51 (4.5–11×109/L) |
| Procalcitonin | 23.17 (<0.15 ng/mL) |
| C reactive protein | 86.5 (0.0–5.0 mg/L) |
| BUN | 22 (2–22 mg/fL) |
| Creatinine | 0.55 (0.55–0.95 mg/dL) |
| Urinalysis | >180 wbc, >180 rbc, >100 protein, 0–10 eosinophils/HPF |
| Urine protein:creatinine (spot, early morning void) | 1.37 (<0.16 mg/mg) |
| C1q binding complexes | 5.5 (<4.4 µg Eq/mL) |
| C1Q | 11.6 (11.8–28.4 mg/dL) |
| C2 | 3.2 (1.6–4.0 mg/dL) |
| C3 | 42 (90–180 mg/dL) |
| C4 | 12 (10–40 mg/dL) |
| CH50 | 12 (42–60 U/mL) |
| Anti-streptolysin O titre | 1944 (<200 IU/mL) |
| Anti-DNAse B antibodies | 8850 (0–120 U/mL) |
Blood urea nitrogen (BUN); White blood cells (wbc); Red blood cells (rbc); High power field (hpf).
Figure 1.
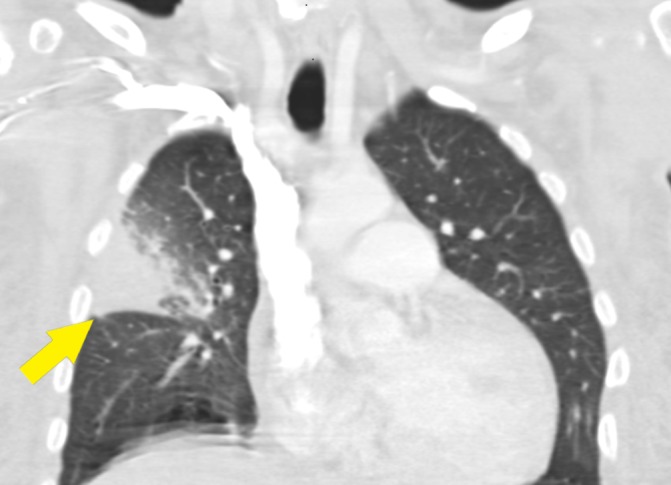
CT chest with intravenous contrast. There is dense consolidation and enhancement of the right upper lobe, which causes mass effect and bowing of the minor fissure (arrow). There are central areas of low attenuation suggesting necrosis or intrapulmonary abscess, and there is surrounding ground-glass opacity involving the majority of the inferior lateral right upper lobe.
Her abdominal pain persisted and serial imaging eventually revealed abdominal wall necrotising fasciitis with bullae in the affected area by day 5 (figure 2). Surgery debrided the wound, and a chest tube was administered for drainage. Intraoperative samples were collected, also growing S. anginosus. Five days after admission, she complained of dark ‘cola-coloured’ urine (figure 3) and developed anasarca. Hypertension became uncontrolled and renal function declined, though there was preserved urine output. Serum testing revealed markedly decreased CH50 and C3, normal C2 and C4, and increased C1q binding complexes (table 1). Additionally, there were elevated titres of antibodies to extracellular streptococcal products (anti-streptolysin-O (ASO) and anti-DNAse B; table 1). With clinical and laboratory evidence of acute nephritis and confirmation of antecedent streptococcal infection, the diagnosis of poststreptococcal glomerulonephritis (PSGN) was made using the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines.1 A renal biopsy was considered, but given strong evidence of PSGN, we abided by KDIGO recommendations to not obtain a renal biopsy. Additionally, the presence of abdominal wall infection was a relative contraindication.
Figure 2.
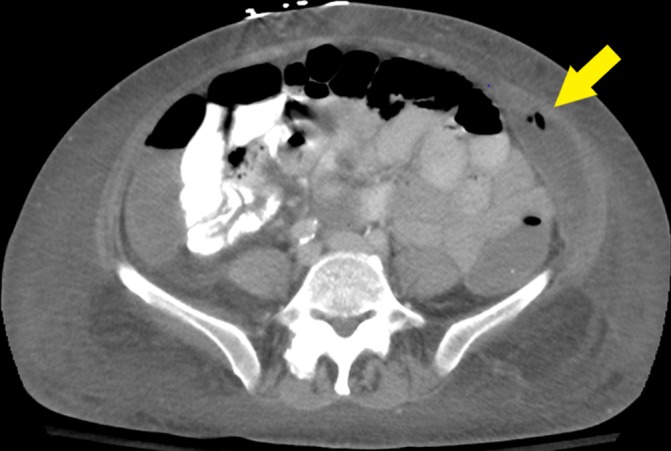
CT abdomen with intravenous contrast. There is an expanding collection in the left extraperitoneal space involving the left transversus abdominis and oblique musculature with internal foci of gas (arrow).
Figure 3.

Urine sample: tea-coloured urine was the first symptom prompting investigation.
The patient was managed conservatively with antibiotic therapy, blood pressure control, diuresis and careful fluid management. Broad-spectrum antibiotics (vancomycin, piperacillin–tazobactam and clindamycin) were downgraded to ceftriaxone and metronidazole when the S. anginosus strain was identified as pansusceptible (testing done for ceftriaxone, clindamycin, penicillin and vancomycin). Anaerobic coverage was maintained empirically with the nature of infections. There was clinical and biochemical improvement with good recovery. The patient was discharged to a nursing facility in stable condition and followed in clinic after.
Investigations
Figure 4 shows the trends in C3 complement and proteinuria measured by spot protein:creatinine ratio on an early morning void sample. The C3 complement levels decreased to a nadir then uptrended slowly, while the proteinuria increased until day 8 and then returned to baseline. On discharge, the C3 complement level had almost normalised at 82 mg/dL.
Figure 4.
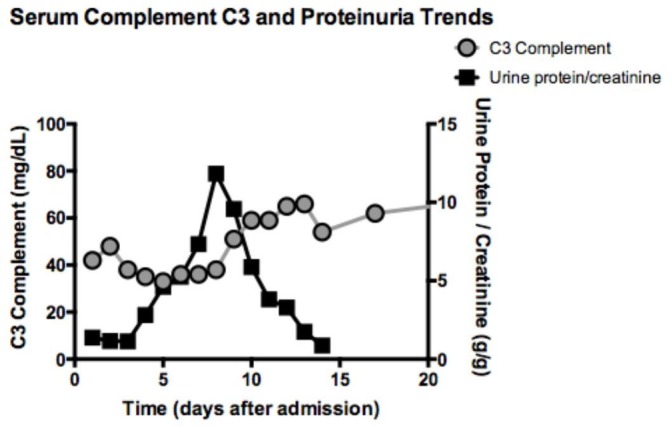
Serum complement C3 and proteinuria trends.
Outcome and follow-up
There was clinical and biochemical improvement with good recovery. The patient was discharged to a nursing facility in stable condition and followed in clinic after. At follow-up 3 months later, the patient had good recovery with normal renal function and the C3 complement level had normalised (132 mg/dL).
Discussion
Of the streptococcal species, S. anginosus is the least investigated of the streptococci. To many physicians, S. anginosus remains shrouded in medical mystery with confusion regarding its classification hampering detailed investigation and diagnosis of infections.2 S. pyogenes is well known as ‘group A beta-hemolytic streptococcus’ (GAS) because it demonstrates haemolysis on blood agar and carbohydrate ‘group’ antigens. However, this dichotomous classification is limited for S. anginosus, which can be α-non-haemolytic, β-non-haemolytic or non-haemolytic, and express A, C, G, or F, or no Lancefield antigen.3 S. anginosus has also been considered a viridans streptococci and associated with Lancefield group F.4 This demonstrates the confusion regarding S. anginosus. In this case, the S. anginosus was typed as Lancefield group A.
Further research to redefine and classify S. anginosus is ongoing using chromogenicity and multilocus sequence analysis,2 5–7 but a paucity of information still exists regarding S. anginosus and their role in human infection. S. anginosus can be pathogenic and is notorious for causing abscesses, particularly in the lungs and abdomen. A 2-year surveillance study by the CDC recently revealed that significant associations for S. anginosus infection included age (<65 years), diabetes mellitus and patients admitted from home.4 Previously known clinical syndromes resulting from S. anginosus infections include bacteraemia, abscess formation, pneumonia, intra-abdominal infection, osteomyelitis and less commonly endocarditis, necrotising fasciitis and streptococcal toxic shock syndrome.4 It remains unclear what clinical differences, if any, exist between α-non-haemolytic, β-non-haemolytic and non-haemolytic S. anginosus group isolates and what the true burden of infection is for cases involving S. anginosus.
A review of the literature documents cases and outbreaks of glomerulonephritis globally from S. pneumoniae, S. zooepidemicus and S. constellatus dating back to the 1960s.8 9 More recently, animal models have also suggested glomerulonephritis is possible from non-GAS strains.10 This case suggests that S. anginosus may harbour or induce nephritogenic antigens. In 2005, Almroth et al 11 examined an outbreak of acute glomerulonephritis among four families in Sweden that coincided with the spread of S. constellatus. There was also infection with S. anginosus, though this strain was not directly implicated. Kidney biopsies revealed an acute diffuse proliferative glomerulonephritis compatible with acute poststreptococcal nephritis and microbiological analysis of renal tissue revealed both S. pyogenes and S. constellatus.
Glomerulonephritis associated with S. zooepidemicus has also been previously reported.12 In 1998, there was a large outbreak (134 patients) of acute glomerulonephritis in Brazil, which was investigated by the CDC. Lancefield group C S. zooepidemicus was identified as the causative organism and linked to the consumption of cheese produced from unpasteurised milk.13 There were four mortalities in the acute phase and five patients (3.7%) required chronic dialysis. Follow-up was continued for 10 years and demonstrated morbidity with patients developing hypertension, reduced renal function and increased microalbuminuria, which peaked within the first 2 years with subsequent stabilisation.14 15 A unique feature of this case is that the patient had two streptococcal infections. In the strictest sense, we are unable to say if the necrotising fasciitis was secondary or second to be identified. In a search of MEDLINE/PubMed, we were unable to find any cases of glomerulonephritis with necrotising fasciitis.
The glomerulonephritis in poststreptococcal disease is thought to be mediated by intraglomerular immune complex formation induced by deposition of nephritogenic streptococcal antigens. In situ formation is augmented by circulating immune complexes, which further activate the complement pathway locally. Therefore, we wondered if S. anginosus strains have been described as antigenically similar to S. pyogenes. A review of the literature revealed that S. anginosus is known to be phenotypically diverse; whole-genome comparative analysis has demonstrated significant variation in genomic size with multiple rearrangements, insertions and deletions.16 A primary virulence factor of S. anginosus includes homologues of streptolysin S, a haemolytic exotoxin described in S. pyogenes.17 18 Although research is limited, streptolysin O and DNase B antibodies have not previously been associated with S. anginosus. However, in the light of our patient having a markedly elevated anti-streptolysin O titre, we hypothesise that streptolysin O and DNAse B may be expressed, potentially as virulence factors, in some S. anginosus strains. This is an exciting observation that we think deserves further research, which has been started.2
Recently, Babbar et al 2 examined α-haemolytic and β-haemolytic S. anginosus isolates from India and Germany. Complementary PCR-based screening for S. pyogenes virulence factors revealed a subgroup of S. anginosus that harbours superantigen and extracellular DNAse coding genes identical to corresponding genes of S. pyogenes. Interestingly, this patient also had elevated levels of C1q binding complexes with decreased serum C1q. Antibodies against complement C1q (anti-C1q) strongly have also been implicated in other cases of nephritis.19 20 These findings deserve further investigation.
Learning points.
Infection with Streptococcus anginosus in this patient was associated with acute nephritis (haematuria, oedema and hypertension), nephrotic syndrome and progressive azotemia.
There was activation of the complement system with decreased complement C3 and C1q and elevated anti-C1q binding complexes.
Testing for streptococcal antigens was strongly positive (streptolysin O, DNAse B), and this deserves further investigation.
Emerging nephritogenic strains of S. anginosus present a significant health concern for both developed and developing countries.
Footnotes
Contributors: SM and KS conceived the idea for the study. SM, KS and SC were directly involved in patient care. SM and SC collected the data and wrote the case report. KS and SC performed the literature review and prepared the supplementary material. All authors contributed to the discussion and approved the final draft of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012;2:139–274. [Google Scholar]
- 2.Babbar A, Kumar VN, Bergmann R, et al. . Members of a new subgroup of Streptococcus anginosus harbor virulence related genes previously observed in Streptococcus pyogenes. Int J Med Microbiol 2017;307:174–81. 10.1016/j.ijmm.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 2002;15:613–30. 10.1128/CMR.15.4.613-630.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broyles LN, Van Beneden C, Beall B, et al. . Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis 2009;48:706–12. 10.1086/597035 [DOI] [PubMed] [Google Scholar]
- 5.Whiley RA, Beighton D. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int J Syst Bacteriol 1991;41:1–5. 10.1099/00207713-41-1-1 [DOI] [PubMed] [Google Scholar]
- 6.Whiley RA, Beighton D, Winstanley TG, et al. . Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol 1992;30:243–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arinto-Garcia R, Pinho MD, Carriço JA, et al. . Comparing matrix-assisted laser desorption ionization-time of flight mass spectrometry and phenotypic and molecular methods for identification of species within the Streptococcus anginosus group. J Clin Microbiol 2015;53:3580–8. 10.1128/JCM.01892-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips J, Palmer A, Baliga R. Glomerulonephritis associated with acute pneumococcal pneumonia: a case report. Pediatr Nephrol 2005;20:1494–5. 10.1007/s00467-005-1994-6 [DOI] [PubMed] [Google Scholar]
- 9.Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med 1999;107:12–27. 10.1016/S0002-9343(99)00103-5 [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Hirota K, Zhang RJ, et al. . Possible role for a polysaccharide antigen shared between Streptococcus pyogenes and S. mutans in the pathogenesis of poststreptococcal glomerulonephritis. Acta Paediatr Jpn 1996;38:470–5. 10.1111/j.1442-200X.1996.tb03529.x [DOI] [PubMed] [Google Scholar]
- 11.Almroth G, Lindell A, Aselius H, et al. . Acute glomerulonephritis associated with Streptococcus pyogenes with concomitant spread of Streptococcus constellatus in four rural families. Ups J Med Sci 2005;110:217–31. 10.3109/2000-1967-067 [DOI] [PubMed] [Google Scholar]
- 12.Barnham M, Thornton TJ, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C). Lancet 1983;1:945–8. 10.1016/S0140-6736(83)92078-0 [DOI] [PubMed] [Google Scholar]
- 13.Balter S, Benin A, Pinto SW, et al. . Epidemic nephritis in Nova Serrana, Brazil. Lancet 2000;355:1776–80. [DOI] [PubMed] [Google Scholar]
- 14.Sesso R, Wyton S, Pinto L. Epidemic glomerulonephritis due to Streptococcus zooepidemicus in Nova Serrana, Brazil. Kidney Int Suppl 2005;97:S132–S136. 10.1111/j.1523-1755.2005.09722.x [DOI] [PubMed] [Google Scholar]
- 15.Pinto SW, Mastroianni-Kirsztajn G, Sesso R. Ten-year follow-up of patients with epidemic post infectious glomerulonephritis. PLoS One 2015;10:e0125313 10.1371/journal.pone.0125313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson AB, Kent H, Sibley CD, et al. . Phylogenetic relationship and virulence inference of Streptococcus Anginosus Group: curated annotation and whole-genome comparative analysis support distinct species designation. BMC Genomics 2013;14:895 10.1186/1471-2164-14-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabata A, Nakano K, Ohkura K, et al. . Novel twin streptolysin S-like peptides encoded in the sag operon homologue of beta-hemolytic Streptococcus anginosus. J Bacteriol 2013;195:1090–9. 10.1128/JB.01344-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asam D, Mauerer S, Walheim E, et al. . Identification of β-haemolysin-encoding genes in Streptococcus anginosus. Mol Oral Microbiol 2013;28:302–15. 10.1111/omi.12026 [DOI] [PubMed] [Google Scholar]
- 19.Kallenberg CG. Anti-C1q autoantibodies. Autoimmun Rev 2008;7:612–5. 10.1016/j.autrev.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Kozyro I, Korosteleva L, Chernoshej D, et al. . Autoantibodies against complement C1q in acute post-streptococcal glomerulonephritis. Clin Immunol 2008;128:409–14. 10.1016/j.clim.2008.04.005 [DOI] [PubMed] [Google Scholar]


