Abstract
There are some disparities between the clinical practice and profiles of cancer in Asia and those in Europe & North America. In Asia, surgical oncologists still have a major role in the multidisciplinary therapy of gastrointestinal stromal tumor (GIST), whereas medical oncologists hold this status in the West. Although the incidence of clinical GIST is considered similar between the two areas, small gastric GISTs are more frequently treated by surgery in East Asia compared with Europe & North America. The diagnosis and treatment of small submucosal tumors (SMTs), including GIST, is important in Asian clinical practice guidelines for GIST. Most items of Asian and Western GIST guidelines are very similar. There are slight differences between the two guidelines in the degree of recommendation, which may come from disparities of clinical practice and available medicines. Importantly, most clinical evidence in the GIST guidelines has been established by clinical trials conducted in Western countries, and the number of clinical trials is still limited in Asia, suggesting that Asian GIST patients may have limited access to investigational drugs after standard therapy. Finally, both Asian and Western GIST guidelines are well-harmonized in some parts, and their contents may reflect the medical circumstances of each region.
Keywords: Gastrointestinal stromal tumor (GIST), guidelines, evidence-based, submucosal tumor (SMT)
Introduction
Gastrointestinal stromal tumor (GIST) is a potentially malignant tumor and the most frequent type of sarcoma in the gastrointestinal tract. The discovery of driver mutations in the KIT or PDGFRA (platelet-derived growth factor receptor alpha) gene and subsequent development of molecularly targeted therapy based on molecular mechanisms of tumor cell proliferation have revolutionized the diagnosis and treatment of GIST, which facilitates scientific research, as well as the publication of clinical guidelines (1,2). Today, a multidisciplinary approach with surgical and medical oncologists, pathologists, gastroenterologists, and radiologists is mandatory to provide optimal treatment for GIST patients. Evidence-based diagnosis and treatment according the guidelines has improved the prognosis of cancer patients (3,4). The clinical guidelines of GISTs were first published by the National Comprehensive Cancer Network (NCCN) in 2004 (5), and by the European Society of Medical Oncology (ESMO) in 2005 (6), followed by clinical practice guidelines in various countries around the world (7-12). Most evidence has been established in Western countries, and Asian patients have supplied limited data on the diagnosis and treatment of GIST. There still exist some differences in the clinical practice and disease spectrum between European & North American countries and Asian countries, especially East Asian countries (12). The GIST experts in Korea, China, Taiwan and Japan consider that some aspects of Western guidelines are not always applicable to Asian patients and that clinical practice in East Asia is somewhat different from that of Western countries. Thus, they have published the Asian consensus GIST guidelines (12).
In this review, we will summarize the clinical practice of both areas and then discuss the similarities and disparities between Western and Asian GIST guidelines, although the fundamental approaches to the diagnostic and treatment strategy for GIST are very similar.
General considerations on clinical practice for GIST in Asia and Europe & North America
The oncological disease spectrum is different between Asia and the West (13,14). In Western countries, breast cancer, colon cancer and prostate cancer are frequent, whereas gastric cancer, esophageal cancer and hepatocellular carcinoma are dominant in East Asian countries. Even in colon cancer and gastric cancer, there still exist some differences in sub-location and histology; right colon cancer is relatively prevalent in the West vs left colon cancer and rectal cancer in the East; proximal gastric cancer with poorly differentiated adenocarcinoma is relatively common in the West vs distal gastric cancer with well differentiation in the East. This may lead to some differences in cancer screening. Gastric cancer screening is emphasized and flourishes in East Asia, while the same holds for health examinations for breast and colon cancer in Western countries. In clinical practice, medical oncologists are crucial and are widely involved in cancer treatment in the West, whereas in the East, surgical oncologists still cover a broad area of oncology because of the limited number of medical oncologists. Surgical oncologists may have a significant role in adjuvant therapy, neoadjuvant therapy and sometimes even imatinib therapy for advanced and/or metastatic GIST.
The true incidence of clinical GIST, which may be symptomatic GIST requiring immediate medical and/or surgical therapy, GIST with considerable recurrent-risk, or metastatic and/or recurrent disease, is considered to be no different between Asian and Western countries (1,15). It is estimated to be no more than 10/million people/year (1,2,6). There are, however, reports describing a high incidence of small GISTs, including mini-GISTs and micro-GISTs (16-21). Almost all these small GISTs show morphologically and clinically indolent features (20,21). Pathological examinations of the stomach and small intestine of middle-aged persons have revealed frequent findings (10% to 35%) of pathological GISTs (micro-GISTs) less than 1 cm in diameter (16-18). Others indicated that fewer than one in one thousand middle-aged adults may potentially have small GISTs less than 2 cm (mini-GISTs) (22). Most of them neither grow nor become clinical GISTs, although the distinction of potentially malignant GISTs from indolent ones appears to be extremely difficult even in pathological examinations. Thus, the true incidence of biological GIST is not known.
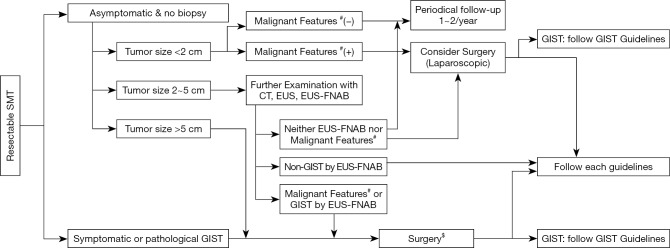
In East Asia, gastric cancer screening sometimes finds an asymptomatic submucosal tumor (SMT) less than 5 cm, which may be pathologically diagnosed as GIST after surgical resection. Thus, the incidence and relative frequency of gastric GIST are higher in East Asian countries compared with Western countries, especially for asymptomatic and small gastric GIST (2,9,22). These circumstances may result in the relatively good prognosis of Asian GIST patients (15). The frequent finding of small and asymptomatic SMTs may also facilitate endoscopic resection of these SMTs and GISTs in East Asia (23,24). Based on the above circumstances, GIST clinical guidelines may start from the diagnosis and treatment of SMT in Asia (Figure 1) (2,9). Small gastric SMTs less than 2 cm without malignant features could be followed by periodical endoscopic ultrasonography (EUS) until the tumors become symptomatic and/or show malignant features in endoscopy and/or EUS. Malignant features of SMT (or GIST) include ulcer formation, internal heterogeneity in EUS, irregular margin, and increase in size during follow-up (2,9,22). These approaches may be applicable for histologically proven gastric GISTs, although the decision should be shared with patients. These decision-making processes are similar to the NCCN guidelines but may be slightly different from the ESMO guidelines (6,8). When small gastric SMTs have malignant features, the guidelines recommend further examination, for example, histological diagnosis by EUS-guided fine-needle aspiration (EUS-FNA) or surgical removal (2,6,9). When EUS-FNA reveals histological GIST, surgery is recommended. For non-gastric GISTs, all guidelines recommend surgical resection regardless of tumor size because recurrence risk and disease progression may be high and frequent in non-gastric GIST, even if it is small (2,6,8,12).
Figure 1.
Diagnostic and therapeutic strategy for small submucosal tumors in the gastrointestinal tract. Small submucosal tumors (SMTs) may necessitate surgery when they produce symptoms or when they have malignant features. The strategy shows that the diagnosis and treatment of asymptomatic and pathologically undetermined SMTs are divided by size. A part of the algorithm is similar to the Japanese clinical practice guidelines for GIST (9). #, Malignant features include ulceration, irregular margins, inhomogeneous parenchyma in endoscopy and EUS and tumor growth during follow-up; $, in this situation, imatinib neoadjuvant therapy may be considered when a large tumor is preoperatively diagnosed as GIST. GIST, gastrointestinal stromal tumor; CT, computed tomography; EUS, endoscopic ultrasonography; EUS-FNAB, endoscopic ultrasonography-guided fine-needle aspiration biopsy.
Pathological diagnosis & genetic analysis
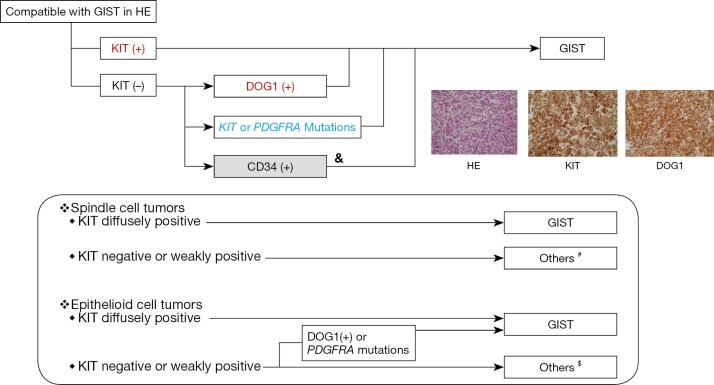
Pathological diagnosis is similar between Asia and the West. In Asian GIST guidelines, the algorithm of the pathological diagnosis is explicitly presented as shown in the upper panel of Figure 2 (2,9,12). Asian GIST guidelines recommend KIT and DOG1 immunostaining and, in addition, genotyping, when required. The guidelines do not always recommend CD34 immunostaining because CD34 is not specific for GIST. In clinical practice, genotyping is not as commonly used in Asian countries compared with hospitals and institutes in Europe & North America. Thus, the Asian guidelines suggest the use of both KIT and DOG1 immunostaining, as well as immunostaining of other markers depending on the tumor (Figure 2 in the lower panel). Both guidelines recommend that mitosis should be expressed with a denominator of 5 mm2 because of different fields of view among microscopes. The Asian GIST guidelines suggest required items in a pathologic report form.
Figure 2.
The algorithm of pathological diagnosis of GIST. &, CD34 is not specific for GIST; #, others include leiomyoma & leiomyosarcoma (desmin-positive), schwannoma (S-100-positive), solitary fibrous tumor (CD34-positive and nuclear STAT6-positive), desmoid (nuclear beta-catenin-positive), inflammatory myofibroblastic tumor (ALK-positive), PEComa (HMB45-positive) and others; $, others include PEComa (HMB45-positive), glomus tumor (smooth muscle actin-positive & vimentin-positive), solitary fibrous tumor (CD34-positive and nuclear STAT6-positive), malignant melanoma (S-100 positive, HMB45-positive), neuroendocrine tumor (several biomarkers), and others. GIST, gastrointestinal stromal tumor.
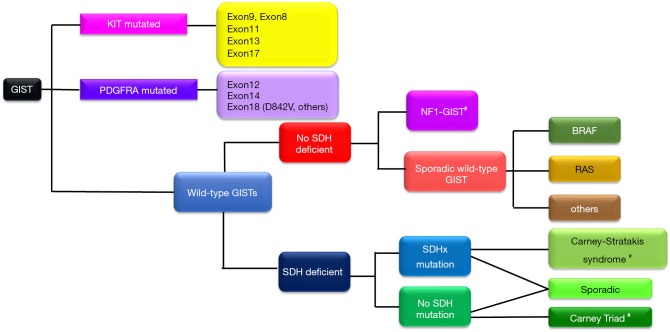
GIST is a heterogeneous disease composed of several genotypes (1,2). Ninety percent of primary GISTs may have mutations in either the KIT (80%) or PDGFRA genes (10%), and 10% have no mutation in either gene (Figure 3) (1,2,6). The latter type is called wild-type GIST. The most frequent KIT mutations are found in the juxtamembrane domain of exon 11, followed by exon 9, and KIT mutations in exons 8, 13 and 17 are rare. Contrary to KIT, kinase domain mutations, especially in exon 18, are most common in PDGFRA. Genotyping is considered to be primarily important as a predictive biomarker of clinical activities of tyrosine kinase inhibitors (TKIs) and secondarily important as a diagnostic biomarker of KIT-negative GIST in immunohistochemistry (1,2,6,8,9,12). For example, no guidelines recommend imatinib adjuvant therapy for high-risk GIST with PDGFRA D842V mutation because this type of mutation is considered to be resistant to all available TKIs, including imatinib, sunitinib, and regorafenib (6,8,9,12). Wild-type GIST may be divided into several genotypes, as shown in Figure 3, and the initial diagnostic step may be SDHB-immunostaining. The GIST experts may consider that wild-type GIST is insensitive to imatinib and do not always recommend imatinib adjuvant therapy for wild-type GIST. Details of wild-type GIST should be found in other reviews and original articles (25-27).
Figure 3.
Genotypes of GIST. Carney-Stratakis syndrome is a dyad of paraganglioma and gastric GIST with autosomal dominance. Carney triad is characterized by three neoplasms of gastric GIST, pulmonary chondroma, and extra-adrenal paraganglioma. A part of the algorithm is similar to (25). #, indicates syndromic GIST. GIST, gastrointestinal stromal tumor; SDH, succinate dehydrogenase; NF1, neurofibromatosis type 1.
There are several risk stratifications and nomograms for GIST (28-33). Among the major risk stratifications and staging systems (Tables 1-4), the Armed Forces Institute of Pathology (AFIP) criteria are indicated to accurately predict recurrence after complete surgery and are recommended by the NCCN and ESMO guidelines (6,8,33-35). However, Asian guidelines recommend the modified NIH (National Institutes of Health) classification (Joensuu classification) because this stratification is suggested to be the most sensitive to select GIST patients who may have benefits from adjuvant therapy (12,36-38). Except for the modified NIH (Joensuu) classification (15), all risk-stratifications and nomograms lack evidence for Asian GIST patients, and there have been few validation studies from Asian countries. Asian guidelines mention that further investigations are still required to find the best risk stratification for Asian GIST patients.
Table 1. Risk classifications—National Institute of Health (NIH) consensus criteria.
| Risk categories | Tumor size (cm) | Mitosis/50 HPF |
|---|---|---|
| Very low | <2 | <5 |
| Low | 2–5 | <5 |
| Intermediate | <5 | 6–10 |
| 5–10 | <5 | |
| High | >5 | >5 |
| >10 | Any | |
| Any | >10 |
HPF, high power field.
Table 2. Risk classifications—the Armed Forces Institute of Pathology (AFIP) criteria (with some modifications).
| Group | Tumor size (cm) | Mitosis/50 HPF | Tumor site | ||
|---|---|---|---|---|---|
| Stomach | Small intestine | Large intestine and rectum | |||
| 1 | ≤2 | ≤5 | None | None | None |
| 2 | 2–5 | Very low | Low | Low | |
| 3a | 5–10 | Low | Moderate | High | |
| 3b | >10 | Moderate | High | ||
| 4 | ≤2 | >5 | None | High | High |
| 5 | 2–5 | Moderate | High | High | |
| 6a | 5–10 | High | High | High | |
| 6b | >10 | High | High | ||
HPF, high power field.
Table 3. Risk classifications—the modified NIH (Joensuu) classification.
| Risk category | Tumor size (cm) | Mitosis/50 HPF | Primary tumor site |
|---|---|---|---|
| Very low | ≤2 | ≤5 | Any |
| Low | 2–5 | ≤5 | Any |
| Intermediate | ≤5 | 6–10 | Gastric |
| 5–10 | ≤5 | Gastric | |
| High | Any | Any | Tumor rupture |
| >10 | Any | Any | |
| Any | >10 | Any | |
| >5 | >5 | Any | |
| ≤5 | >5 | Non gastric | |
| >5–10 | ≤5 | Non gastric |
HPF, high power field.
Table 4. Risk classifications—American Joint Committee on Cancer (AJCC) staging.
| Stage | T | N | M | Mitotic index |
|---|---|---|---|---|
| Gastric | ||||
| Stage I | T1, T2, T3 | N0 | M0 | Low |
| Stage II | T1, T2 | N0 | M0 | High |
| T4 | N0 | M0 | Low | |
| Stage III | T3, T4 | N0 | M0 | High |
| Stage IV | Any T | N1 | M0 | Any |
| Any T | Any N | M1 | Any | |
| Non-gastric | ||||
| Stage I | T1, T2 | N0 | M0 | Low |
| Stage II | T3 | N0 | M0 | Low |
| Stage III | T4 | N0 | M0 | Low |
| T1, T2, T3, T4 | N0 | M0 | High | |
| Stage IV | Any T | N1 | M0 | Any |
| Any T | Any N | M1 | Any | |
T1, <2 cm; T2, 2–5 cm; T3, 5–10 cm; T4, >10 cm; N0, no lymph node metastasis; N1, regional lymph node metastasis; M0, no distant metastasis; M1, distant metastasis; mitotic index low: <5/50 HPF, high: >5/50 HPF. HPF, high power field.
Surgical treatment
Surgery is still a mainstay for a potential permanent cure even in the era of tyrosine kinase inhibitors. Indications of multidisciplinary therapy of neoadjuvant and adjuvant therapy may be individualized. Before neoadjuvant therapy, pathological diagnosis using biopsy samples is mandatory (9,12). Asian guidelines recommend luminal biopsy, such as EUS-FNA (2,9,12), and Western guidelines recommend fine-needle biopsy through the abdominal wall based on the results from retrospective studies indicating that such biopsies did not increase recurrent risk when appropriate surgery and/or medical therapy were done after biopsy (6,8,39).
In surgical treatment, macroscopically and microscopically clear surgical margins are required for complete resection of GIST without injuring the pseudocapsule, even if it is small. Thus, Asian guidelines do not recommend endoscopic resection of small GIST because it has a potential risk of pseudocapsule damage, which may be predisposed to recurrence (9,12). The guidelines recommend laparoscopic surgery for small GIST less than 5 cm when it is in a favorable location (2,6,9,12). Laparoscopic surgery is less painful and less invasive and shows better cosmetic results, as well as earlier recovery, than open surgery (40-43). Furthermore, oncologic outcomes are similar between laparoscopic and open surgery.
The prognostic factors for recurrence after complete surgery have been rigorously investigated in both Western and Asian countries (5-12), and four factors are recognized as independent prognostic factors: tumor size (cm), mitosis (per 50 HPF or per 5 mm2), tumor location (gastric vs non-gastric) and tumor rupture (15,28-32). Among the four factors, tumor rupture is the most ominous prognostic factor, and most ruptured GIST may have recurrences during follow-up (1,2,15). Thus, all guidelines suggest imatinib adjuvant therapy for GIST patients with tumor rupture, and some experts may consider that these patients should have adjuvant therapy for much longer than three years. The definition of “tumor rupture”, however, has been subjective, and the diagnosis of tumor rupture may depend on the surgeon. Hence, the preliminary data indicate different prognostic outcomes between intraoperative and preoperative rupture. Recently, Hølmebakk et al. (44) classified major and minor tumor ruptures. A major defect included tumor spillage, tumor fracture, piecemeal resection, bowel perforation at the tumor site with blood-tinged ascites at laparotomy, microscopic tumor invasion into neighboring structures, and surgical biopsy; and a minor defect included iatrogenic peritoneal laceration on the tumor (injury to the pseudocapsule) and serosal laceration at the tumor site. GISTs with major findings showed poorer prognosis than those with only minor findings. These criteria do not consider minor defects and fine-needle biopsies to be true “tumor rupture” (44,45). However, macroscopic injuries to the pseudocapsule exposing tumor cells were shown to have similarly poor prognosis as a major defect (37,46). Finally, we may consider that “tumor rupture” includes tumor perforation and tumor fracture with blood-tinged ascites, piecemeal resection during operation, microscopic tumor invasion into neighboring structures, and macroscopic injuries to the pseudocapsule exposing tumor cells into the peritoneal cavity.
The follow-up strategy after complete surgery also depends on risk classification, and the most careful follow-up is required for high-risk GIST patients. Patients with intermediate-risk GISTs may accept a more relaxed follow-up. In Western countries, patients with very low-risk GISTs may be considered to have no follow-up after complete surgery because they have had no relapse (6,8,47,48). The evidence for the follow-up strategy has been established in Western countries, and Asian GIST patients have not supplied their own data. Hence, Asian guidelines indicate that further investigations are required to establish an optimized surveillance schedule with CT for Asian GIST patients.
Multidisciplinary treatment
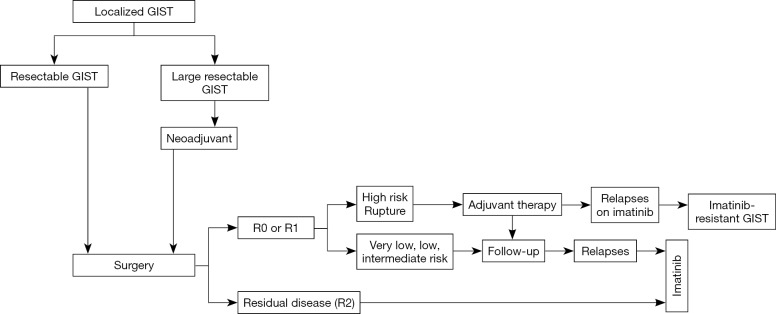
There is no large difference in adjuvant and/or neoadjuvant therapy recommended by GIST experts between the East and the West (Figure 4). In the ESMO guidelines, however, neoadjuvant therapy is concisely described, and the NCCN guidelines suggest that neoadjuvant treatment may be considered for patients who may require extensive surgery with resection of surrounding organs and/or surgery with significant risks (6,8). Both guidelines indicate that the decision to use neoadjuvant therapy may be made on an individual basis. In contrast, the Asian guidelines describe details of neoadjuvant therapy, including purpose, early evaluation, and duration of preoperative imatinib therapy (12,49). After preoperative imatinib, surgery is recommended at the time of best response or sufficient shrinkage, which usually takes 6 to 12 months of therapy (49). A preoperative drug holiday is not always required in the absence of significant drug-related adverse events. After complete resection, adjuvant therapy for three years or more is recommended for high-risk and/or ruptured GIST based on the disease evaluation before imatinib treatment. In adjuvant therapy, NCCN and ESMO guidelines consider that patients with significant risk of recurrence may have adjuvant therapy after discussion with experts from multiple disciplines and that shared decision with patients is important for adjuvant therapy (6,8). Both guidelines may allow some patients with intermediate-risk GISTs to have adjuvant therapy, depending on the patient’s situation and point of view in addition to recurrence risk. In contrast, Asian guidelines describe that candidates for adjuvant therapy may be high-risk GISTs, and patients with intermediate-risk GISTs have no sufficient evidence at present (12).
Figure 4.
Treatment of localized GIST. R0, microscopically and macroscopically no residual disease; R1, microscopic-positive and macroscopic-negative margin; R2, macroscopically residual disease. GIST, gastrointestinal stromal tumor.
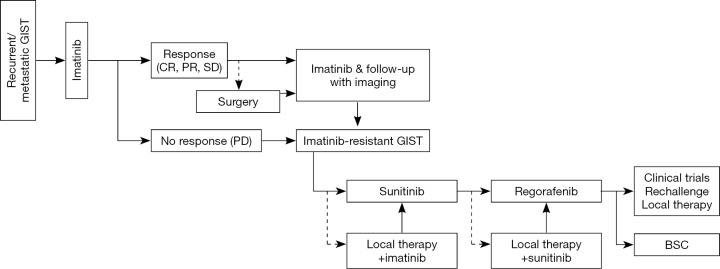
Compared with the Western guidelines, the Asian consensus guidelines indicate a more aggressive approach for advanced GIST patients, such as en bloc resection of extra-gastrointestinal GIST, even if it requires multi-visceral resection, and surgery for resectable residual diseases of metastatic and/or recurrent GIST under imatinib therapy (Figure 5) (12). Based on retrospective analyses and sub-analysis of discontinued clinical trials suggesting potential improvement of PFS and OS by surgical resection of residual tumors responding to imatinib, the Asian guidelines indicate en bloc resection of residual disease of advanced GIST patients after 4 to 12 months of imatinib therapy when applicable (12,50-53). The guidelines also suggest surgical resection or intervention by radiofrequency ablation (RFA) or by trans-arterial embolization (TAE) might be used when GIST patients show limited (or focal) progression of the disease in the presence of TKI (50-52,54). The primary approach to metastatic and/or recurrent GIST is imatinib, and the Asian guidelines tend to consider multidisciplinary therapy to improve the prognosis when applicable. Neither Asian nor Western guidelines suggest front-line debulking surgery for metastatic and/or recurrent disease (6,8,9,12).
Figure 5.
Treatment of recurrent/metastatic GIST. GIST, gastrointestinal stromal tumor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; BSC, best supportive care.
Medical therapy
For medical treatment, Asian and Western guidelines are very similar in recommending 1st-line imatinib, 2nd-line sunitinib and 3rd-line regorafenib (6,8,9,12). However, most of the evidence supporting those recommendations has been established by clinical trials conducted in the Western countries (55-57), and Asian GIST patients may still require their own evidence for some aspects, such as dose optimization. Asian patients are generally smaller than Caucasians and may have a different profile of adverse events. For example, Asian patients may show relatively frequent and severe hand-foot syndrome and hematotoxicity of the decrease in platelet and neutrophil counts when they receive sunitinib or regorafenib, whereas diarrhea is more frequent in Caucasians (56-60). The NCCN guidelines describe the details of medical therapy, including dose optimization, management of drug toxicities, mechanisms of drug resistance and treatment strategy for refractory GISTs, including investigational agents. After standard treatment, the guidelines recommend clinical trials for investigational agents, but in Asia, these trials are limited (12). In these situations, the Asian guidelines encourage a repeated challenge of TKI or use of TKI beyond PD (progressive disease) if applicable (61).
Conclusions
Between Asian and Western countries, there are some disparities in the medical system, clinical practice and disease profiles in oncology. In Asia, surgical oncologists have a major role in the multidisciplinary therapy, whereas medical oncologists are more prominent in the West. Although the incidence of clinical GIST appears to be similar between the two, the number of small GISTs treated by surgery seems to be high in Asia. Thus, the diagnostic and treatment strategies for small SMTs and GISTs are important in clinical practice in East Asia. Major parts of GIST guidelines are very similar between Asia and the West. However, there exist slight differences between their guidelines in the degree of recommendation, which may come from disparities in clinical practice and available medicines. Importantly, most clinical evidence in the GIST clinical guidelines has been established by clinical trials conducted in Western countries, and the number of clinical trials is still limited in Asia. This indicates that Asian GIST patients may have limited evidence based on their own data and may have limited access to new drugs after standard therapy. Finally, we may conclude that the GIST guidelines are well harmonized and reflect the particular medical circumstances in each region.
Acknowledgements
Funding: This work is supported in part by a Grant-in-Aid (16H05419 and 16K15600) for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by a Grant (28-A-16) from the National Cancer Center Research and Development Fund.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. 10.1016/S0140-6736(13)60106-3 [DOI] [PubMed] [Google Scholar]
- 2.Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. 10.1007/s10120-015-0526-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi CR, Vecchiato A, Mastrangelo G, et al. Adherence to treatment guidelines for primary sarcomas affects patient survival: a side study of the European CONnective TIssue CAncer NETwork (CONTICANET). Ann Oncol 2013;24:1685-91. 10.1093/annonc/mdt031 [DOI] [PubMed] [Google Scholar]
- 4.Derbel O, Heudel PE, Cropet C, et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS One 2017;12:e0158406. 10.1371/journal.pone.0158406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri GD, Benjamin R, Blanke CD, et al. NCCN GIST Task Force. NCCN Task Force report: optimal management of patients with gastrointestinal stromal tumor (GIST)--expansion and update of NCCN clinical practice guidelines. J Natl Compr Canc Netw 2004;2 Suppl 1:S1-26. [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41. 10.6004/jnccn.2010.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blay JY, Bonvalot S, Casali P, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566-78 10.1093/annonc/mdi127 [DOI] [PubMed] [Google Scholar]
- 8.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. 10.1093/annonc/mdu255 [DOI] [PubMed] [Google Scholar]
- 9.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416-30. 10.1007/s10147-008-0798-7 [DOI] [PubMed] [Google Scholar]
- 10.Kang YK, Kang HJ, Kim KM, et al. Korean GIST Study Group (KGSG) Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat 2012;44:85-96. 10.4143/crt.2012.44.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh CN, Hwang TL, Huang CS, et al. Taiwan Surgical Society of Gastroenterology Clinical practice guidelines for patients with gastrointestinal stromal tumor in Taiwan. World J Surg Oncol 2012;10:246. 10.1186/1477-7819-10-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat 2016;48:1155-66. 10.4143/crt.2016.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allemani C, Weir HK, Carreira H, et al. CONCORD Working Group Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 15.Joensuu H, Vehtari A, Riihima¨ki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 16.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006;37:1527-35. 10.1016/j.humpath.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 17.Abraham SC, Krasinskas AM, Hofstetter WL, et al. "Seedling" mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol 2007;31:1629-35. 10.1097/PAS.0b013e31806ab2c3 [DOI] [PubMed] [Google Scholar]
- 18.Agaimy A, Wünsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 2007;31:113-20. 10.1097/01.pas.0000213307.05811.f0 [DOI] [PubMed] [Google Scholar]
- 19.Agaimy A, Wünsch PH, Dirnhofer S, et al. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol 2008;32:867-73. 10.1097/PAS.0b013e31815c0417 [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Gasparotto D, Toffolatti L, et al. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs)of small size. Am J Surg Pathol 2010;34:1480-91. 10.1097/PAS.0b013e3181ef7431 [DOI] [PubMed] [Google Scholar]
- 21.Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110-8. 10.1002/cncr.30239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5: 20-3. 10.1007/BF00591381 [DOI] [PubMed] [Google Scholar]
- 23.Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc 2016;83:318-26. 10.1016/j.gie.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 24.An W, Sun PB, Gao J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc 2017;31:4522-31. 10.1007/s00464-017-5511-3 [DOI] [PubMed] [Google Scholar]
- 25.Nishida T. Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs? Transl Gastroenterol Hepatol 2017;2:92. 10.21037/tgh.2017.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A 2011;108:314-8. 10.1073/pnas.1009199108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type gastrointestinal stromal tumors: a report from the national institutes of health gastrointestinal stromal tumor clinic. JAMA Oncol 2016;2:922-8. 10.1001/jamaoncol.2016.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459-65. 10.1053/hupa.2002.123545 [DOI] [PubMed] [Google Scholar]
- 29.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 30.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Edge SB, Byrd DR, Compton CC, et al. AJCC staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 32.Gold JS, Go¨nen M, Gutie’rrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10:1045-52. 10.1016/S1470-2045(09)70242-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol 2011;35:1646-56. 10.1097/PAS.0b013e31822d63a7 [DOI] [PubMed] [Google Scholar]
- 34.Goh BK, Chow PK, Yap WM, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol 2008;15:2153-63. 10.1245/s10434-008-9969-z [DOI] [PubMed] [Google Scholar]
- 35.Bischof DA, Kim Y, Behman R, et al. A nomogram to predict disease-free survival after surgical resection of GIST. J Gastrointest Surg 2014;18:2123-9. 10.1007/s11605-014-2658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour—the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 2011;37: 890-6. 10.1016/j.ejso.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 37.Yanagimoto Y, Takahashi T, Muguruma K, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GIST) after complete resection: indications for adjuvant therapy. Gastric Cancer 2015;18:426-33. 10.1007/s10120-014-0386-7 [DOI] [PubMed] [Google Scholar]
- 38.Joensuu H, Eriksson M, Hall KS, et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer 2014;120:2325-33. 10.1002/cncr.28669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksson M, Reichardt P, Sundby Hall K, et al. Needle biopsy through the abdominal wall for the diagnosis of gastrointestinal stromal tumour - Does it increase the risk for tumour cell seeding and recurrence? Eur J Cancer 2016;59:128-33. 10.1016/j.ejca.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 40.Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc 2007;21:875-8. 10.1007/s00464-006-9065-z [DOI] [PubMed] [Google Scholar]
- 41.Bischof DA, Kim Y, Dodson R, et al. Open versus minimally invasive resection of gastric GIST: a multi-institutional analysis of short- and long-term outcomes. Ann Surg Oncol 2014;21:2941-8. 10.1245/s10434-014-3733-3 [DOI] [PubMed] [Google Scholar]
- 42.Chen K, Zhou YC, Mou YP, et al. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc 2015;29:355-67. 10.1007/s00464-014-3676-6 [DOI] [PubMed] [Google Scholar]
- 43.MacArthur KM, Baumann BC, Nicholl MB. Laparoscopic versus open resection for gastrointestinal stromal tumors (GISTs). J Gastrointest Cancer 2017;48:20-24. 10.1007/s12029-016-9861-1 [DOI] [PubMed] [Google Scholar]
- 44.Hølmebakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg 2016;103:684-691. 10.1002/bjs.10104 [DOI] [PubMed] [Google Scholar]
- 45.McCarter MD, Antonescu CR, Ballman KV, et al. American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant Gist Study Team Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg 2012;215:53-9. 10.1016/j.jamcollsurg.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Nakajima K, Nishitani A, et al. An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 2007;12:369-374. 10.1007/s10147-007-0705-7 [DOI] [PubMed] [Google Scholar]
- 47.Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumor treated with or without adjuvant imatinib after surgery. Eur J Cancer 2015; 51:1611-7. 10.1016/j.ejca.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 48.Joensuu H, Reichardt P, Eriksson M, et al. Gastrointestinal stromal tumor: a method for optimizing the timing of CT scans in the follow-up of cancer patients. Radiology 2014;271:96-103. 10.1148/radiol.13131040 [DOI] [PubMed] [Google Scholar]
- 49.Kurokawa Y, Yang HK, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117:25-32. 10.1038/bjc.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raut CP, Wang Q, Manola J, et al. Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib malate. Ann Surg Oncol 2010;17:407-15. 10.1245/s10434-009-0784-y [DOI] [PubMed] [Google Scholar]
- 51.DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. 10.1097/01.sla.0000236630.93587.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6. 10.1097/01.sla.0000242710.36384.1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomized trial in China. Eur J Cancer 2014;50:1772-8. 10.1016/j.ejca.2014.03.280 [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa J, Kanda T, Hirota S, et al. Surgical interventions for focal progression of advanced gastrointestinal stromal tumors during imatinib therapy. Int J Clin Oncol 2007;12:212-7. 10.1007/s10147-007-0657-y [DOI] [PubMed] [Google Scholar]
- 55.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. 10.1056/NEJMoa020461 [DOI] [PubMed] [Google Scholar]
- 56.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 57.Demetri GD, Reichardt P, Kang YK, et al. GRID study investigators Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirao K, Nishida T, Doi T, et al. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs 2010;28:866-75. 10.1007/s10637-009-9306-9 [DOI] [PubMed] [Google Scholar]
- 59.Komatsu Y, Ohki E, Ueno N, et al. Safety, efficacy and prognostic analyses of sunitinib in the post-marketing surveillance study of Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol 2015;45:1016-22. 10.1093/jjco/hyv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komatsu Y, Doi T, Sawaki A, et al. Regorafenib for advanced gastrointestinal stromal tumors following imatinib and sunitinib treatment: a subgroup analysis evaluating Japanese patients in the phase III GRID trial. Int J Clin Oncol 2015;20:905-12. 10.1007/s10147-015-0790-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2013;14:1175-82. 10.1016/S1470-2045(13)70453-4 [DOI] [PMC free article] [PubMed] [Google Scholar]