Abstract
We describe a rare case of severe autoimmune haemolytic anaemia (AIHA) in the setting of underlying chronic lymphocytic leukaemia receiving intravenous immunoglobulin, history of warm IgG autoantibody and treatment with nivolumab for advanced non-small cell lung cancer. In this report, we describe AIHA as a potential serious immune-related adverse event from immune checkpoint inhibitors, discuss other potential contributing factors and review previously described cases of AIHA in patients receiving programmed death 1 (PD-1) inhibitors. In the era of immunotherapy, we hope to add literature to raise awareness of potential immune-related sequelae such as AIHA. We aim to highlight the importance of close monitoring for prompt identification and management of potentially fatal AIHA and immune-related adverse events of PD-1 inhibitors by holding immunotherapy and treating with high-dose steroids, particularly in subgroups which may be at increased risk.
Keywords: lung cancer (oncology), haematology (drugs and medicines), haematology (incl blood transfusion), immunology, unwanted effects / adverse reactions
Background
Advanced non-small cell lung cancer (NSCLC), particularly those refractory to systemic chemotherapies, has previously had limited therapeutic options. Targeted therapies and immune checkpoint inhibitors have emerged as first-line and second-line therapeutic alternatives for NSCLC. Programmed death-1 (PD-1) checkpoint inhibitors are increasingly being used for a wide range of solid tumours and haematological malignancies. Despite their favourable safety profile compared with cytotoxic chemotherapy, immunotherapies are associated with a new spectrum of immune-related adverse events. Although usually manageable with interruption of immunotherapy and immunosuppression, these adverse events can be severe or even fatal. Previously reported immune-related adverse events of PD-1 inhibitors involve dermatological manifestations, colitis, endocrinopathies, pneumonitis and hepatotoxicity.1 Anaemia is an adverse effect associated with the use of PD-1 and PD-L1 inhibitors.2–6 We now present a rare case of autoimmune haemolytic anaemia (AIHA) associated with the use of nivolumab as well as various cases reported in the literature.
Case presentation
We present a man in his early 60s with history of diabetes mellitus type 2 and chronic lymphocytic leukaemia (CLL) who was subsequently diagnosed with poorly differentiated adenocarcinoma of the left lower lung. He was initially diagnosed with CLL Rai stage I after presenting with leucocytosis and waxing and waning cervical lymphadenopathy with confirmatory biopsy in 2009. He was treated with six cycles of fludarabine, mitoxantrone, dexamethasone and rituximab and achieved complete radiological remission.
In 2011, a repeat positron emission tomography (PET) scan revealed progressive lymphadenopathy and a new 1 cm left lower lung nodule. A lymph node biopsy confirmed CLL relapse, but his asymptomatic CLL was monitored without additional treatment. In 2013, a surveillance PET scan revealed progressive diffuse lymphadenopathy, left hilar uptake and left lower lung collapse. Biopsy of the left lower lung revealed poorly differentiated adenocarcinoma negative for epidermal growth factor receptor mutation, anaplastic lymphoma kinase or ROS1 rearrangement. It was initially staged as IIIA cT3N2Mx. As his course was complicated by a left empyema requiring hospitalisation, he initially received 2 weeks of palliative radiation to possibly relieve the obstruction. After clinical improvement in 2014, he received concomitant chemoradiation with cisplatin and docetaxel. Pleural biopsy performed during thoracotomy for empyema drainage showed adenocarcinoma and he received consolidation chemotherapy with docetaxel for three cycles.
About 4 months later, he presented with clinical and radiological CLL recurrence, and was started on ibrutinib. One month later, PET scan revealed new hypermetabolic mediastinal lymphadenopathy and supraclavicular lymph nodes, and biopsy of the left supraclavicular lymph node confirmed metastatic adenocarcinoma. As such, he received first-line platinum doublet chemotherapy with carboplatin and pemetrexed for four cycles followed by pemetrexed maintenance with good clinical response. In 2015, he was switched from ibrutinib to ofatumumab due to bleeding complications in the left open thoracotomy site, felt to be related to ibrutinib. He had a good response. However, both ofatumumab and pemetrexed were discontinued about 4 months later after the development of cardiac tamponade requiring pericardiocentesis and a decline in his performance status, limiting the duration of these agents.
On disease progression on platinum-based chemotherapy, he was started on nivolumab for his metastatic NSCLC. Additionally, because he remained with CLL progression, ofatumumab was resumed in early 2016 and later switched to bendamustine due to CLL progression resulting in clinically stable disease. Additionally, he had been receiving 20 g of intravenous immunoglobulin (IVIG) monthly for CLL-associated hypogammaglobulinaemia since 2014. Nivolumab had been well tolerated, and he demonstrated good clinical response with stable NSCLC on serial PET scans. However, 2 weeks after the 21st dose of nivolumab, he presented to the hospital with 3 days of progressive shortness of breath, jaundice and confusion. He was hypotensive, tachycardic and ill-appearing with generalised jaundice and scleral icterus, mildly distant heart sounds, diminished breath sounds at the bases bilaterally, mild splenomegaly and otherwise normal abdominal and skin examination. He was afebrile with no obvious signs of infection and although oriented only to self, had no focal neurological deficits.
Investigations
Laboratory work up was consistent with haemolysis given haemoglobin 4.3 g/dL, total bilirubin 6.5 mg/dL, direct bilirubin 0.2 mg/dL, elevated lactate dehydrogenase (LDH) 335 U/L, haptoglobin <10 mg/dL, reticulocyte count 17%, fibrinogen 404, prothrombin time 22.2, international normalised ratio 1.97 and partial thromboplastin time 38.7.
Peripheral blood smear demonstrated reticulocytosis and spherocytosis with no schistocytes.
On admission, blood type was A Rh(+), direct antiglobulin test (DAT) was positive for IgG and negative for complement. Eleven days after admission, DAT was positive for IgG and anti-Jka IgG (3+) and negative for complement. Of note, the patient had received 5 units of Jka negative, Kell negative and little C negative packed red blood cells (PRBCs) between these two tests.
A prior DAT in 2013 was positive for warm IgG antibodies; however, an antibody screen performed about 2 months prior to admission was negative for autoantibodies and alloantibodies.
Other than mild hyperglycaemia and elevated bilirubin, chemistry was unremarkable.
Blood and urine cultures were negative.
Chest CT revealed left lower lobe mass, right pleural effusion, moderate pericardial effusion and left lower lobe atelectasis without evidence of pneumonia.
Abdominal CT revealed cholelithiasis but no cholecystitis.
Echocardiogram revealed low normal systolic function and moderate diastolic dysfunction.
Differential diagnosis
The presentation of jaundice and laboratory findings of haemolytic anaemia with positive DAT is consistent with AIHA. Possible aetiologies include drug-induced haemolytic anaemia, AIHA associated with lymphoproliferative disorder, purine analogue associated AIHA, transfusion reaction, AIHA associated with IVIG, AIHA associated with venous thromboembolic disease, sepsis induced haemolytic anaemia or while less likely, paroxysmal cold haemoglobinuria, connective tissue disease, cold agglutinin induced haemolytic anaemia or hereditary spherocytosis.
Treatment
The patient was admitted to the intensive care unit and treated with methylprednisolone sodium succinate 80 mg intravenously daily for 2 weeks, a total of 7 units of PRBCs and folic acid supplementation. This was followed by a gradual prednisone taper over several months.
Outcome and follow-up
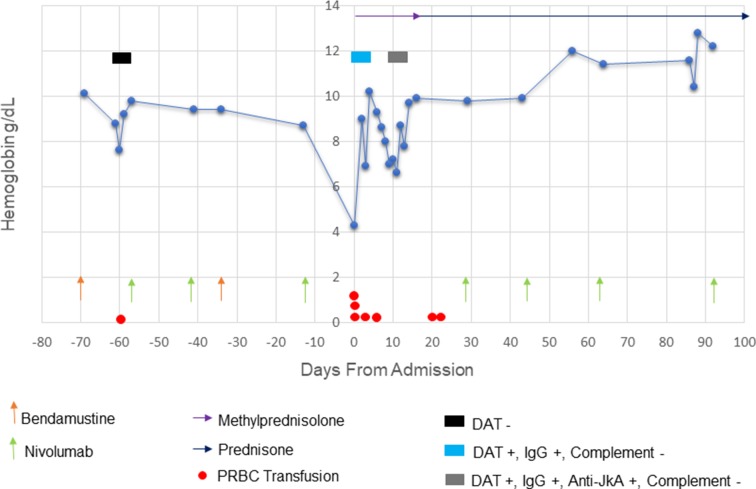
The patient was admitted to the hospital for a total of 15 days, and had resolution of acute anaemia after approximately 2 weeks. The patient’s haemoglobin trend is demonstrated in figure 1. Given limited therapeutic options and good clinical response of NSCLC, he was restarted on nivolumab without recurrence. Unfortunately, 5 months after discharge, the patient died due to progression of NSCLC.
Figure 1.
Plot of haemoglobin trend, serology and relevant therapies. DAT, direct antiglobulin test; PRBC, packed red blood cells.
Discussion
Lung cancer is the second most commonly diagnosed cancer and is also the leading cause of cancer death in the USA.7 Approximately, 70% of lung cancer is locally advanced or metastatic at the time of diagnosis, representing a challenge to effective treatment.8 Systemic therapies for advanced NSCLC include chemotherapy, therapy targeted to available actionable mutations or immunotherapy. Cytotoxic, platinum-based doublet chemotherapy used to be the standard first-line treatment for patients with good performance status and advanced NSCLC without actionable driver mutations. Recently, the combination of carboplatin and pemetrexed with pembrolizumab has been approved as a first-line option for patients with advanced non-squamous NSCLC and single agent pembrolizumab also has been approved in select patients having advanced NSCLC with PD-L1 tumour expression >50% tumour proportion score.9 10 In the era of immunotherapy, recent data has shown a 5-year survival of 16% for patients with stage IV NSCLC.11
Second-line therapeutic options include nivolumab, pembrolizumab, atezolizumab, docetaxel, pemetrexed, gemcitabine or ramucirumab and docetaxel.12 Nivolumab is a human IgG4 PD-1 immune checkpoint inhibitor antibody that binds to PD-1 receptors with high affinity on T cells and inhibits T-effector cell inactivation. The presence of PD-L1 and PD-L2 on tumour cells contributes to tumour escape via this pathway. Therefore, inhibitors of this pathway reactivate T effector cells to eliminate tumour cells.13 Generally, nivolumab is well-tolerated, but some serious immune-related adverse events have been reported.1
AIHA occurs when antibodies of IgG, IgM or rarely IgA bind to red blood cell (RBC) surface antigens and lead to RBC destruction via complement or the reticuloendothelial system. Studies of AIHA suggest that the activation of autoreactive helper T cells by antigen presenting cells is important in the pathogenesis of AIHA.14 Secondary causes of AIHA include infections, autoimmune and connective tissue diseases, haematological malignancies and related therapies, prior blood transfusions, medications, stem cell or organ transplantations. The diagnosis of AIHA is typically made by laboratory evidence of haemolysis with an isolated decrease in haemoglobin, positive DAT or Coomb’s test, indirect hyperbilirubinaemia, elevated LDH, reticulocytosis, reduced haptoglobin and peripheral smear with spherocytosis. Timely corticosteroid treatment and transfusions are first-line therapy in AIHA. Cases refractory to corticosteroids may use immunosuppressive drugs, IVIG, rituximab or even surgical splenectomy.15 Refractory AIHA cases can become complicated or even fatal, often secondary to venous thromboembolism, renal failure, complications related to aggressive transfusion therapy, severe infections or cardiac compromise.
We suspect that AIHA in our patient may be an immune-mediated adverse event of PD-1 inhibitors either by reactivation of prior RBC autoantibodies or the de novo production of RBC autoantibodies. It has been reported that immune checkpoint inhibitors may induce the production of de novo RBC and other autoantibodies, which is supported by preclinical studies demonstrating the production of autoantibodies in PD-1 deficient mice.16–19 Table 1 summarises the eight published cases of AIHA suggested to be associated with PD-1 inhibitors.20–27 In addition to our patient with CLL and prior antibodies, published cases include one patient with Hodgkin’s lymphoma, a patient with CLL and two patients with prior RBC antibodies. Onset of AIHA was variable ranging from after 2 to 21 doses of PD1 inhibitor. Unfortunately, the presence of RBC antibodies prior to the administration of nivolumab is not available for many of the published cases.
Table 1.
Summary of published cases of AIHA associated with the use of PD-1 inhibitors
| Authors | Age/gender | Indication of PD-1 inhibitor | PD-1 inhibitor (dose) |
No of doses prior to event | Prior RBC Ab |
Lymphoproliferative disorder | Haemoglobin (g/dL) Nadir |
Blood type DAT |
Treatment | Outcome |
| Presented Case | Early 60s Male |
Advanced NSCLC | Nivolumab (3 mg/kg) |
21 | Prior IgG auto-Ab* | CLL | 4.3 | A+; IgG AntiJKA† |
Methylprednisolone 80 mg intravenous daily for 2 weeks, then prednisone taper of 4 weeks | Acute anaemia resolved after 2 weeks. PD-1 inhibitor resumed after complete recovery of anaemia without recurrence of haemolysis |
| Kong et al 21 | 85 Male |
Metastatic melanoma | Nivolumab (3 mg/kg) |
5 | Prior allo-Ab and auto-Ab‡ | No | 6.4 | O+; IgG |
Prednisone 110 mg×2 weeks, tapered over a total of 80 days | Anaemia resolution. PD-1 inhibitor was not resumed |
| Schwab et al 25 | 82 Male |
Cutaneous squamous cell carcinoma | Nivolumab (3 mg/kg) |
8 | None reported | CLL§ | Not available | Not available; IgG, C3 |
Prednisolone 80 mg/dayx2 weeks | Anaemia resolution after 1 month. PD-1 inhibitor not resumed |
| Tardy et al 26 | 75 Female |
Stage IIIB Hodgkin’s lymphoma | Nivolumab (3 mg/kg) |
2 | None reported | Hodgkin’s lymphoma | 6.4 | Not available; IgG |
Prednisone 2 mg/kg×10 days, then 1 mg/kg×2 weeks, then tapered over 3 months | Anaemia resolution after 1 month. PD-1 inhibitor resumed without further recurrence of haemolysis |
| Palla et al 24 | 70 Male |
Advanced NSCLC | Nivolumab (3 mg/kg) |
2 | None reported | No | 5.5 | Not available; C3 |
Prednisone 1.5 mg/kg×5 days | Death 10 days after admission |
| Khan et al 20 | 43 Female |
Metastatic melanoma | Nivolumab and ipilimumab (unknown dose) | 2 | None reported | No | 5.6 | Not available; IgG, C3d |
Methylprednisolone 1 g intravenous dailyx3 days, then prednisone taper over several weeks. | Anaemia resolution after 2 months. Nivolumab and ipilimumab were resumed resulting in AIHA recurrence |
| Nair et al 23 | 52 Female |
Malignant melanoma | Pembrolizumab (unknown dose) | 3 | None reported | No | 6.3 | Not available; IgG |
High dose steroids, followed by prednisone 20 mg taper over 8 weeks, and IVIG x1. | AIHA with pure red cell aplasia resolved after treatment. PD-1 inhibitor was not resumed |
| Lott et al 22 | 67 Male |
Advanced NSCLC | Pembrolizumab (10 mg/kg) | 18 | None reported | No | 3.2 | Not available; IgG |
Prednisone, azathioprine, cyclophosphamide and IVIG therapy, then prednisone taper and cyclophosphamide | Developed Evan’s syndrome treated with rituximab and steroids, with eventual resolution of anaemia and thrombocytopenia. PD-1 inhibitor was not resumed |
| Le Burel et al 27 | 72 Female |
Melanoma | Unknown | 5 | Cold agglutinins | No | 8.2 | Not available; warm agglutinins |
Corticosteroids (2 mg/kg) |
Improvement |
*The patient had a previous DAT positive for warm IgG in 2013. However, the antibody screen about 2 months prior to the event was negative.
†DAT was negative for alloantibodies on admission, but anti-Jka was positive after 11 days of admission and after receiving five units of PRBCs.
‡Prior DAT in 2011 was positive for anti-C, anti-e and multiple other weak alloantibodies. Additionally, the patient had a previous DAT positive for warm IgG in 2011. However, the antibody screen in 2012 was negative.
§CLL was previously treated with fludarabine and ibrutinib, though noted to be in stable remission during AIHA.
Ab, anitgen; AIHA, autoimmune haemolytic anaemia; CLL, chronic lymphocytic leukaemia; DAT, direct antiglobulin test; IVIG, intravenous immunoglobulin; NSCLC, non-small cell lung cancer; PD-1, programmed death-1; PRBCs, packed red blood cells; RBC, red blood cell.
There are different potential causes for AIHA in our patient. While AIHA can occur in any stage of CLL, it has been associated with male gender, age >65 years, absolute lymphocyte count >60×109/L and more aggressive disease with Binet stage B or C.28 29 Our patient had stable CLL during the episode of AIHA, with a normal white blood cell count and no history of prior autoimmune cytopenias or alloantibodies. Treatment with fludarabine, a purine analogue, has been associated with AIHA in CLL, however, his last treatment was in 2009.29 Review of the patient’s medications did not reveal any medications commonly associated with AIHA.30 Additionally, haemolytic anaemia has been described as a rare complication of IVIG through the passive transfer of blood group alloantibodies.31 32 Per a case series of IVIG-related haemolytic anaemia, a high cumulative dose of IVIG therapy, female gender, non-O blood group and positive inflammatory serological markers may predispose IVIG-related haemolysis.33 Other than non-O blood groups of A Rh+, these do not apply to our male patient who received low dose monthly IVIG.
Alternatively, it is possible that the patient developed an atypical delayed transfusion-related haemolytic anaemia from a blood product transfusion he received about 2 months prior of which donor phenotype is unknown. Our patient developed anti-Jka alloantibodies 11 days after admission and after receiving five units of Jka negative, Kell negative and little C negative PRBCs. Delayed haemolytic transfusion reactions are rare occurring in 0.2%–2.6% of patients, but arise when a patient lacking minor RBC antigens is immunised via transfusion of blood products with the corresponding antigen and subsequently receives blood products containing the antigen.34 A low titre of alloantibody may result in a compatible crossmatch. Over one-third to one-half of patients who have autoantibodies are reported to have underlying alloantibodies as the presence of warm autoantibodies may mask alloantibodies.35
Novel immunotherapy drugs that have the promise to treat a wide variety of malignancies may predispose patients to potentially serious but often manageable immune-related adverse events. It is important to monitor for these immune-related adverse effects such as AIHA throughout the entire course of treatment as onset is variable. It is also essential to identify and treat these adverse effects promptly and effectively by immediately holding immunotherapy and initiating high dose steroids to improve outcomes. History of RBC antibodies or lymphoproliferative disorders associated with the presence of autoreactive T cells may be risk factors for the production or reactivation of RBC autoantibodies and AIHA with exposure to PD-1 inhibitors. These findings warrant further studies to evaluate AIHA as a potential adverse effect of PD-1/PD-L1 inhibitors with special attention to potential subgroups at increased risk.
Footnotes
Contributors: All authors contributed extensively to the article. SDA and WP identified the case, performed the literature review, drafted and edited the manuscript. RM and TJH reviewed the draft and provided critical revisions. RM was also significantly involved in the care of the patient.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol 2016;2:1346–53. 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 2. Opdivo (R) [Package Insert]. Princeton, NJ: Bristol-Myers Squibb Company, 2017. https://packageinserts.bms.com/pi/pi_opdivo.pdf [Google Scholar]
- 3. Keytruda (R) [Package Insert]: Merck & Co., Inc., Whitehouse Station, NJ, 2017. http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf [Google Scholar]
- 4. Tecentriq (R) [Package Insert]. San Franciscio, CA: Genentech, Incl, A Member of the Roche Group, 2017. [Google Scholar]
- 5. Bavencio (R) [Package Insert]: EMD Serono, Inc. and Pfizer Inc., NY, 2017. [Google Scholar]
- 6. Imfinzi (R) [Package Insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2017. [Google Scholar]
- 7.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Advances in experimental medicine and biology 2016;893:1–19. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: a cancer j for clinicians 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 9.Langer CJ, Gadgeel SM, Borghaei H, et al. . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med Overseas Ed 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 11.Brahmer J, Horn L, Jackman D, eds Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): clinical characteristics of long-term survivors. American Association for Cancer Research Annual Meeting, 2017. [Google Scholar]
- 12.Ettinger DS, Wood DE, Akerley W, et al. . NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 4.2016. J National Comprehensive Cancer Network 2016;14:255–64. 10.6004/jnccn.2016.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Li F, Jiang F, et al. . A Mini-Review for Cancer Immunotherapy: Molecular Understanding of PD-1/PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int J Mol Sci 2016;17:1151 10.3390/ijms17071151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagiolo E. Immunological tolerance loss vs. erythrocyte self antigens and cytokine network disregulation in autoimmune hemolytic anaemia. Autoimmun Rev 2004;3:53–9. 10.1016/S1568-9972(03)00085-5 [DOI] [PubMed] [Google Scholar]
- 15.Park SH. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Res 2016;51:69–71. 10.5045/br.2016.51.2.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooling L, Sherbeck J, Mowers J, et al. . Development of red blood cell autoantibodies following treatment with checkpoint inhibitors: a new class of anti-neoplastic, immunotherapeutic agents associated with immune dysregulation. Immunohematology 2017;33:15. [PubMed] [Google Scholar]
- 17.Nishimura H, Nose M, Hiai H, et al. . Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999;11:141–51. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 18.Okazaki T, Tanaka Y, Nishio R, et al. . Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477–83. 10.1038/nm955 [DOI] [PubMed] [Google Scholar]
- 19.Raskin J, Masrori P, Cant A, et al. . Recurrent dysphasia due to nivolumab-induced encephalopathy with presence of Hu autoantibody. Lung Cancer 2017;109:74–7. 10.1016/j.lungcan.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Khan U, Ali F, Khurram MS, et al. . Immunotherapy-associated autoimmune hemolytic anemia. J Immunother Cancer 2017;5:15 10.1186/s40425-017-0214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong BY, Micklethwaite KP, Swaminathan S, et al. . Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 2016;26:202–4. 10.1097/CMR.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 22.Lott A, Butler M, Leighl N, et al. . Evan’s Syndrome Associated with Pembrolizumab Therapy in Metastatic Non-Small Cell Lung Cancer. Blood 2015;126:4543. [Google Scholar]
- 23.Nair R, Gheith S, Nair SG. Immunotherapy-Associated Hemolytic Anemia with Pure Red-Cell Aplasia. N Engl J Med Overseas Ed 2016;374:1096–7. 10.1056/NEJMc1509362 [DOI] [PubMed] [Google Scholar]
- 24.Palla AR, Kennedy D, Mosharraf H, et al. . Autoimmune Hemolytic Anemia as a Complication of Nivolumab Therapy. Case Rep Oncol 2016;9:691–7. 10.1159/000452296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab KS, Heine A, Weimann T, et al. . Development of Hemolytic Anemia in a Nivolumab-Treated Patient with Refractory Metastatic Squamous Cell Skin Cancer and Chronic Lymphatic Leukemia. Case Rep Oncol 2016;9:373–8. 10.1159/000447508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tardy MP, Gastaud L, Boscagli A, et al. . Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol 2017;35:875–7. 10.1002/hon.2338 [DOI] [PubMed] [Google Scholar]
- 27.Le Burel S, Champiat S, Mateus C, et al. . Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34–44. 10.1016/j.ejca.2017.05.032 [DOI] [PubMed] [Google Scholar]
- 28.Mauro FR, Foa R, Cerretti R, et al. . Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood 2000;95:2786–92. [PubMed] [Google Scholar]
- 29.Molica S, Polliack A. Autoimmune hemolytic anemia (AIHA) associated with chronic lymphocytic leukemia in the current era of targeted therapy. Leuk Res 2016;50:31–6. 10.1016/j.leukres.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Garratty G. Drug-induced immune hemolytic anemia. Hematology 2009;2009:73–9. 10.1182/asheducation-2009.1.73 [DOI] [PubMed] [Google Scholar]
- 31.Pintova S, Bhardwaj AS, Aledort LM. IVIG — A Hemolytic Culprit. N Engl J Med Overseas Ed 2012;367:974–6. 10.1056/NEJMc1205644 [DOI] [PubMed] [Google Scholar]
- 32.Späth PJ, Granata G, La Marra F, et al. . On the dark side of therapies with immunoglobulin concentrates: the adverse events. Front Immunol 2015;6:11 10.3389/fimmu.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daw Z, Padmore R, Neurath D, et al. . Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion 2008;48:1598–601. 10.1111/j.1537-2995.2008.01721.x [DOI] [PubMed] [Google Scholar]
- 34.Cohn J. Chapter 138: Blood Procurement and Red Cell Transfusion : Kaushansky K, Lichtman MA, Prchal JT, Levi M, Press OW, et al; Williams Hematology. 9th edn New York: McGraw-Hill Education LLC, 2016. [Google Scholar]
- 35.Chaudhary RK, Das S. Autoimmune hemolytic anemia: From lab to bedside. Asian J Transfus Sci 2014;8:5 10.4103/0973-6247.126681 [DOI] [PMC free article] [PubMed] [Google Scholar]