Abstract
Metaplastic breast cancer (MBC) in men is an extremely rare entity. MBC is typically very aggressive with a poor prognosis. In men, it has only been reported three times in the literature. We report a 47-year-old man who presented with right-sided breast erythema and nipple inversion. Mammogram revealed a 2.4 cm spiculated mass. Initial pathology was inconclusive; however, right-sided simple mastectomy showed invasive metaplastic carcinoma with adenosquamous histology. He received adjuvant chemotherapy with 4 cycles of dose dense Adriamycin and cyclophosphamide followed by 12 weeks of paclitaxel and chest wall radiation. Although oestrogen receptor status was 1%, tamoxifen was not given due to recent diagnosis of pulmonary embolism. Two years after treatment, he is currently living with no signs of recurrence. This case will serve as a useful addition to the current literature discussing successful diagnosis, treatment and prognosis of a man with MBC.
Keywords: oncology, breast cancer, pathology
Background
Breast cancer is the most frequently diagnosed cancer.1 In men, breast cancer only accounts for less than 1% of diagnoses.1 Metaplastic breast cancer (MBC), characterised by two or more histological cell types, is also seen in less than 1% of breast cancer diagnoses.2 MBC in men has been limited to only three cases described in the literature.3–5 We report the youngest case of MBC in a man who successfully underwent mastectomy, adjuvant chemotherapy with Adriamycin and cyclophosphamide followed by paclitaxel and radiation to the chest wall. Complications of pulmonary embolism (PE) prevented axillary node dissection and administration of hormonal therapy with tamoxifen; however, despite this, he had a complete response and is still in remission 24 months later.
Case presentation
A 47-year-old man presented with 1 month duration of right-sided breast swelling, erythema, skin changes and nipple inversion. He denied any complaints or changes regarding the left breast or chest wall.
His medical history was significant for obesity, hypertension, hyperlipidaemia, depression, anxiety, chronic obstructive pulmonary disease and chronic back pain. He denied any current tobacco, alcohol or recreational drug use; however, he did previously smoke 4 packs per day for 30 years and at times drank heavily. He denied any family history of breast cancer.
Physical examination revealed stable vital signs and right-sided breast swelling, erythema and nipple inversion. No obvious breast mass was palpated. A right-sided, 1 cm axillary lymph node was palpated with mild tenderness. Laboratory data were within normal limits.
A right-sided mammogram showed a 2.4 cm spiculated nodule with associated nipple retraction and skin thickening (figure 1). Biopsies had findings of gynecomastia and significant infiltrative chronic inflammatory cells. Concern for breast cancer remained, and thus, he received a right-sided simple mastectomy.
Figure 1.
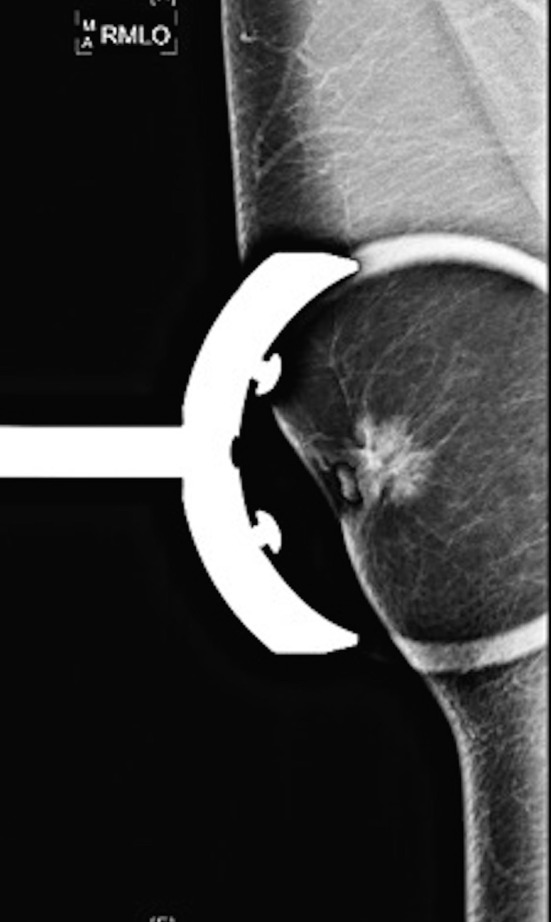
Right-sided mammogram with a 2.4 cm spiculated nodule with associated nipple retraction and skin thickening.
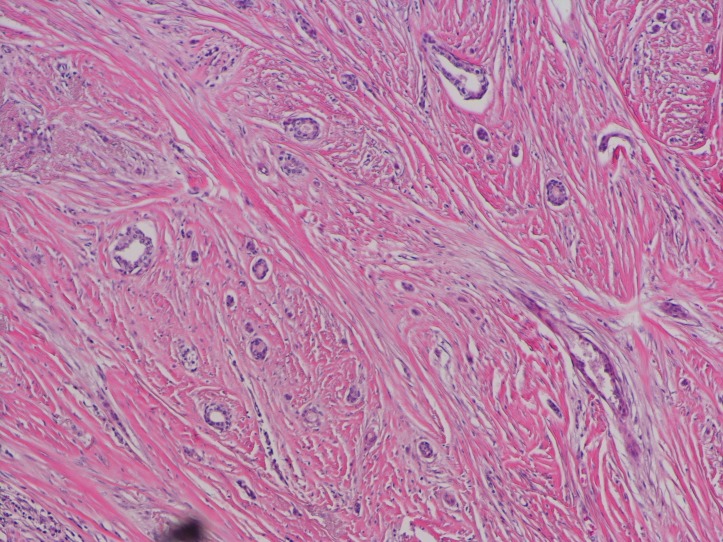
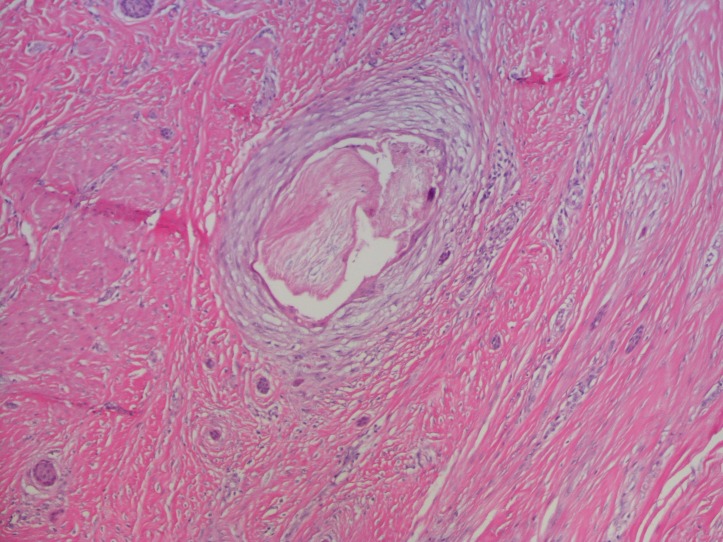
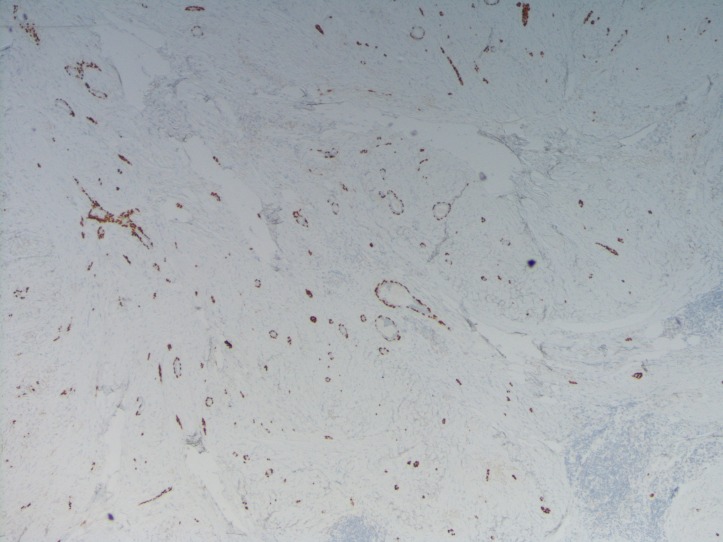
Pathology showed a 1.5 cm invasive metaplastic carcinoma with features of low-grade adenosquamous histology (figures 2 and 3). Oestrogen receptor was 1%, progesterone receptor was negative and human epidermal growth factor receptor 2 was negative by immunohistochemistry. E-cadherin and p63 were positive (figure 4).
Figure 2.
H&E stain showing small glands infiltrating stroma.
Figure 3.
H&E stain with area of squamous differentiation.
Figure 4.
Immunohistochemistry stain p63 demonstrates positive infiltrating.
Metastatic work-up was obtained after the patient was diagnosed with invasive MBC due to a palpable lymph node. Nuclear medicine bone scan and MRI brain showed no evidence of metastatic disease. CT of the abdomen and pelvis showed a small ill-defined mass in the right hepatic lobe that was further characterised by MRI and found to be a haemangioma and cyst. CT of the chest with contrast revealed a small low-density mass in the anterior mediastinum. Finally, a positron emission tomography (PET)/CT showed no uptake except within the small anterior mediastinal mass (standard uptake value 2.0)
With concern for possible metastatic disease due to minimal PET avidity of the mass, he received a thymectomy. Pathology revealed a multilocular 2.5 cm thymic cyst that was negative for both primary or secondary malignancy. His postoperative course after thymectomy was complicated by a PE, and he was started on Coumadin. Subsequently, due to the PE and being on anticoagulation, he did not receive an axillary node dissection as he was deemed high risk for the procedure.
Prior to receiving adjuvant chemotherapy for stage IIA MBC, an echocardiogram was obtained, and his ejection fraction was estimated to be 50%–55% with normal left ventricle wall motion. He also received genetic testing and did not harbour any abnormal mutations.
Differential diagnosis
gynecomastia
pseudogynecomastia
infection
lipoma
pseudoangiomatous stroma hyperplasia
granular cell tumour
fibromatosis
syringomatous adenoma of the nipple
metastatic disease from another primary.
Treatment
The patient received 4 cycles of dose dense Adriamycin and cyclophosphamide with Neulasta for bone marrow convalescence followed by 12 cycles of weekly Taxol. He then received radiation to the right chest wall. It was decided not to administer tamoxifen due to his history of PE and his oestrogen receptor status being only 1%.
Outcome and follow-up
Currently, 2 years after surgery, the patient is doing well. He occasionally complains of some pain at the site of his right-sided simple mastectomy; however, pain medications help. He had no issues with his left breast and his recent mammogram of the left breast was negative.
Discussion
Breast cancer is the most frequently diagnosed cancer globally and is the most commonly diagnosed cancer in women.1 Rarely, breast cancer can be diagnosed in men and accounts for less than 1% of all new breast cancers each year.1 This year in the USA, approximately 2470 men will be diagnosed and 460 men will die from breast cancer.1
Risk factors for developing male breast cancer include family history of breast cancer in a male first-degree relative, increased oestrogen stimulation by consuming oestrogen-containing compounds, hepatic dysfunction, obesity, marijuana use, thyroid disease, Klinefelter syndrome, primary testicular conditions such as orchitis, cryptorchidism or testicular injury and inherited mutations such as the BRCA gene mutation.6 Other genes including the tumour suppressor gene PTEN, mutations in TP53, PALB2 mutations and mutations in mismatch repair have also been associated with an increased risk of breast cancer in men.7 Therefore, genetic testing is recommended by the National Comprehensive Cancer Center Network in all men diagnosed with breast cancer.
Most men with breast cancer present with a painless, firm mass in the subareolar region commonly involving the nipple. Diagnostic evaluation and staging is similar to women and mammography and biopsy should be done.8
Mammography is the standard imaging modality to evaluate for any breast abnormalities.8 Langlands et al 9 reviewed mammograms of 71 patients with MBC. Findings revealed that in those with MBC, irregularly shaped masses, spiculated masses and calcifications were less frequently than in other breast cancers. Yang et al 10 demonstrated that MBC has more benign features like round or oval mass with circumscribed margins on imaging.
Pathology remains the gold standard for diagnosis. Histologically, most breast cancers in men are invasive ductal carcinomas, accounting for 90% of cases, while lobular cancers account for 1.5% of cases. MBC is rare and represents less than 1% of all breast cancers.2 4 11–13 MBC is a neoplasm characterised by two or more histological cell types, most commonly epithelial and mesenchymal. According to WHO classification 2012, MBCs are classified as metaplastic carcinoma of no special type, low-grade adenosquamous carcinoma, fibromatosis like carcinoma, squamous cell carcinoma, spindle cell carcinoma, metaplastic carcinoma with mesenchymal differentiation (chondroid differentiation, osseous differentiation), mixed metaplastic carcinoma and myoepithelial carcinoma.12–14 The most common type of MBC is squamous cell carcinoma accounting for 0.5%–3.7% of all the breast cancers followed by spindle cell carcinoma and matrix-producing carcinoma.14 The main pathological feature of this cancer is epithelial and mesenchymal transition.15 16 Electron microscopy and immunohistochemistry may show myoepithelial origin of the cells.4 11 13–15 17 Although male breast cancers are typically oestrogen receptor positive, metaplastic breast cancers are typically agressive and triple negative.4 11 13–15 17
MBC in men is limited to case reports. After extensive literature review, only three cases have been described. Rehman3 described a case of a 75-year-old man that presented with a painless lump on the right side of his chest with ipsilateral axillary and inguinal adenopathy. Biopsy did not reveal pathology and subsequently received a palliative right mastectomy and biopsy of the axillary and inguinal masses revealing poorly differentiated metaplastic, triple negative, breast carcinoma with sarcomatous differentiation (carcinosarcoma) and malignant deposits in axillary and inguinal lymph nodes. He completed adjuvant chemotherapy and radiation but died within 6 months of surgery. Barr and Jane Clayton4 discussed a case of a 59-year-old man with a presumed right-sided breast abscess. Incision and drainage was performed and biopsies of the base of the cavity were obtained revealing poorly differentiated carcinoma that was TTF1 positive, suggesting origin from lung or thyroid. Local control of the ulcerating mass was needed and a right mastectomy with level I axillary dissection was performed. Pathology confirmed invasive metaplastic carcinoma, oestrogen and progesterone receptor negative and one out of five lymph nodes positive for macrometastasis. PET/CT showed a hypermetabolic lesion in the apex of the lung that was resected and showed poorly differentiate carcinoma with sarcomatous features consistent with metastasis. The patient received six cycles of 5-fluorouricil, epirubicin and cyclophosphamide. Ffollow-up imaging revealed complete response and he was referred for radiotherapy to the chest wall and supraclavicular fossa. Finally, Kuo et al 5 analysed a case series of eight patients with metaplastic carcinoma of the breast, with one patient being male. This patient was a 73-year-old man who received a modified radical mastectomy. Pathology showed a 5 cm adenosquamous carcinoma of the breast with no lymph node invasion that was oestrogen and progesterone receptor positive. He was treated with adjuvant tamoxifen and died of distant metastasis 4 months postoperatively.
Although the ideal treatment is unknown for metaplastic breast cancer, it is treated similarly to invasive ductal carcinoma (IDC). Surgery is the treatment of choice for MBC. Tumour size greater than 5 cm is a contraindication to breast conservation surgery, and thus, most MBCs are treated with radical mastectomy due to their greater sizes compared with IDC.3 11–14 17 18 They are more resistant to chemotherapy than IDC,11 18 and the exact role of chemotherapy and radiotherapy is uncertain because of rarity of this disease.13 18 Hormone therapy is usually unnecessary and has no role due to high prevalence of triple-negative receptor status.11 12 18
As demonstrated in the previous cases described in the literature, metaplastic breast cancer usually has a poor prognosis as compared with IDC due to high proliferation index, increased tumour size, histopathological tumour heterogeneity, triple negativity and lack of effective targeted therapies.3 14 Poor prognostic factors include age less than 40 years, skin invasion and squamous cell component. Tumour size, tumour grade and hormonal status does not affect the prognosis in contrast to invasive ductal carcinoma and invasive lobular carcinoma. Lymph node status and lymphovascular invasion affects the outcome. Histological subtypes do not confer prognosis,13 and survival at 5 years is approximately 38%–65%.11 12
In summary, this is a rare case report of MBC in a 47-year-old man. This case emphasises the work-up, diagnosis and successful treatment plan for a male patient with MBC. The presentation and course of this patient’s care will be a useful adjunct to the current literature for determining treatment for male patients with MBC.
Learning points.
Breast cancer should be considered when a male patient presents with breast swelling, erythema and nipple inversion.
Genetic testing is recommended in all male patients diagnosed with breast cancer.
Metaplastic breast cancer (MBC) usually has a poor prognosis as compared with invasive ductal carcinoma as it is usually more aggressive and the receptor status is negative.
According to the National Comprehensive Cancer Network guidelines, treatment plans for MBC in men follow the same guidelines for women with more common, ductal-type or lobular-type breast cancers.
Footnotes
Contributors: HK participated in the clinical management of the patient in the case report and preparation of the manuscript. HJ participated in the preparation of the manuscript. TD participated in determining the pathological diagnosis of the disease, provided the pathological slides, including immunohistochemical and H&E stains. YL participated in the management of the patient and editing of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017;67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Gibson GR, Qian D, Ku JK, et al. Metaplastic breast cancer: clinical features and outcomes. Am Surg 2005;71:725–30. [PubMed] [Google Scholar]
- 3.Rehman A. Triple-negative phenotype of poorly-differentiated metaplastic breast carcinoma in a male: an oncological rarity. J Coll Physicians Surg Pak 2013;23:370–2. doi:05.2013/JCPSP.370372 [PubMed] [Google Scholar]
- 4.Barr JG, Jane Clayton ES, Sotheran W. A case of metaplastic breast cancer in a man. J Surg Case Rep 2013;2013:rjs047 10.1093/jscr/rjs047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo SH, Chen CL, Huang CS, et al. Metaplastic carcinoma of the breast: analysis of eight Asian patients with special emphasis on two unusual cases presenting with inflammatory-type breast cancer. Anticancer Res 2000;20:2219–22. [PubMed] [Google Scholar]
- 6.Thomas DB. Breast cancer in men. Epidemiol Rev 1993;15:220–31. 10.1093/oxfordjournals.epirev.a036108 [DOI] [PubMed] [Google Scholar]
- 7.Ding YC, Steele L, Kuan CJ, et al. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat 2011;126:771–8. 10.1007/s10549-010-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dershaw DD, Borgen PI, Deutch BM, et al. Mammographic findings in men with breast cancer. AJR Am J Roentgenol 1993;160:267–70. 10.2214/ajr.160.2.8424331 [DOI] [PubMed] [Google Scholar]
- 9.Langlands F, Cornford E, Rakha E, et al. Imaging overview of metaplastic carcinomas of the breast: a large study of 71 cases. Br J Radiol 2016:20140644 10.1259/bjr.20140644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WT, Hennessy B, Broglio K, et al. Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol 2007;189:1288–93. 10.2214/AJR.07.2056 [DOI] [PubMed] [Google Scholar]
- 11.Shah DR, Tseng WH, Martinez SR. Treatment options for metaplastic breast cancer. ISRN Oncol 2012;2012:1–4. 10.5402/2012/706162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, et al. Management and outcomes in metaplastic breast cancer. Clin Breast Cancer 2016;16:437–43. 10.1016/j.clbc.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Fernández Pérez MA, Viqueira Rodriguez I, Tello Royloa A, et al. Metaplastic breast carcinoma with unusual presentation: review of three cases. Breast Care 2015;10:404–7. 10.1159/000441288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian T, Lin Q, Wu Z, et al. Metaplastic carcinoma of the breast: imaging and pathological features. Oncol Lett 2016;12:3975–80. 10.3892/ol.2016.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose T, Honda J, Bando Y, et al. A case of matrix-producing carcinoma of the breast. World J Surg Oncol 2008;6:60 10.1186/1477-7819-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi L, Paglicci C, Caprio G, et al. Matrix-producing carcinoma of the breast: a case report. Case Rep Oncol 2013;6:245–9. 10.1159/000351119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edenfield J, Schammel C, Collins J, et al. Metaplastic breast cancer: molecular typing and identification of potential targeted therapies at a single institution. Clin Breast Cancer 2016;S1526-8209:30197–5. [DOI] [PubMed] [Google Scholar]
- 18.Mituś JW, Sas-Korczyńska B, Kruczak A, et al. Metaplastic breast cancer with rapidly progressive recurrence in a young woman: case report and review of the literature. Arch Med Sci 2016;12:1384–8. 10.5114/aoms.2016.62917 [DOI] [PMC free article] [PubMed] [Google Scholar]