Abstract
A 33-year-old, 8-week pregnant woman presented with mottling, pain and swelling of her left leg. Ultrasound Doppler scan revealed a large left iliofemoral deep vein thrombosis and the patient was diagnosed with phlegmasia cerulea dolens. After 24 hours of treatment with unfractionated heparin, there were minimal improvements in her symptoms. Catheter-directed thrombolysis was performed, following multidisciplinary consultation with the patient. An underlying May-Thurner lesion was identified and successfully stented. Radiation exposure to the fetus was minimised with the use of intravenous ultrasound and very low-dose fluoroscopy. Total radiation exposure to the fetus is 1.38 mGy, which is equivalent to 8 months of background radiation exposure. No immediate complication occurred and patient’s symptoms completely resolved. On further follow-up, her iliofemoral veins remained patent with good flow and there were no recurrence of symptoms. A healthy infant was successfully delivered at 40 weeks gestation.
Keywords: interventional radiology, venous thromboembolism, pregnancy
Background
Venous thromboembolisms occur in approximately 1–2 per 1000 pregnancies.1 In pregnancy, the risk of developing a deep vein thrombosis (DVT) is increased fivefold, and in the postpartum period, this risk is increased to 20-fold.2 In addition, DVTs in pregnancy occurs more proximally and can present as an acute ischaemic limb such as phlegmasia cerulea dolens (PCD).3 4 During pregnancy, the management of proximal DVTs can be challenging because of the high risk of developing post-thrombotic syndrome (PTS) after conservative medical treatment and the risk of causing maternal and fetal complications with aggressive therapy. The National Institute for Health and Care Excellence guideline states that catheter-directed thrombolysis (CDT) can be considered to treat symptomatic proximal DVTs but does not specify its use in pregnancy.5 CDT has been shown to be effective in treating proximal DVTs but the evidence of its use in pregnancy remains limited.6
Case presentation
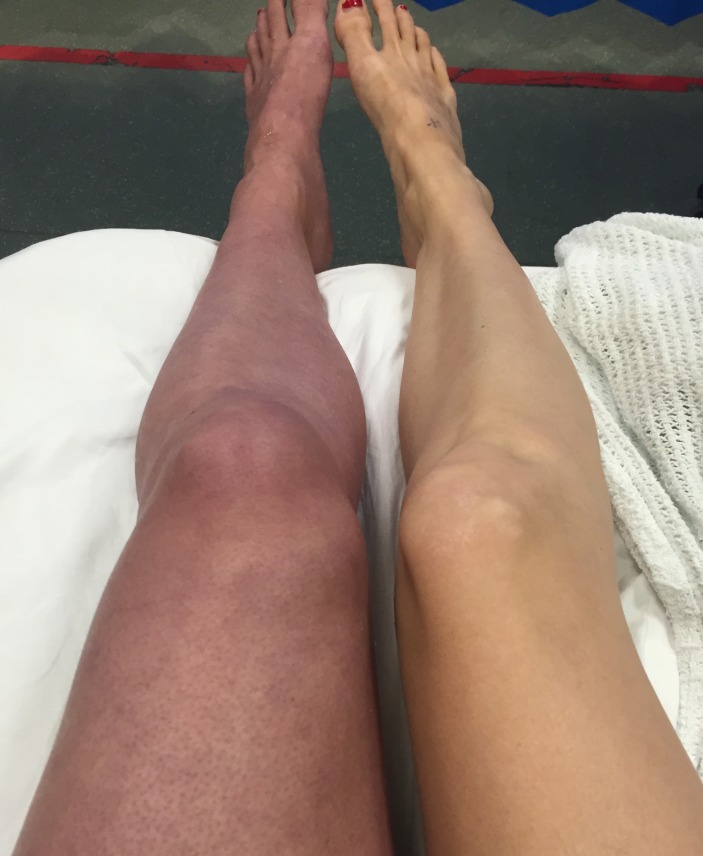
A 33-year-old, 8-week pregnant woman presented with severe unilateral leg pain and swelling. The pain started as a dull ache 7 days ago in her left groin extending to her left buttock. That morning, the pain in her left leg became increasingly severe and constant and her leg became mottled. She denied any chest pain or shortness of breath. There was no previous history of DVT or prothrombotic conditions. This was her second pregnancy and her first pregnancy was uneventful. She did not take any regular medications other than vitamins. On examination, her left leg felt cold, painful to palpate and turning from a dusky pink to blue in colour (figure 1). Her popliteal, posterior tibial and dorsalis pedis pulses were impalpable due to leg swelling. Sensation was reduced on the whole left leg but motor function was preserved. Examination of the respiratory system and the right leg was unremarkable.
Figure 1.
Patient’s legs on initial presentation.
Investigations
Initial observations were as follows: temperature was 37°C, heart rate was 130 beats per minute, blood pressure was 105/70 mm Hg, respiratory rate was 21 breaths per minute and oxygen saturation was 96%. Her haematological results were normal other than an elevated C reactive protein of 54. A venous blood gas revealed a lactate of 1.6. Her urine dipstick was normal with a positive beta human chorionic gonadotropin. An ultrasound Doppler scan (figure 2) performed on her left leg showed a large thrombus in her left femoral vein extending up to her left common iliac vein. The arterial system of the left leg was normal with no arterial embolism or thrombosis seen. A diagnosis of PCD secondary to an acute proximal vein thrombosis was made.
Figure 2.
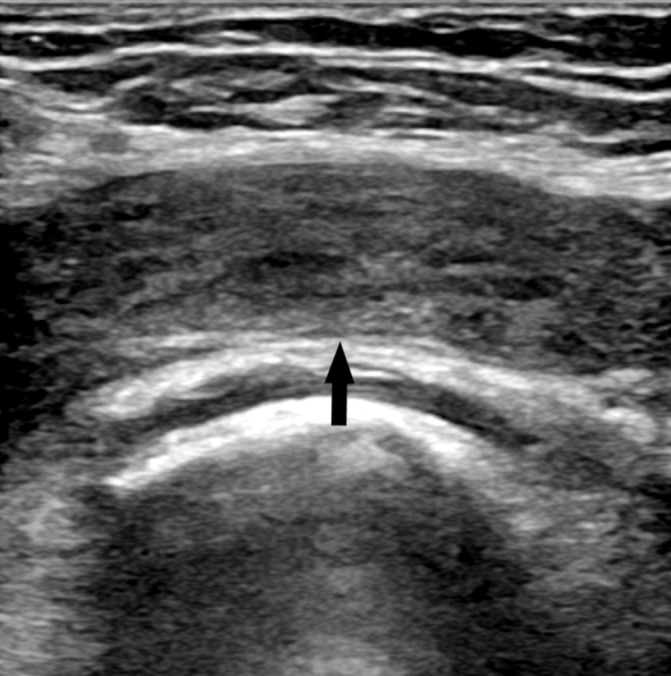
Ultrasound Doppler scan showed extensive echogenic left common femoral vein thrombus on presentation.
Treatment
Initially, this was treated conservatively using intravenous infusion of unfractionated heparin and leg elevation. After 24 hours of intravenous unfractionated heparin, there were minimal improvements and the limb remained threatened. Following extensive discussion with the obstetrics, vascular and interventional radiology teams, surgical thrombectomy, conservative treatment and CDT were considered. Overall, CDT was deemed most favourable. The patient was heavily counselled regarding the risks and benefits of the procedure. In particular, the risk of thrombolysis and radiation exposure to the fetus and mother, which the evidence is limited.
With the patient’s consent, the procedure was performed by the interventional radiology team with the focus on minimising fetal radiation exposure. The team has 5 years experience treating acute iliofemoral DVTs using CDT. At 8 weeks gestation, the gravid uterus is estimated to be below the level of the pubic symphysis and radiation exposure to the area was avoided. Access into the left popliteal vein was performed using ultrasound guidance. Minimal, unmagnified and heavily collimated low-dose fluoroscopy was used to negotiate a hydrophilic guidewire (Boston Scientific, Massachusetts, USA) through the thrombosed left femoral vein. Only one short digital subtraction angiography (DSA) run was performed to confirm the position of the catheter in the inferior vena cava (IVC) at the level of the diaphragm. A Cragg-McNamara infusion catheter (Micro Therapeutics, California, USA) was negotiated through the thrombosed left femoral and iliac veins into the IVC (figure 3). The position of the catheter within the left iliofemoral vein was confirmed with intravenous contrast and 5 mg of recombinant tissue plasminogen activator (rTPA) was administered. The catheter was left within the left leg venous system for further localised infusions of rTPA at 0.75 mg/hour for a total of 48 hours. The patient tolerated the procedure well with no immediate complications.
Figure 3.
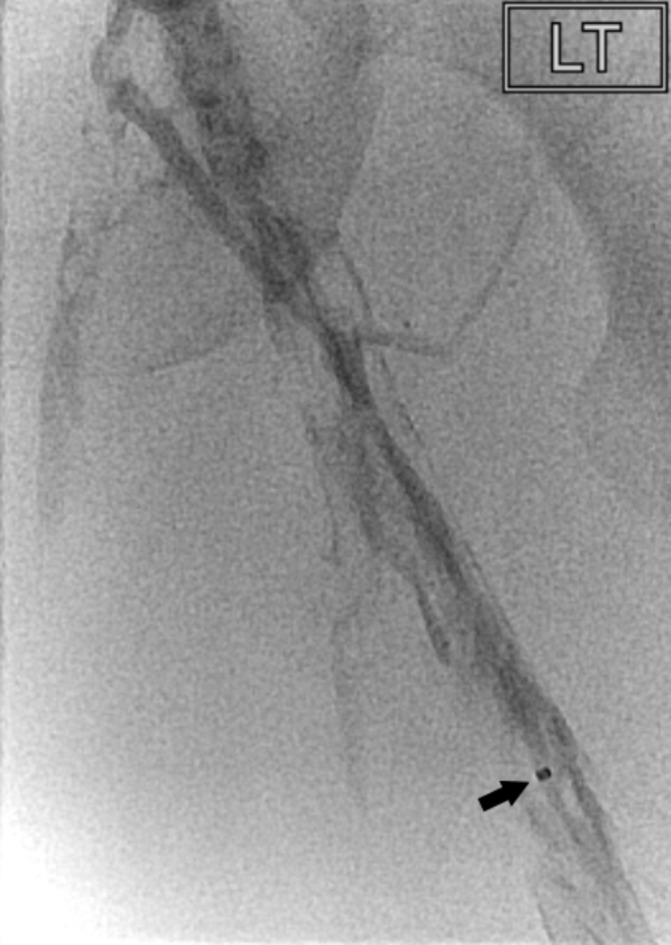
Venogram showing filling defects in the left femoral vein. The distal marker (arrowed) of the Cragg-McNamara catheter (Micro Therapeutics) is evident and the proximal marker was positioned at the confluence of the common iliac veins.
Outcome and follow-up
Her symptoms improved remarkably after 24 hours of DVT, with reduction in swelling and mottling (figure 4). A left leg venogram performed the next day showed considerable improvement in appearances with clearance of the femoral vein with mild residual filling defects within the iliac vein (figure 5). Day 2 postprocedure, another repeat venogram showed complete clot resolution. An intravascular ultrasound (IVUS) catheter (Volcano, California, USA) was used to identify the May-Thurner lesion. Venoplasty was performed using a 16 mm Atlas Gold balloon (Bard Peripheral Vascular, Arizona, USA) (figure 6). With the help of IVUS (figure 7), a 16 mm Veniti Stent (Veniti, Missouri, USA) was positioned and deployed (figure 8). No immediate complication occurred during the procedure.
Figure 4.
Patient receiving catheter-directed thrombolysis with catheter in situ (arrowed).
Figure 5.
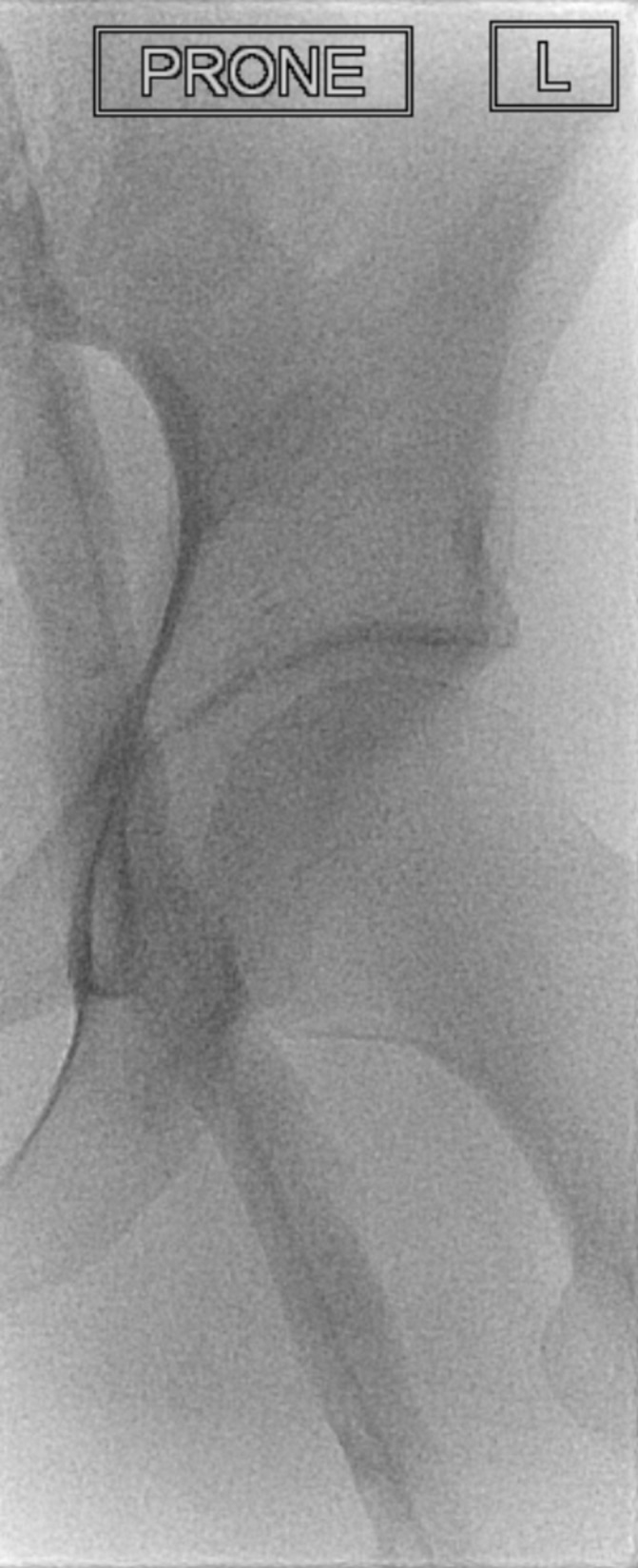
Repeat venogram on day 2 of catheter-directed thrombolysis showing considerable improvement with clearance of thrombus in the femoral vein.
Figure 6.
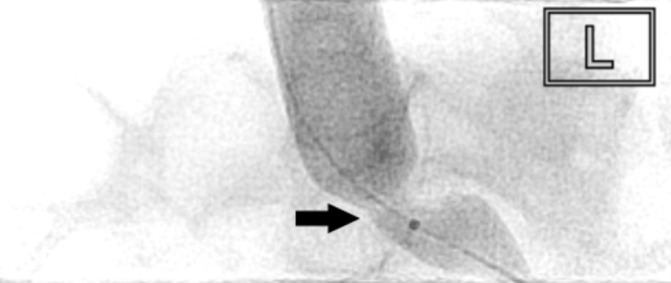
Angioplasty of May-Thurner lesion (arrowed) with 16 mm Atlas Gold balloon (Bard Peripheral Vascular).
Figure 7.
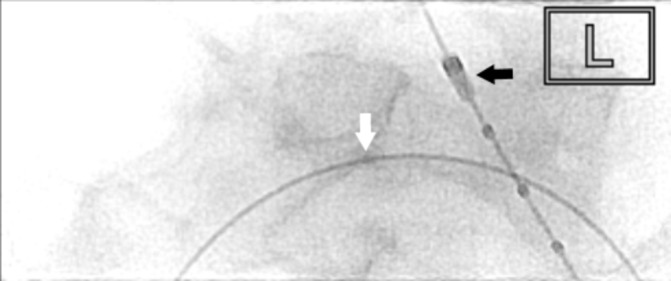
Intravascular ultrasound catheter (black arrow) (Volcano) was used to visualise the passage of guidewire (white arrow) to the right femoral vein to confirm position of confluence of inferior vena cava.
Figure 8.
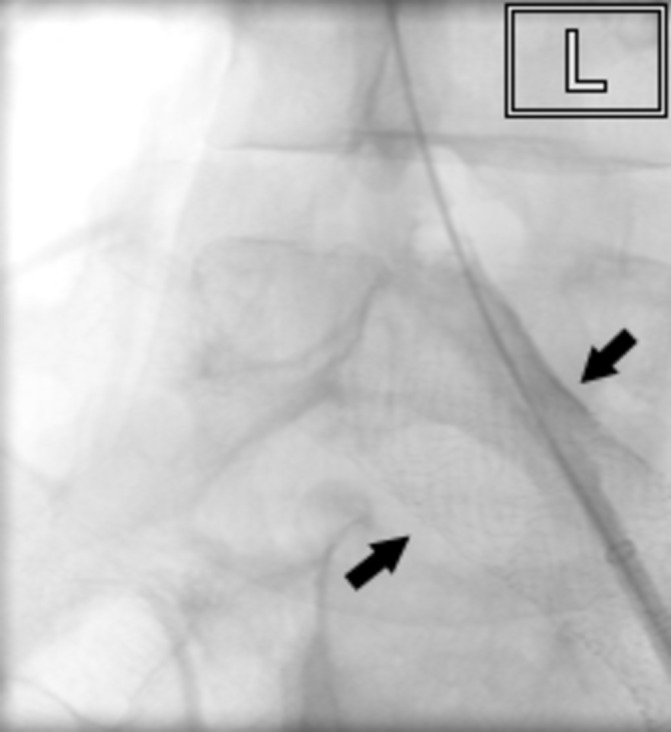
16 mm Veniti stent (arrowed) (Veniti) deployed within the left femoral vein.
On day 1 poststenting, ultrasound Doppler scan of her left lower limb showed good flow in external iliac and common femoral veins with no thrombus and the stent widely patent. The dose-affected products from each procedure were 1404 mGy/cm2, 26 mGy/cm2 and 1961 mGy/cm2, respectively. Total screening times were 45 s, 11 s and 81 s, respectively. Only two DSA runs were performed without direct exposure to the fetus, the other during the third procedure. IVUS was used to guide stent position and deployment. The radiation dose to the fetus from all the examinations performed was calculated by the medical physics department at the Surrey Regional Radiation Protection Service and was found to be approximately 1.38 mGy. This is a low dose and corresponds to about 8 months of exposure to natural background radiation.
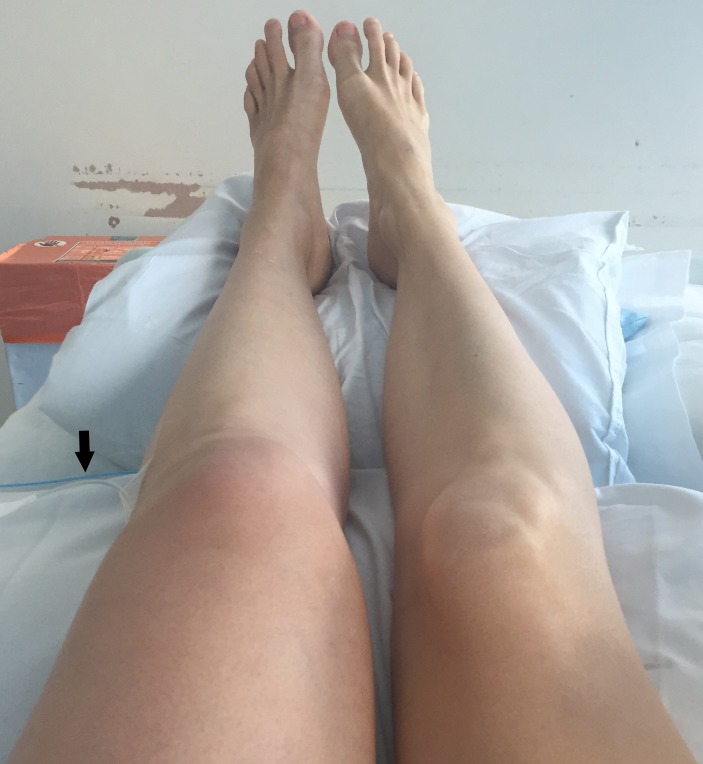
After the procedure, the patient’s symptoms completely resolved. On discharge, the patient continued having low-molecular-weight heparin (LMWH) until the end of her pregnancy, with regular antifactor Xa tests. She also wore thigh-length class II compression stockings throughout the pregnancy and continued taking aspirin for 6 weeks postdischarge. She was followed-up regularly with Duplex scans at 2 weeks, 6 weeks and 6 months. These demonstrated patent and normal phasic flow in the left iliofemoral veins. On follow-up, she had no in-stent stenosis or recurrence of leg swelling throughout the rest of her pregnancy (figure 9). All her 4-weekly growth scans showed normal growth, with no evidence of uterine bleeding or fetal abnormalities. A healthy female infant was delivered via spontaneous vaginal delivery at 40 weeks. No abnormalities were identified on initial baby examination. Head circumference and birth weight were within normal limits at 34 cm and 3.8 kg, respectively.
Figure 9.
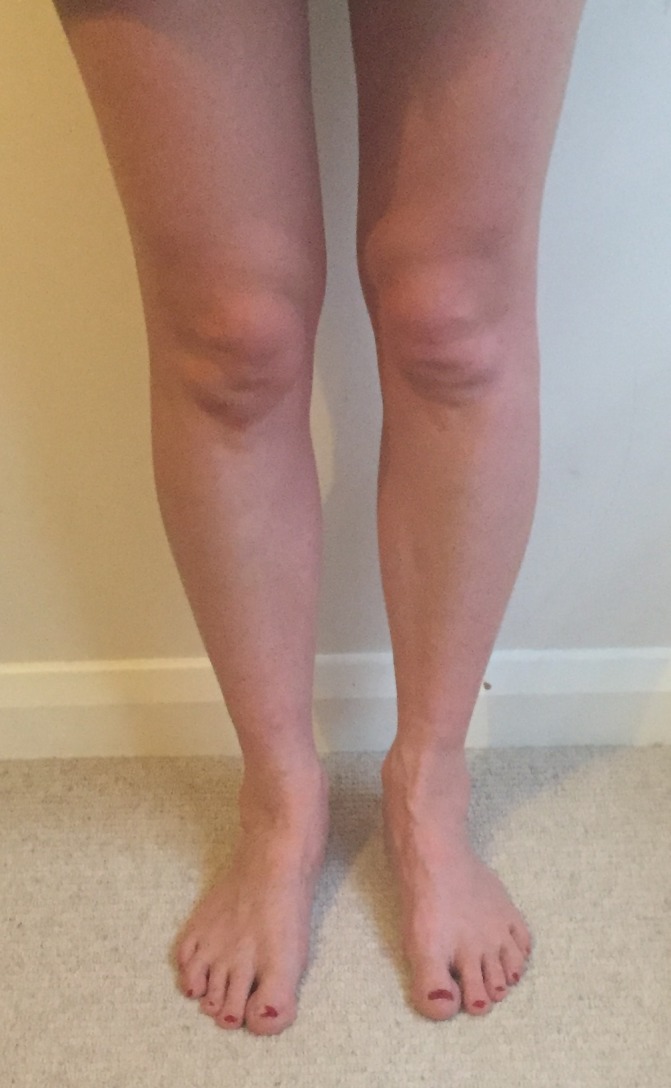
Patient’s legs after discharge from hospital with no recurrence of symptoms.
Discussion
Iliac vein compression syndrome, also known as May-Thurner syndrome, is an anatomical variant where the left common iliac vein is compressed between the overlying right common iliac artery and the fifth lumbar vertebrae. The stenosis and compression of the lumen promotes venous stasis and predisposes to clot formation. The prothrombotic state in pregnancy further increases this risk. PCD is a severe manifestation of an extensive occlusion of the proximal venous system in a limb, usually due to a thrombus. The swelling and increased venous pressures can impair arterial supply causing acute limb ischaemia and eventually leading to gangrene. In the first instance, anticoagulation using intravenous unfractionated heparin is preferred due to its prompt anticoagulation effects and easy reversibility. In this case, we opted for a more aggressive approach to quickly relieve the venous obstruction and reduce the risk of recurrent DVTs and the development of PTS.
Acute iliofemoral DVTs have a high risk of developing PTS with trials showing that one in four patients develop PTS within 2 years when managed with anticoagulation alone.7 8 PTS can have a significant long-term impact on an individual’s quality of life due to chronic pain, swelling, oedema and venous ulceration. PTS is thought to be caused by venous hypertension due to persistent venous obstruction/insufficiency after inflammatory destruction of venous valves following a DVT.9 A systemic review showed that systemic thrombolysis offers a significant advantage, reducing the incidence of PTS by one-third.10 This may be due to early recanalisation reducing the inflammatory destruction of valves following a DVT.
Pregnancy is a relative contraindication to thrombolysis because of the lack of trials demonstrating its safety. In animal studies, rTPA did not demonstrate any teratogenic effects when given at therapeutic doses.11 12 This is because drugs that have molecular weights of greater than 1000 Da, such as rTPA (molecular weight of 60 000 Da), do not cross the placenta.13 14 In a review of 28 patients who received systemic rTPA for various indications, there were 11 patients in their first trimester. Two spontaneous abortion occurred after rTPA including one in the first trimester.15 However, it is difficult to establish if this is a direct consequence of rTPA because of the severity of the underlying maternal medical condition, such as shock. The rate of maternal complications was found to be similar to that in non-pregnant patients.
A catheter-directed approach offers the advantage of localised delivery of the thrombolytic agent to the clot, thus reducing the total dose administered systematically. Despite the dose reduction, evidence suggests that CDT is as effective as systemic thrombolysis in reducing the incidence of PTS.10 Current evidence on the use of CDT in pregnancy is limited but have demonstrated successful clot lysis with no complications to the fetus.16 A limited observational study of 13 patients with pregnancy-related iliofemoral DVTs managed using a strategy of thrombus removal, either CDT or thrombectomy, demonstrated complete or near complete clot resolution with only one reoccurrence on follow-up.17 This was likely due to non-compliance with LMWH after discharge resulting in recurrence and this patient subsequently underwent an elective termination of pregnancy. The other 12 patients in the study had normal pregnancies. A similar lower incidence of PTS was observed in patients with pregnancy-related DVT who were treated with CDT compared with anticoagulation only.18
The radiation exposure to the fetus was 1.38 mGy compared with 55 mGy that was reported in a previous study.17 IVUS enabled us to minimise the number of DSA runs because they significantly increase the radiation exposed to the fetus. The low fluoroscopy settings resulted in lower image quality but still provided sufficient radiographic imaging for the procedure to be performed safely and effectively. Radiation exposure can cause fetal complications such as prenatal death, intrauterine growth restriction, microcephaly, mental retardation, organ malformation and childhood cancers.19 In animal studies and atomic bomb survivors, fetal abnormalities were observed at exposures above 100 mGy during the 8–15 week period.19 The American College of Obstetricians and Gynaecologists guideline states that radiation exposures at 50 mGy or less have not been associated with an increase in fetal abnormalities or fetal loss.20 The low dose in this case is unlikely to cause any abnormalities. None were observed but long-term sequelae requires further follow-up.
In our department, it is routine practice to not use an IVC filter in patients being treated for acute proximal DVTs using CDT, when the thrombus is confined to the iliofemoral veins. This is due to the lack of evidence supporting routine use in these cases.21
Endovascular treatment and stenting of May-Thurner lesions have been shown to be successful with low recurrence rates.22 23 Current evidence shows that the gravid uterus does not cause any structural damage to the stent and any compression is relieved postpartum.24
In the postprocedural period, we advised the continuation of LMWH at least until delivery, as this has been shown to be safe and effective in preventing recurrence.25
In conclusion, this is an interesting case where a pregnancy-related acute iliofemoral DVT is successfully managed via CDT with good outcome. Our case demonstrated that this procedure can be performed with low levels of radiation to the fetus. Our case also demonstrates the short-term benefits of CDT with patency of the iliofemoral veins with 6 months of follow-up. Further follow-up is still required to assess the long-term benefits.
Learning points.
Acute iliofemoral deep vein thrombosis in pregnancy can be safely treated using catheter-directed thrombolysis (CDT) producing a good outcome for both mother and infant.
CDT can be successfully performed with low radiation exposure to the fetus.
The use of intravascular ultrasound in interventional radiology can help minimise the radiation exposure and is especially important in pregnant patients.
Footnotes
Contributors: THS: drafted the case report, research and review of current evidence. AC: revised the article, research and review of current evidence.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5. 10.1016/j.ajog.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706. 10.7326/0003-4819-143-10-200511150-00006 [DOI] [PubMed] [Google Scholar]
- 3.Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010;182:657–60. 10.1503/cmaj.091692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698–707. 10.7326/0003-4819-149-10-200811180-00004 [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Clinical Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Nice guideline (CG144), 2012. [PubMed] [Google Scholar]
- 6.Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999;211:39–49. 10.1148/radiology.211.1.r99ap4739 [DOI] [PubMed] [Google Scholar]
- 7.Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997;349:759–62. 10.1016/S0140-6736(96)12215-7 [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141:249–56. [DOI] [PubMed] [Google Scholar]
- 9.Kahn SR. The post-thrombotic syndrome: progress and pitfalls. Br J Haematol 2006;134:357–65. 10.1111/j.1365-2141.2006.06200.x [DOI] [PubMed] [Google Scholar]
- 10.Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2016;11:CD002783 10.1002/14651858.CD002783.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima N, Naya M, Imoto H, et al. Reproduction study of GMK-527 (rt-PA) - (III) Teratogenic Study in rabbits treated intravenously with GMK-527. Jpn Pharmacol Ther 1988;16:107–23. [Google Scholar]
- 12.Tanaka M, Mizuno F, Ohtsuka T, et al. Study in rats treated intravenously with GMK-527 (II) Teratogenicity study in rats treated intravenously with GMK-527. Jpn Pharmacol Ther 1988;16:93–106. [Google Scholar]
- 13.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 2004;43:487–514. 10.2165/00003088-200443080-00001 [DOI] [PubMed] [Google Scholar]
- 14.Gartman EJ, Gartland E. The use of thrombolytic therapy in pregnancy. Obstet Med 2013;6:105–11. 10.1177/1753495X13488771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271–6. 10.1007/s11239-006-5709-z [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy P, Martin CB, Kay HH, et al. Catheter-directed thrombolysis for thromboembolic disease during pregnancy: a viable option. J Matern Fetal Med 1999;8:24–7. [DOI] [PubMed] [Google Scholar]
- 17.Herrera S, Comerota AJ, Thakur S, et al. Managing iliofemoral deep venous thrombosis of pregnancy with a strategy of thrombus removal is safe and avoids post-thrombotic morbidity. J Vasc Surg 2014;59:456–64. 10.1016/j.jvs.2013.07.108 [DOI] [PubMed] [Google Scholar]
- 18.Spectre G, Bloom A, Roth B, et al. Pharmacomechanical catheter directed thrombolysis for pregnancy related proximal lower extremity deep venous thrombosis: Effectiveness in the prevention of post thrombotic syndrome. Blood 2016;128:1448. [Google Scholar]
- 19.McCollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics 2007;27:909–17. 10.1148/rg.274065149 [DOI] [PubMed] [Google Scholar]
- 20. ACOG Committee on Obstetric Practice. Guidelines for diagnostic imaging during pregnancy. ACOG Committee opinion no. 299, September 2004 (replaces no. 158, September 1995). Obstet Gynecol;2004:647–51. [DOI] [PubMed] [Google Scholar]
- 21.Protack CD, Bakken AM, Patel N, et al. Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement. J Vasc Surg 2007;45:992–7. 10.1016/j.jvs.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000;11:823–36. 10.1016/S1051-0443(07)61796-5 [DOI] [PubMed] [Google Scholar]
- 23.Patel NH, Stookey KR, Ketcham DB, et al. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J Vasc Interv Radiol 2000;11:1297–302. 10.1016/S1051-0443(07)61304-9 [DOI] [PubMed] [Google Scholar]
- 24.Hartung O, Barthelemy P, Arnoux D, et al. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg 2009;50:355–9. 10.1016/j.jvs.2009.01.034 [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen M, Broholm R, Bækgaard N. Pregnancy after catheter-directed thrombolysis for acute iliofemoral deep venous thrombosis. Phlebology 2013;28 Suppl 1:34–8. 10.1177/0268355513477286 [DOI] [PubMed] [Google Scholar]