Abstract
Immune checkpoint inhibitors have promising clinical activity across multiple gastrointestinal cancers and immune-mediated hepatotoxicity is particularly relevant for this group of patients. In this article we will review the recognition, workup and management of suspected checkpoint inhibitor related immune-hepatitis.
Keywords: Immunotherapy, immune checkpoint inhibitors, gastrointestinal cancers, immune-mediated toxicity
Introduction
Checkpoint inhibitors harness the body’s immune system and enhance the systemic antitumor immune response. The currently approved immune checkpoint inhibitors can be broadly classified into three categories: (I) CTLA-4 inhibitor: currently the only FDA approved drug in this class is Ipilimumab. It was also the first immune checkpoint inhibitor to be approved after improved survival was shown in patients with metastatic melanoma (1); (II) programmed-cell death-1 (PD-1) inhibitors: pembrolizumab was initially approved for patients with metastatic melanoma and has subsequently shown activity in and has received approval for patients with non-small cell lung cancer, Hodgkin’s lymphoma and head & neck cancer. Nivolumab a human Ig4 anti-PD1 monoclonal antibody was also initially approved for treatment in metastatic melanoma and has since gained FDA approval for non-small cell lung cancer, renal cell cancer, head & neck cancer, Hodgkin Lymphoma and urothelial cancer (2), (III) PD ligand 1 (PDL1) inhibitors: atezolizumab is the first drug in this class which received initial FDA approval for metastatic urothelial carcinoma and has since has been approved for metastatic non-small cell lung cancer. Avelumab received approval for Merkel cell carcinoma in March of 2017 and durvalumab received approval for urothelial carcinoma on May 1st 2017 (2).
In addition, there is emerging data showing efficacy of immune checkpoint inhibitors in microsatellite unstable colon cancer, gastric cancer, hepatocellular cancer, hematological malignancies and triple negative breast cancer (3-7). Immune checkpoint inhibitors work by blocking the T lymphocyte inhibition and restoring cytotoxic T-cell activity, which acts against greater variety of antigens (8). A downside of employing this strategy is the risk of loss of self-tolerance and subsequent development of a range of autoimmune disorders. The most common adverse events are dermatologic, endocrine, pulmonary, gastrointestinal, and rheumatologic (9). Hepatic toxicity is a rare, but clinically significant, toxicity which is particularly relevant to patients with gastrointestinal cancers. In this paper, we will review the diagnosis and management of checkpoint inhibitor related hepatic toxicity.
Incidence
Patients with gastrointestinal cancers may be at risk for higher rate of hepatotoxicity due to underlying patient risk factors and comorbid conditions. Prior systemic and liver directed cancer treatments can further effect liver function. This makes the recognition of immune-related hepatotoxicity more challenging. Hepatotoxicity generally presents as asymptomatic elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST). Isolated bilirubin elevation is rare, but can occur after prolonged AST and ALT elevation. Hepatotoxicity can occur at any time after treatment but is most commonly seen after 6–12 weeks of the therapy (10). Liver biopsy reveals a pan lobular active hepatitis with a predominant CD8-positive inflammatory infiltrate. This mirrors autoimmune hepatitis and is suggestive of predominant injury to hepatocytes (11). More rarely, predominant injury to bile ducts can be seen with mild portal mononuclear infiltrate around proliferated bile ductules (12).
The rate of autoimmune hepatotoxicity varies between different checkpoint inhibitors. The incidence of autoimmune hepatotoxicity, of all grades, with CTLA-4 inhibitors is between 3–9% (10). The rate is lower with PD-1 inhibitors, ranging between 1–3% with grade 3–4 hepatitis being rare (13,14). A recent meta-analysis also supports a higher rate of all- and high-grade hepatotoxicity with CTLA-4 inhibitors compared with PD-1 inhibitors (15). Combining CTLA-4 inhibitors with PD-1 inhibitors substantially increases the risk of hepatotoxicity, with ALT elevation rates approaching 11–20% and grade 3–4 events rising to 11% (7,16,17). A higher rate of elevated transaminases are seen when checkpoint inhibitors are combined with traditional chemotherapy or targeted therapies (18,19).
Work up of suspected checkpoint inhibitor related autoimmune hepatitis
Baseline testing: viral hepatitis serologies, transaminase levels (ALT and AST) and bilirubin should be tested prior to starting therapy with checkpoint inhibitors. Viral hepatitis serology include Hepatitis-B virus (HBV) surface Antigen (HBsAg), Hepatitis-B core antibody (HBcAb) and Hepatitis C virus (HCV) antibody. A positive HBsAg or HBcAb serology should prompt checking HBV DNA and a positive HCV antibody should be followed by HCV RNA levels. Clinicians should obtain hepatology consultation for all patients with positive viral serology to consider viral hepatitis treatment either prior to, or concurrently with, checkpoint inhibitors. The decision to treat viral hepatitis will depend on the viral load, liver enzymes, and underlying liver condition. An ‘HBcAb positive/HBV DNA negative’ panel suggests prior exposure to HBV and although the rate of potential HBV reactivation is low, it should be considered as the patient undergoes therapy.
Monitoring on therapy: clinicians should have a low threshold to work up suspected drug related hepatotoxicity to differentiate checkpoint inhibitor-induced immune related toxicity from other causes of hepatocyte damage. These include the effect of concurrent or prior treatments, underlying malignancy, infection or inflammation. In patients with known HBV or HCV infection, isolated increases in transaminases, particularly ALT, could be from activation of the immune system leading to increased immune response to HCV or HBV and subsequent decrease in viral load. There has been concern that immune modulation from checkpoint inhibitors could lead to an increase in viral replication.
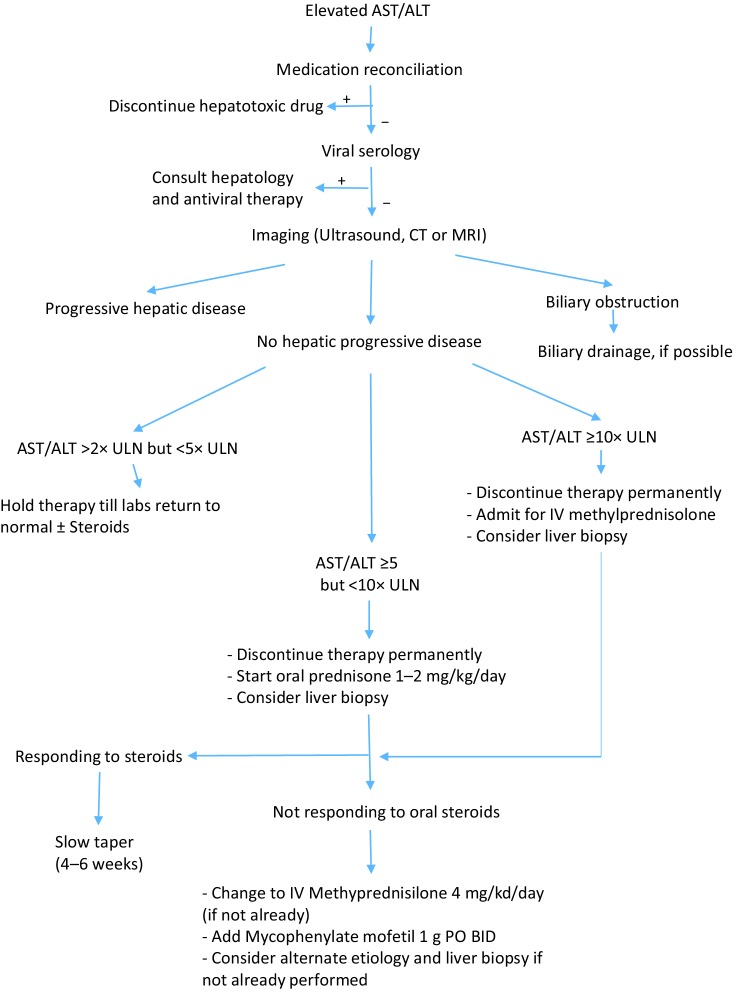
Transaminase (ALT and AST) and bilirubin levels should be checked prior to each dose of therapy. An increase of ALT or AST of more than 2 times the upper limit of normal (ULN) should prompt work up of hepatotoxicity (Figure 1). Medication reconciliation including evaluation for alternative therapy/herbal medications should be performed and hepatotoxic drugs discontinued. Workup should include basic viral hepatitis serologies (HAV, HBV, and HCV) depending on baseline serologies and immune status. Additional workup should include antinuclear antibodies (ANA), smooth muscle antibody (SMA), Epstein Barr virus (EBV) IgM, cytomegalovirus (CMV) PCR depending on the clinical context and possible preexisting liver dysfunction.
Figure 1.
Workup and management of suspected checkpoint inhibitor related immune hepatotoxicity. AST, aspartate aminotransferase; ALT, alanine aminotransferase; MRI, Magnetic resonance imaging; ULN, upper limit of normal.
Imaging: ultrasound or CT scan imaging can evaluate progression of hepatic metastasis and possible biliary obstruction. Hyperprogressive disease where in accelerated progression in an otherwise slowly progressive disease occurs after starting checkpoint inhibitors has been observed in up to 9% of patients on anti-PD-1/PD-L1 drugs and should be considered in the differential (20).
Biopsy: liver biopsy can help differentiate between various etiologies of hepatocyte damage. Individual patient circumstances, including the results of laboratory and imaging findings listed above, can drive the decision of when to proceed with a biopsy. Early tissue biopsy can help differentiate between various etiologies of hepatitis but there is significant overlap between features of autoimmune hepatitis and checkpoint inhibitor related injury. The treatment of suspected immune related hepatitis should not be delayed for tissue confirmation and it should be recognized that these may mask features of immune related injury.
Liver biopsy should be strongly considered in patients with negative viral hepatitis serologies who have persistent grade 2 hepatotoxicity despite 3–4 days of adequate steroid therapy, and in patients with grade 3 and 4 toxicity. Clinicians should tailor their testing to balance the risk and cost of biopsy with additional yield of clinically relevant information.
Treatment
Treatment with checkpoint inhibitors should be interrupted for AST/ALT elevations between 2 and 5 times ULN, and permanently discontinued for elevations greater than 5 times ULN (1,21,22).
Prompt treatment of suspected checkpoint-inhibitor related immune hepatotoxicity is critical to ensure patient safety. Patients with elevation between 5–10 times ULN of AST/ALT and negative viral serology, should start treatment with oral prednisone 1–2 mg/kg/day PO and monitored with frequent blood testing. Treatment with IV methylprednisolone 2–3 mg/kg/day should be considered if there is no improvement in 3–5 days. Treatment with Mycophenylate mofetil can be added after 3–5 days of maximal steroid treatment if no improvement is seen.
Patients with (I) continually rising AST/ALT levels despite adequate oral prednisone therapy, or (II) those that increase to >10× ULN (with or without bilirubin elevation >5× ULN) should be admitted to the inpatient setting. IV Methylprednisolone 4 mg/kg/day should be started emergently, a Hepatology consult should be obtained and a liver biopsy considered. LFTs should be monitored daily until AST/ALT falls to <8× ULN. Mycophenylate mofetil 1 g twice daily should be started if the LFT’s don’t respond to IV corticosteroids after 2 days. Refractory immune related toxicity that does not responded to IV steroids and Mycophenylate has been reported to respond to antithymocyte globulin therapy (23). Of note, Infliximab, which is usually used in severe autoimmune adverse reactions, should be avoided in autoimmune hepatitis as it is hepatotoxic.
A slow taper of the steroid dose over no less than 4 weeks can be started once hepatitis begins to improve. Pneumocystis carinii prophylaxis should be considered for patients receiving prednisone equivalent of 20 mg or more daily for 4 weeks or more (24). Of note, hepatitis can recur even after discontinuation of checkpoint inhibitors and some patients require repeated courses of steroids.
Restarting checkpoint inhibitors: The decision to restart checkpoint inhibitor after an episode of suspected treatment related hepatotoxicity depends on the degree of hepatocyte damage and duration of toxicity. Restarting checkpoint inhibitor therapy is not recommended for patients (I) with peak AST/ALT elevation of >5× ULN, (II) whose levels do not improve to grade 1/baseline, and (III) who show signs of liver decompensation such as increasing INR. For patients with peak elevation between 2 and 5× ULN, restarting checkpoint inhibitors can be considered if levels return to baseline/grade 1 and individual package insert guidelines should be followed.
Conclusions
Checkpoint inhibitor-induced immune related hepatitis is a clinically significant toxicity for patients with GI cancer. All patients receiving therapy should be monitored closely and prompt recognition of hepatitis is essential to ensure that appropriate treatment is started in a timely manner. Clinicians should maintain a low threshold for working up and treating suspected immune hepatitis as delays can lead to permanent discontinuation of cancer therapy.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Administration USFaD. Hematology/Oncology (Cancer) Approvals & Safety Notifications. 2017. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm. Accessed 06/21/2017.
- 3.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Sangro B, Yau TC, et al. Nivolumab dose escalation and expansion in patients with advanced hepatocellular carcinoma (HCC): The CheckMate 040 study. J Clin Oncol 2017;35:226. 10.1200/JCO.2017.35.4_suppl.22628056206 [DOI] [Google Scholar]
- 6.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pianko MJ, Liu Y, Bagchi S, et al. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig 2017;4:32. 10.21037/sci.2017.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 2007;110:2614-27. 10.1002/cncr.23086 [DOI] [PubMed] [Google Scholar]
- 9.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 10.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691-7. 10.1200/JCO.2012.41.6750 [DOI] [PubMed] [Google Scholar]
- 11.Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol 2015;39:1075-84. 10.1097/PAS.0000000000000453 [DOI] [PubMed] [Google Scholar]
- 12.Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013;31:1071-7. 10.1007/s10637-013-9939-6 [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 14.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Lie P, Guo M, et al. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer 2017;141:1018-28. 10.1002/ijc.30678 [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammers H, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014;32:4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013;368:1365-6. 10.1056/NEJMc1302338 [DOI] [PubMed] [Google Scholar]
- 19.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 20.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chmiel KD, Suan D, Liddle C, et al. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol 2011;29:e237-40. 10.1200/JCO.2010.32.2206 [DOI] [PubMed] [Google Scholar]
- 24.Network NCC. Prevention and Treatment of Cancer-Related Infections. 2017. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 06/21/2017.