Abstract
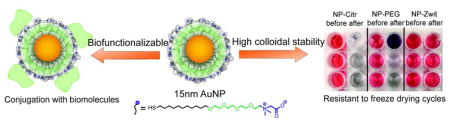
Gold nanoparticles provide an excellent platform for biological and material applications due to their unique physical and chemical properties. However, decreased colloidal stability and formation of irreversible aggregates while freeze-drying nanomaterials, limits their use in real world applications. Here, we report a new generation of surface ligands based on a combination of short oligo (ethylene glycol) chain and zwitterions capable of providing non-fouling characteristics, while maintaining colloidal stability and functionalization capabilities. Additionally, conjugation of these gold nanoparticles with avidin can help developing a universal toolkit for further functionalization of nanomaterials.
Keywords: Stability, Gold nanoparticles, UV, DLS, ion strength, freeze-drying, surface plasmon resonance, biofluids
Graphical abstract
INTRODUCTION
Gold nanomaterials have been used in numerous applications, from the creation of sensors and catalysts,1,2,3 to their applications as drug delivery vehicles and diagnostics agents.4,5,6 Among the different sizes of gold nanoparticles (AuNPs), medium/large size (≥10 nm) gold nanoparticles have been extensively investigated due to their unique physical properties.7,8 For example, the well-defined surface plasmon resonance (SPR) of these particles has been employed for sensing, where a variety of sensors have been developed by following changes of the SPR during the sensing event.9,10,11 By the use of this colorimetric strategy, not only the detection of small molecules and ions is possible, but also more complex systems such as proteins, cells, and by extension diseases can be detected.12,13,14,15
The sensitivity and specificity of these AuNP systems can be tuned by functionalizing the particle surface with different ligands (e.g. protein receptors).16 This facile change of the AuNP surface identity has also offered opportunities for the utilization of these nanomaterials in biological applications. The use of proteins as one of the surface ligands, for example, has allowed the development of targeted carriers to deliver drugs in specific locations.17,18,19 Likewise, the conjugation of AuNPs with DNA strands has permitted the utilization of AuNPs for imaging and therapy, where detection or regulation of genes has been achieved.20
Despite of the potential of AuNPs to serve as sensor and delivery platforms, the use of these material in real world applications is limited by their lack of long-term colloidal stability, in particular the formation of irreversible aggregates when they are subjected to physical and chemical changes (e.g. contact with biofluids, freeze-drying, and ion strength gradients). Particle stability is an important prerequisite for the use of AuNPs for in vitro and in vivo applications, and as such this stability issue complicates the use of AuNPs in biological environments.21,22 In addition, this lack of stability limits the industrial implementation of these systems, given that the low stability complicates various steps of the manufacturing and shipping processes.23 To counter the challenge of instability, several approaches have been investigated.24,25,26 The most successful method to date is the use of non-ionic poly(ethylene glycol) (PEG) to functionalize the AuNPs, a technique that is capable of conferring some degree of stability to AuNPs.27,28 However, the absence of reactive centers in this molecule combined with its amphiphilic nature hinders the systematic functionalization of PEG stabilized AuNPs.29,30 For example, the presence of PEG allows the internalization of hydrophobic functionalities masking their effects, while the amphiphilic nature of PEG alters the bioactivity of proteins.31,32 In addition, a report presents evidence of the recognition of PEG structures by the immune system, triggering a rapid elimination from the body, thus limiting its use.33
In our previous studies we have shown that zwitterionic ligands provided very tractable small (2 nm core) AuNPs.34 Here we present the use of zwitterionic ligand to provide high colloidal stability to medium and large AuNPs. Through appropriate carboxybetaine ligand design we have developed particles with ‘plug and play’ streptavidin connections for ready conjugation. Crucially, AuNPs decorated with these ligands can be stored as powders, exhibiting resistance to freeze-drying as well as changes in ionic strength while still maintaining functionalization capabilities. These conjugates do not lose their activity and stability after freeze-drying, facilitating the functionalization of nanomaterials with other proteins or mixture of molecules. As such, this NP coverage confers nanomaterials the capability of being used as building blocks, allowing not only an easy and fast functionalization, but also enabling the development of new technologies for therapeutic applications that cannot be achieved with unstable nanoparticles.
RESULTS AND DISCUSSION
Fabrication and characterization of different size AuNPs
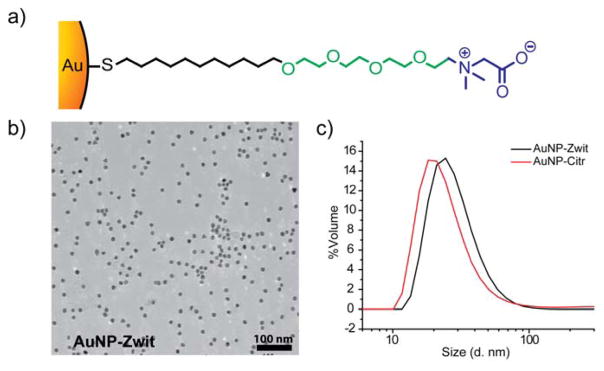
The chemical design of the NP ligand comprises the combination of a short oligo (ethylene glycol) (OEG) chain and a carboxybetaine zwitterionic headgroup (Figure 1a). In previous studies, we demonstrated that by the use of a similar design we were capable of reducing protein adsorption over small nanoparticles (<10 nm), 34 as well as confer increased blood circulation times comparative to PEG.35 That made us aware of the possibilities of increasing colloidal stability by the use of this approach. However, chemical diversity was limited to pre-functionalization synthesis, as sulfobetaines do not offer readily activatable chemical groups. To overcome this difficulty in functionalization, in our current design we employed a carboxybetaine group,36,37,38 a moiety that allowed us to vary the end group at will after the final AuNP synthesis (ligand synthesis, AuNP functionalization and characterization are described in the supporting information, Figures S1–S5). Incorporation of carboxybetaines as zwitterionic headgroups conferred high colloidal stability to the AuNPs. In particular, it allowed us to design “plug and play” systems that could prevent non-specific protein adsorption and remain biofunctionalizable at the same time. We demonstrated the utility of these particles using three different gold NP core sizes, 15 nm, 25 nm, and 35 nm diameter, given the significant amount of applications that have been developed for particles of this size. It should also be pointed out that 15 nm as the current industrial AuNP standard. The functionalization of the 15 nm core with the zwitterionic ligand did not affect the core size of the AuNPs and increase in the hydrodynamic size is accounted to the ligand attachment (Figure 1b, c)
Figure 1.
a) Chemical structure of the AuNP ligand, composed of an oligo (ethylene glycol) chain followed by a carboxybetaine zwitterionic group. b) TEM of the 15 nm AuNPs after functionalization with the engineered ligand (AuNP-Zwit). c) DLS profiles of AuNP-Zwit and AuNP-Citr (without ligand functionalization) in phosphates buffer (PB).
Stability studies of zwitterionic-AuNPs
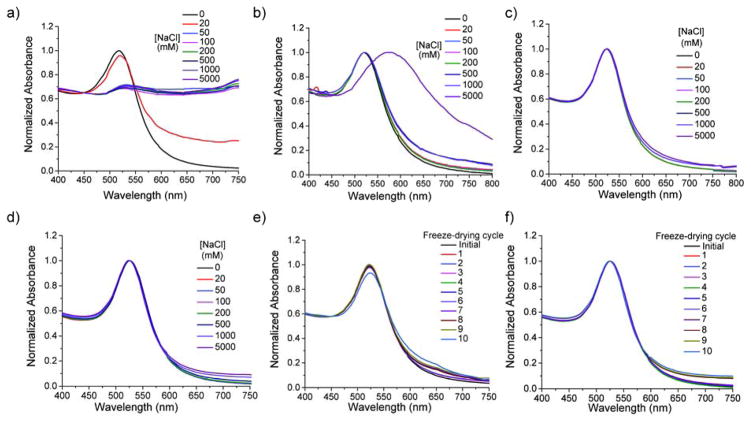
We first tested the stability of AuNPs functionalized with our ligand (AuNP-Zwit) comparative to citrate-capped AuNPs (AuNP-Citr) and thiolated-PEG functionalized AuNPs (AuNP-PEG), highly used in biological applications. To this end, we varied the ionic strength and observed changes in color (SPR peak), indicative of AuNP aggregation.39,40,41 As expected, citrate capped AuNPs aggregated with increasing ionic strength, with a change in the original SPR peak observed as the salt concentration increased (Figure 2a, 2b, 2c and 2d). AuNP-PEG also showed signs of aggregation at high salt concentrations. We only observed a decrease in stability at extreme salt concentrations for the largest gold core (35 nm, Figure S6). On the other hand and as observed in Figure 2c and 2d, our ligand was capable of conferring stability to AuNPs even at extreme ion concentrations (5M), where no apparent changes in the color were observed. Additionally, zwitterionic NPs were stable even after incubation for 24 hours at high salt concentration (up to 5mM), which is evidenced by the UV spectrum in Figure S6 d). This further corroborates long term storage stability conferred by our zwitterionic ligands.
Figure 2.
a) UV spectra of AuNP-Citr, b) 15 nm AuNP-PEG, c) 15 nm AuNP-Zwit and d) 25 nm AuNP-Zwit at different ion strengths, evidencing the enhanced stability conferred by the zwitterionic ligand. e) UV spectrum of 15 nm and f) 25 nm AuNP-Zwit, after successive cycles of freeze-drying.
One of the principal barriers for the industrial and general use of AuNPs is their poor capability of enduring freeze-drying cycles.23 This is one of the major reasons of why most of the commercially available AuNPs are sold as solutions. In addition, many reports have shown that when AuNPs are subjected to lyophilization,42 larger AuNPs agglomerates are formed, and these aggregates cannot be dispersed again in solution.43 Variations in the stabilizing agent helps in controlling shape and the size of the final structures, though complete stability was achievable only in few cases.44,45 For example, one report showed that 15 nm AuNPs are capable of resisting freeze-drying cycles if PEG structures were used as the surface ligands. However, as we described before, this technique does not facilitate the post-functionalization of AuNPs with ligands of interest, limiting its applicability.
Effective stabilization of NPs often requires bulky PEG ligands (~5Kd) which alter their mobility.46 We were interested in observing if AuNPs decorated with our ligand were capable of enduring this stressing process. For this purpose we subjected the AuNP-Zwit to freeze-drying, and records of the UV spectrum were taken before and after the process. As observed in Figure 2e–f (and Figure S7), the initial UV spectrum is essentially the same to the one obtained for AuNP-Zwit redispersed in water after lyophilization. This signal recovery was observed even after repeating the freeze-drying cycle 10 consecutive times, evidencing the degree of colloidal stability that the zwitterionic ligand can confer to the different AuNP sizes. On the contrary, AuNP-PEG and citrate-capped AuNPs could not be redispersed after the first lyophilization cycle (Figure S8).
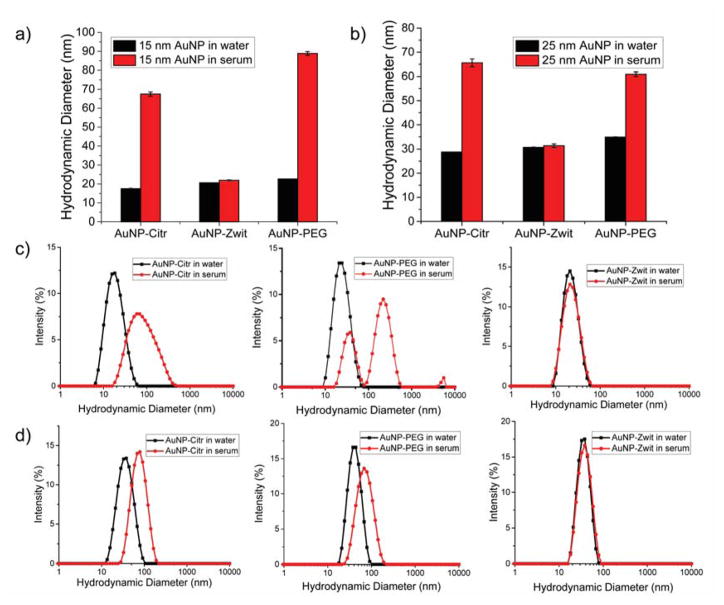
Understanding the association of NPs with proteins plays an important role in biosensing and biomedical applications. Avoiding interactions such as formation of a protein corona is important for designing ‘stealthy’ particles for delivery applications. The undesirable adsorption of proteins on NP surface not only reduces its targeting capabilities but it also promotes their elimination from the body. We tested stability of our AuNPs in biological fluids (human serum) by dynamic light scattering (DLS), as this is critical a requirement for their use in applications in vitro and in vivo.47,48 As depicted in the DLS profiles (Figure 3), no aggregates were observed for AuNP-Zwit while AuNP-Citr and AuNP-PEG formed larger aggregates, evidencing the enhanced stability conferred by the zwitterionic ligand.
Figure 3.
Comparison of NP size after 24 hr incubation in serum a) 15 nm AuNPs b) 25 nm AuNPs. Particle size distribution for NPs in the presence and absence of serum proteins (50%) evidencing the stability of AuNP-Zwit c) DLS profiles of 15 nm AuNP-Citr, AuNP-PEG and AuNP-Zwit and d) DLS profiles of 25 nm AuNP-Citr, AuNP-PEG and AuNP-Zwit respectively.
Bio-functionalization of AuNPs
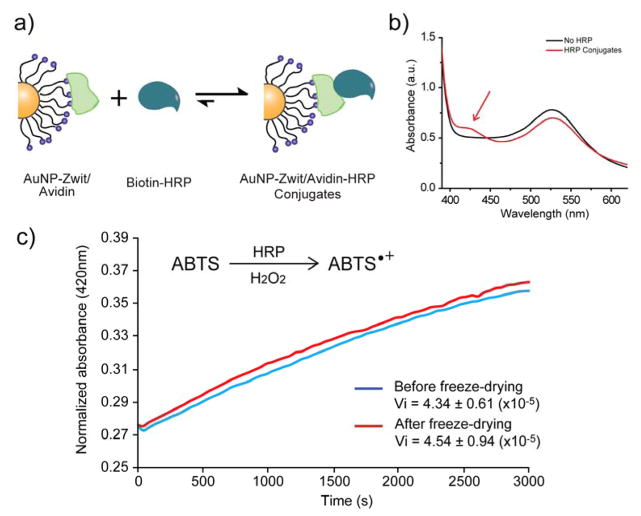
After the colloidal stability of our system was tested, we then proceeded to functionalize the surface of the AuNPs with biomacromolecules according to our initial design (inclusion of the carboxylic group in the zwitterionic pair). For this experiment we selected the 15 nm gold core as this is size is the one that be found from commercial sources. Since our intention was to create a ‘plug-and-use’ system of AuNPs, we covalently linked AuNP-Zwit to avidin by the use of EDC/NHS coupling.49,50 Each NP could bind to 9 avidin molecules as determined using biotin-FITC binding experiments (described in Supporting Information).51 The purpose of using avidin was to further allow the system to form conjugates with any biotin-functionalized biomacromolecule in an easy and fast way (Figure 4a).52 To assess if this arrangement was possible, we mixed biotin-HRP (horseradish peroxidase) with the avidin-functionalized AuNP-Zwit. After removing the free HRP through the use of numerous centrifugation/redispersion cycles, we tested if the conjugation was successful by introducing ABTS (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) to the solution and observing if coloration was observed (indicating the presence of HRP). As observed in Figure 4b, a significant change in color was observed comparative to the controls, evidencing the formation of the conjugates. We then determined if the AuNP-Zwit/avidin conjugates were capable of enduring freeze-drying cycles. As such, we lyophilized the AuNP-Zwit/avidin conjugates and analyzed if the avidin maintained its capability of binding to biotin-HRP. As depicted in figure 4b, this was the case and the AuNP-HRP conjugates were still formed. Furthermore, the kinetics of substrate consumption was similar to the one observed before the lyophilization, indicating no lost in the structural conformation required for the avidin-biotin recognition (Figure 4c). It is important to note that the formulation of these conjugates was done by simply mixing AuNPs-Zwit/avidin with biotin-HRP, followed by a fast cleaning process with centrifugation. This convenient conjugation procedure puts this approach closer to the idea of ‘disperse and use’ commonly required for the majority of therapeutic formulations.
Figure 4.
a) Scheme of the conjugation of AuNP-Zwit/avidin with biotin-functionalized HRP. b) UV spectrum of AuNPs alone and conjugated with HRP after the addition of ABTS (substrate). c) Normalized substrate transformation by the AuNP-Zwit/avidin-HRP conjugates before and after freeze-drying. As evidenced by the initial rates of reaction (Vi), the AuNP-Zwit/avidin conjugates are capable of enduring freeze-drying stress without losing activity.
CONCLUSION
In summary, we have introduced a new nanoparticle coverage specifically designed to provide enhanced stability to AuNPs while still offering functionalization capabilities. AuNPs functionalized with this ligand are stable in biological fluids, are able to endure strong changes in ionic strength, and can be redispersed after freeze-drying cycles. In addition, these AuNPs can be functionalized with biomacromolecules, and by the specific use of avidin, any biotin-functionalized macromolecule (from the large catalog that is commercially available) can be used to confer the AuNPs the desired biological properties. Finally these AuNPs-avidin conjugates can be lyophilized without affecting their activity, which allows their transport as powders for future formulations. As such, this approach will allow the convenient development of therapeutic formulations, and will facilitate the introduction of these technologies into the industrial scale.
Supplementary Material
Acknowledgments
This work was supported by the grants from the NIH (EB014277) and the Center for Hierarchical Manufacturing (CMMI-1025020).
Footnotes
Notes
The authors declare no competing financial interests.
Nanoparticle synthesis, characterization, and additional experimental details and figures are described in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Eustis S, El-Sayed MA. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem Soc Rev. 2006;35:209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 2.Corma A, Garcia H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem Soc Rev. 2008;37:2096–2126. doi: 10.1039/b707314n. [DOI] [PubMed] [Google Scholar]
- 3.Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold Nanoparticles in Chemical and Biological Sensing. Chem Rev. 2012;112:2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J Phys Chem B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 5.Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-Time Vital Optical Imaging of Precancer Using Anti-Epidermal Growth Factor Receptor Antibodies Conjugated to Gold Nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- 6.Rosi NL, Mirkin CA. Nanostructures in Biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 7.Daniel MC, Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 8.Lv W, Wang Y, Feng W, Qi J, Zhang G, Zhang F, Fan X. Robust and Smart Gold Nanoparticles: One-Step Synthesis, Tunable Optical Property, and Switchable Catalytic Activity. J Mater Chem. 2011;21:6173–6178. [Google Scholar]
- 9.Nath N, Chilkoti A. A Colorimetric Gold Nanoparticle Sensor to Interrogate Biomolecular Interactions in Real Time on a Surface. Anal Chem. 2002;74:504–509. doi: 10.1021/ac015657x. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, El-Sayed MA. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J Phys Chem B. 2006;110:19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Z, Le NDB, Gupta A, Rotello VM. Cell Surface-Based Sensing with Metallic Nanoparticles. Chem Soc Rev. 2015;44:4264–4274. doi: 10.1039/c4cs00387j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CC, Yang Z, Lee KH, Chang HT. Synthesis of Highly Fluorescent Gold Nanoparticles for Sensing Mercury (II) Angew Chemie. 2007;119:6948–6952. doi: 10.1002/anie.200700803. [DOI] [PubMed] [Google Scholar]
- 13.Ai K, Liu Y, Lu L. Hydrogen-Bonding Recognition-Induced Color Change of Gold Nanoparticles for Visual Detection of Melamine in Raw Milk and Infant Formula. J Am Chem Soc. 2009;131:9496–9497. doi: 10.1021/ja9037017. [DOI] [PubMed] [Google Scholar]
- 14.Su H, Ma Q, Shang K, Liu T, Yin H, Ai S. Gold Nanoparticles as Colorimetric Sensor: A Case Study on E. Coli O157:H7 as a Model for Gram-Negative Bacteria. Sensors Actuators B Chem. 2012;161:298–303. [Google Scholar]
- 15.El-Sayed IH, Huang X, El-Sayed MA. Surface Plasmon Resonance Scattering and Absorption of Anti-EGFR Antibody Conjugated Gold Nanoparticles in Cancer Diagnostics: Applications in Oral Cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 16.Homola J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem Rev. 2008;108:462–493. doi: 10.1021/cr068107d. [DOI] [PubMed] [Google Scholar]
- 17.Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and Brain Uptake of Transferrin-Containing Nanoparticles by Tuning Avidity to Transferrin Receptor. Proc Natl Acad Sci. 2013;110:8662–8667. doi: 10.1073/pnas.1307152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold Nanoparticles in Delivery Applications. Adv Drug Deliv Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Tebbe M, Kuttner C, Männel M, Fery A, Chanana M. Colloidally Stable and Surfactant-Free Protein-Coated Gold Nanorods in Biological Media. ACS Appl Mater Interfaces. 2015;7:5984–5991. doi: 10.1021/acsami.5b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 21.Khlebtsov N, Dykman L. Biodistribution and Toxicity of Engineered Gold Nanoparticles: A Review of in Vitro and in Vivo Studies. Chem Soc Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 22.Boisselier E, Astruc D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 23.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv Drug Deliv Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. Zwitterionic SAMs That Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir. 2001;17:2841–2850. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Huang H, Jin Q, Ji J. Mixed Charged Zwitterionic Self-Assembled Monolayers as a Facile Way to Stabilize Large Gold Nanoparticles. Langmuir. 2011;27:5242–5251. doi: 10.1021/la2002223. [DOI] [PubMed] [Google Scholar]
- 26.Pillai PP, Huda S, Kowalczyk B, Grzybowski BA. Controlled pH Stability and Adjustable Cellular Uptake of Mixed-Charge Nanoparticles. J Am Chem Soc. 2013;135:6392–6395. doi: 10.1021/ja4001272. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of Anchoring Ligands and Particle Size on the Colloidal Stability and in Vivo Biodistribution of Polyethylene Glycol-Coated Gold Nanoparticles in Tumor-Xenografted Mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Shipton MK, Ryan J, Kaufman ED, Franzen S, Feldheim DL. Synthesis, Stability, and Cellular Internalization of Gold Nanoparticles Containing Mixed Peptide-Poly(ethylene Glycol) Monolayers. Anal Chem. 2007;79:2221–2229. doi: 10.1021/ac061578f. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Wang S, Beizhong J. Synthesis and Characterization of Macroinitiator-Amino Terminated PEG and Poly(g-Benzyl-L-Glutamate)-PEO-Poly(g-Benzyl-L-Glutamate) Triblock Copolymer. Polym Adv Technol. 2004;15:617–621. [Google Scholar]
- 30.Akagi T, Higashi M, Kaneko T, Kida T, Akashi M. Hydrolytic and Enzymatic Degradation of Nanoparticles Based on Amphiphilic Poly(gamma-Glutamic Acid)-Graft-L-Phenylalanine Copolymers. Biomacromolecules. 2006;7:297–303. doi: 10.1021/bm050657i. [DOI] [PubMed] [Google Scholar]
- 31.Sekiguchi S, Niikura K, Matsuo Y, Ijiro K. Hydrophilic Gold Nanoparticles Adaptable for Hydrophobic Solvents. Langmuir. 2012;28:5503–5507. doi: 10.1021/la300299x. [DOI] [PubMed] [Google Scholar]
- 32.Keefe AJ, Jiang S. Poly(zwitterionic)protein Conjugates Offer Increased Stability without Sacrificing Binding Affinity or Bioactivity. Nat Chem. 2012;4:59–63. doi: 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct Polymer Architecture Mediates Switching of Complement Activation Pathways at the Nanosphere−Serum Interface: Implications for Stealth Nanoparticle Engineering. ACS Nano. 2010;4:6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 34.Moyano DF, Saha K, Prakash G, Yan B, Kong H, Yazdani M, Rotello VM. Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nanoparticles. PLoS One. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaisocherová H, Yang W, Zhang Z, Cao Z, Cheng G, Piliarik M, Homola J, Jiang S. Ultralow Fouling and Functionalizable Surface Chemistry Based on a Zwitterionic Polymer Enabling Sensitive and Specific Protein Detection in Undiluted Blood Plasma. Anal Chem. 2008;80:7894–7901. doi: 10.1021/ac8015888. [DOI] [PubMed] [Google Scholar]
- 37.Park J, Nam J, Won N, Jin H, Jung S, Jung S, Cho SH, Kim S. Compact and Stable Quantum Dots with Positive, Negative, or Zwitterionic Surface: Specific Cell Interactions and Non-Specific Adsorptions by the Surface Charges. Adv Funct Mater. 2011;21:1558–1566. [Google Scholar]
- 38.Aldeek F, Muhammed MAH, Palui G, Zhan N, Mattoussi H. Growth of Highly Fluorescent Polyethylene Glycol- and Zwitterion-Functionalized Gold Nanoclusters. ACS Nano. 2013;7:2509–2521. doi: 10.1021/nn305856t. [DOI] [PubMed] [Google Scholar]
- 39.Yusa S, Fukuda K, Yamamoto T, Iwasaki Y, Watanabe A, Akiyoshi K, Morishima Y. Salt Effect on the Heat-Induced Association Behavior of Gold Nanoparticles Coated with Poly(N-Isopropylacrylamide) Prepared via Reversible Addition−Fragmentation Chain Transfer (RAFT) Radical Polymerization. Langmuir. 2007;23:12842–12848. doi: 10.1021/la702741q. [DOI] [PubMed] [Google Scholar]
- 40.Rouhana LL, Jaber JA, Schlenoff JB. Aggregation-Resistant Water-Soluble Gold Nanoparticles. Langmuir. 2007;23:12799–12801. doi: 10.1021/la702151q. [DOI] [PubMed] [Google Scholar]
- 41.Tatumi R, Fujihara H. Remarkably Stable Gold Nanoparticles Functionalized with a Zwitterionic Liquid Based on Imidazolium Sulfonate in a High Concentration of Aqueous Electrolyte and Ionic Liquid. Chem Commun (Camb) 2005;1:83–85. doi: 10.1039/b413385d. [DOI] [PubMed] [Google Scholar]
- 42.Haba Y, Kojima C, Harada A, Ura T, Horinaka H, Kono K. Preparation of Poly(ethylene Glycol)-Modified Poly(amido Amine) Dendrimers Encapsulating Gold Nanoparticles and Their Heat-Generating Ability. Langmuir. 2007;23:5243–5246. doi: 10.1021/la0700826. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Li P, Li D, Guo S, Wang E. Effect of Freeze−Thawing on Lipid Bilayer-Protected Gold Nanoparticles. Langmuir. 2008;24:3407–3411. doi: 10.1021/la703737q. [DOI] [PubMed] [Google Scholar]
- 44.Mangeney C, Ferrage F, Aujard I, Marchi-Artzner V, Jullien L, Ouari O, Rékaï ED, Laschewsky A, Vikholm I, Sadowski JW. Synthesis and Properties of Water-Soluble Gold Colloids Covalently Derivatized with Neutral Polymer Monolayers. J Am Chem Soc. 2002;124:5811–5821. doi: 10.1021/ja010796h. [DOI] [PubMed] [Google Scholar]
- 45.Lévy R, Thanh NTK, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, Brust M, Fernig DG. Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles. J Am Chem Soc. 2004;126:10076–10084. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- 46.García I, Sánchez-Iglesias A, Henriksen-Lacey M, Grzelczak M, Penadés S, Liz-Marzán LM. Glycans as Biofunctional Ligands for Gold Nanorods: Stability and Targeting in Protein-Rich Media. J Am Chem Soc. 2015;137:3686–3692. doi: 10.1021/jacs.5b01001. [DOI] [PubMed] [Google Scholar]
- 47.Yang W, Zhang L, Wang S, White AD, Jiang S. Functionalizable and Ultra Stable Nanoparticles Coated with Zwitterionic Poly(carboxybetaine) in Undiluted Blood Serum. Biomaterials. 2009;30:5617–5621. doi: 10.1016/j.biomaterials.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 48.Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Keefe AJ, Giarmarco M, Brault ND, Jiang S. Simple and Robust Approach for Passivating and Functionalizing Surfaces for Use in Complex Media. Langmuir. 2012;28:9707–9713. doi: 10.1021/la301691d. [DOI] [PubMed] [Google Scholar]
- 50.Muro E, Pons T, Lequeux N, Fragola A, Sanson N, Lenkei Z, Dubertret B. Small and Stable Sulfobetaine Zwitterionic Quantum Dots for Functional Live-Cell Imaging. J Am Chem Soc. 2010;132:4556–4557. doi: 10.1021/ja1005493. [DOI] [PubMed] [Google Scholar]
- 51.Meiser F, Cortez C, Caruso F. Biofunctionalization of Fluorescent Rare-Earth-Doped Lanthanum Phosphate Colloidal Nanoparticles. Angew Chemie Int Ed. 2004;43:5954–5957. doi: 10.1002/anie.200460856. [DOI] [PubMed] [Google Scholar]
- 52.Hsu SM, Raine L, Fanger H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.