Abstract
Background
Studies in male combat veterans have suggested PTSD is associated with shorter telomere length (TL). We examined the cross-sectional association of PTSD with TL in women exposed to traumas common in civilian life.
Methods
Data are from a substudy of the Nurses’ Health Study II (N=116). PTSD and subclinical PTSD were assessed in trauma-exposed women using diagnostic interviews. An array of health behaviors and conditions were assessed. DNA was extracted from peripheral blood leukocytes (collected 1996–1999). Telomere repeat copy number to single gene copy number (T/S) was determined by quantitative real-time PCR telomere assay. We used linear regression models to assess associations and examine whether a range of important health behaviors (e.g., cigarette smoking) and medical conditions (e.g., hypertension) previously associated with TL might explain a PTSD-TL association. We further examined whether type of trauma exposure (e.g., interpersonal violence) was associated with TL and whether trauma type might explain a PTSD-TL association.
Results
Relative to not having PTSD, women with a PTSD diagnosis had shorter log-transformed TL (β=−0.112, 95% confidence interval=−0.196, −0.028). Adjustment for health behaviors and medical conditions did not attenuate this association. Trauma type was not associated with TL and did not account for the association of PTSD with TL.
Conclusions
Our results add to growing evidence that PTSD may be associated with more rapid cellular aging as measured by telomere erosion. Moreover, the association could not be explained by health behaviors and medical conditions assessed in this study, nor by type of trauma exposure.
Keywords: Posttraumatic Stress Disorders, Telomere, Biological markers, Trauma
INTRODUCTION
Posttraumatic stress disorder (PTSD), characterized by severe distress in response to trauma exposure, is associated with incident chronic disease, including cardiovascular disease (CVD) and type 2 diabetes (Lee et al., 2016; Andrea L Roberts et al., 2015; Sumner et al., 2015), as well as disease progression (Gander & von Kanel, 2006; Révész, Milaneschi, Verhoeven, & Penninx, 2014) and earlier mortality (Boscarino, 2006). Telomere erosion has been proposed as a potentially important mechanism by which traumatic stress and the severe distress that often follows is biologically embedded, resulting in accelerated aging, chronic disease, and early death (Lindqvist et al., 2015; Schutte & Malouff, 2014b). Telomeres are repetitive structures at the end of eukaryotic chromosomes that protect chromosome ends from deterioration during cell division. Telomere erosion leads to genomic instability, cell reproductive senescence and programmed cell death (apoptosis). (Wong et al., 2010) As shorter telomeres have been found in persons with versus without CVD (Starr et al., 2007), diabetes (Zee, Castonguay, Barton, Germer, & Martin, 2010), and health risk factors, such as obesity and smoking (A. Valdes et al., 2005), and telomere length also appears to predict earlier mortality (Cawthon, Smith, O'Brien, Sivatchenko, & Kerber, 2003), recent research suggests telomere length may serve as a biomarker of accelerated aging and increased risk of earlier development of chronic disease.
Psychosocial stress and distress are hypothesized to accelerate telomere erosion via chronic activation of relevant biological processes, including oxidative stress, inflammation, and decreased telomerase activity (Epel et al., 2004). Empirical work generally finds shorter telomere length associated with measures of stress or distress, including perceived stress, (Epel, et al., 2004; Parks et al., 2009) psychosomatic distress (Zahran et al., 2015), and salivary biomarkers of stress (Zahran, et al., 2015) (although studies are not uniformly consistent (Jodczyk, Fergusson, Horwood, Pearson, & Kennedy, 2014; Savolainen, Eriksson, Kajantie, Lahti, & Räikkönen, 2015; Verhoeven, van Oppen, Puterman, Elzinga, & Penninx, 2015)), suggesting that telomere shortening may be one mechanism by which PTSD leads to increased risk of disease and premature death.
Seven empirical studies have examined the association of PTSD with leukocyte telomere length (TL) (Boks et al., 2015; Jergović et al., 2014; Ladwig et al., 2013; Malan, Hemmings, Kidd, Martin, & Seedat, 2011; O'Donovan et al., 2011; I Shalev et al., 2014; Zhang et al., 2014). Three studies were conducted with male combat veterans (Boks, et al., 2015; Jergović, et al., 2014; Zhang, et al., 2014); two found PTSD associated cross-sectionally with shorter telomeres (Jergović, et al., 2014; Zhang, et al., 2014) and one found no association (Boks, et al., 2015). Only two studies used population-based samples, and findings were mixed. One examined TL in association with any internalizing disorder, measured by combining PTSD with generalized anxiety disorder, overanxious disorder of childhood, and depression (I Shalev, et al., 2014). This study found significantly shorter TL and more rapid telomere erosion over time among men with versus without any internalizing disorders, but no associations were evident in women. The only population-based study (among 3000 German men and women) that examined PTSD specifically reported that participants with versus without PTSD had shorter TL. (Ladwig, et al., 2013) A recent meta-analysis considering six of the studies reported a medium to large effect that was significant with a pooled Cohen’s d 0.76 (95% confidence interval (CI)=0.25–1.28)(Lohr et al.). An additional meta-analysis including five of these studies found a mean difference of −1.27 standard deviations in TL for participants with versus without PTSD (Darrow et al., 2016).
Current knowledge of the association of PTSD and TL has several limitations. There is ongoing uncertainty whether health-related effects of PTSD are largely due to effects of trauma or to the resultant psychological distress, and few studies of TL have separately examined the effects of trauma versus psychological sequelae of trauma (Audrey R Tyrka et al., 2016). If effects are primarily due to trauma, then in fact type of trauma may matter with regard to linkages with TL. While PTSD is more prevalent among women (Tolin & Foa, 2006), only three studies have been able to consider associations of PTSD and TL among civilian women exposed to traumatic stressors common in everyday life (Ladwig, et al., 2013; O'Donovan, et al., 2011; I Shalev, et al., 2014). Finally, in most studies relevant health risk factors and conditions linked to both PTSD and TL have not been considered. Only two studies considered some relevant health risk factors and conditions linked to both PTSD and TL and conceptualized them as potential confounders (Ladwig, et al., 2013; I Shalev, et al., 2014). In prior work PTSD has predicted health risk factors and conditions, including elevated BMI (Kubzansky et al., 2014), smoking (Cook, Jakupcak, Rosenheck, Fontana, & McFall, 2009; Fu et al., 2007), unhealthy diet (Breslau, Davis, Peterson, & Schultz, 1997; Hall, Hoerster, & Yancy, 2015), as well as hypertension (Kibler, Joshi, & Ma, 2009) and high cholesterol, (Heppner et al., 2009; Kulenovic, Kucukalic, & Malec, 2008) that have been associated with shorter TL (Buxton et al., 2011; Cherkas, Hunkin, Kato, & et al., 2008; Du et al., 2012; Harris et al., 2006; Harris, Martin-Ruiz, von Zglinicki, Starr, & Deary, 2012; A. M. Valdes et al., 2005).
The present study examines the association of PTSD symptoms with TL in a sample of civilian women exposed to stressors common in civilian life. Based on prior work examining PTSD in relation to CVD risk reporting a dose-response relation (Kubzansky, Koenen, Jones, & Eaton, 2009; Kubzansky, Koenen, Spiro, Vokonas, & Sparrow, 2007; Sumner, et al., 2015), we hypothesized sub-clinical PTSD symptomatology would be associated with shorter TL and that PTSD symptom count would demonstrate a dose-response relation with TL. We examine whether higher prevalence of health risk factors in women with PTSD might account for an association of PTSD with TL, including BMI (Buxton, et al., 2011; Kim et al., 2009; Nordfjäll et al., 2008), smoking (McGrath, Wong, Michaud, Hunter, & De Vivo, 2007; A. M. Valdes, et al., 2005), alcohol consumption (Pavanello et al., 2011), antidepressant use, type 2 diabetes, hypertension (Lung, Ku, & Kao, 2008), physical activity (Du, et al., 2012; Savela et al., 2013) and high cholesterol. (Strandberg et al., 2011; Sun et al., 2012) We investigate the association of type of trauma exposure with TL, and whether type of trauma exposure accounts for possible differences in TL by PTSD status. Moreover, as depression and PTSD are highly comorbid (Bleich, Koslowsky, Dolev, & Lerer, 1997; Campbell et al., 2007; Gill, Page, Sharps, & Campbell, 2008), and depression is associated with shorter TL in several (Hartmann, Boehner, Groenen, & Kalb, 2010; Schutte & Malouff, 2015; Simon et al., 2006) but not all studies (Ladwig, et al., 2013), we explore the possibility that having PTSD and depression combined is associated with even shorter TL compared with only PTSD.
MATERIALS AND METHODS
Sample
The Nurses’ Health Study II (NHSII) comprises 116,430 female nurses enrolled in 1989 at ages 25–42 years and followed biennially. Blood was collected in 1996–1999 when women were ages 32–52 years (Tworoger, Sluss, & Hankinson, 2006). In 2008, the PTSD Substudy was conducted (Koenen et al., 2009; A. L. Roberts et al., 2012). 60,804 NHSII participants were mailed a supplementary questionnaire that assessed trauma exposure and PTSD symptoms; 54,224 participants returned the questionnaire (response rate=89%); 80% reported at least one lifetime traumatic event, 53% of whom agreed to be interviewed in depth and from whom 2,112 probable cases of PTSD and 2,001 matched controls (all trauma exposed), were identified. Seventy-three percent of these women (N=3,013) completed a PTSD diagnostic interview. The protocol has been published. (Koenen, et al., 2009) The present analyses include women who completed the PTSD diagnostic interview and who also served as medically healthy controls in other NHSII substudies that measured TL. One case-control study was for type 2 diabetes (n=74) and the other was for CVD (n=24); TL measures were available in an additional sample of healthy women selected for another NHSII substudy because they were exposed to childhood abuse (n=20) (Mason, Prescott, Tworoger, DeVivo, & Rich-Edwards, 2015).
PTSD assessment
Lifetime PTSD case status and PTSD symptom counts were ascertained via diagnostic telephone interview, previously validated against the Clinician-Administered PTSD Scale (Blake et al., 1998) in another cohort (Monica Uddin et al., 2010). Respondents were asked to identify events they had experienced from a list of 25 potentially traumatic events (e.g., natural disaster, mugging) as well as “any other very stressful situation or event”. Respondents then selected the event they considered to be their worst and reported the year it occurred. For analyses considering trauma type explicitly, based on research regarding trauma severity (A. L. Roberts, et al., 2012), we grouped worst trauma types in five categories: 1) rape; 2) interpersonal violence; 3) other event to self; 4) sudden death of a loved one; 5) other event to a loved one. To be a PTSD case, respondents must have experienced their worst trauma before the blood draw and met all DSM-IV criteria for PTSD, as assessed according to 17 symptoms reflecting re-experiencing of the trauma, avoidance/numbing, arousal and several additional questions. (Kessler & Ustun, 2004) Participants were cued to think of the period following the event during which symptoms were most intense, and then asked whether they had ever been bothered by each of the 17 symptoms (rated each on a scale from 1:“not at all” to 5:“extremely”). Respondents were classified as a PTSD case if they reported experiencing one or more of the 5 re-experiencing symptoms, 3 or more of the 7 avoidance/numbing symptoms, and 2 or more of the 5 arousal symptoms at least “moderately.” Additional questions assessed the other three DSM-IV criteria: intense fear, horror, or helplessness in response to the event, symptom duration of at least one month, and clinically significant impairment in functioning due to symptoms.
To assess reliability, a blind review of audiotapes from 50 interviews was conducted by a licensed clinical psychologist who is an experienced diagnostician specializing in PTSD treatment. Reliability was assessed by comparing this diagnosis with that made via computer algorithm from the structured interviews using Cohen’s kappa statistic. The kappa was 1.0 (perfect reliability).
Respondents were considered affected by lifetime PTSD if all six DSM-IV criteria were met. Respondents were considered as having subclinical PTSD symptoms if they did not meet all diagnostic criteria and met at least one of the DSM-IV criteria: re-experiencing (≥1 symptom at least “moderately”), avoidance/numbing (≥3 symptoms at least “moderately”), or arousal (≥2 symptoms at least “moderately”), or had clinically significant impairment (McLaughlin et al., 2015). All other respondents were considered as not having PTSD. We additionally summed PTSD symptoms to create a continuous score (possible range, 17–85) (Solovieff et al., 2014) and divided this score into quintiles to examine a possible dose-response between PTSD symptoms and TL. We included in our analyses only women who reported the year of their worst event as before their blood draw. Women with no or few symptoms comprised the reference group but because few women were eligible, to enlarge this group, we included women whose worst trauma followed the blood draw but who reported low levels of PTSD symptoms (in the lowest PTSD symptom quintile).
Our diagnostic interview also assessed lifetime depression and age of first onset via a modified version of the Patient Health Questionnaire (PHQ-9) using DSM-IV coding criteria (Kroenke, Spitzer, & Williams, 2001). Participants were considered as having depression if they met criteria for major depressive disorder and reported onset before the year of their blood draw. The PHQ-9 has excellent internal consistency (α=0.87). We ascertained the validity of our mental health assessments in a separate cohort, the Detroit Neighborhood Health Study, via clinical interviews among a random sub-sample of 51 participants. (M. Uddin et al., 2010) A licensed psychologist conducted 1-hour in-person interviews, using the Clinician-Administered PTSD Scale for DSM-IV to evaluate PTSD (Blake, et al., 1998) and the Structured Clinical Interview for DSM-IV Disorders to evaluate depression. (First, Spitzer, Gibbon, & Williams, 1996) The psychologist was blinded to information obtained from the main study. Comparison of the clinical interviews with the PCL-C and PHQ-9 from the main study showed excellent concordance for both disorders. (M. Uddin, et al., 2010)
Telomere assay
Procedures for telomere assays in NHS samples have been described elsewhere (McGrath, et al., 2007). Briefly, genomic DNA was extracted from peripheral blood leukocytes using the QIAmp (Qiagen) 96-spin blood protocol. PicoGreen DNA quantitation was conducted using a Molecular Devices 96-well spectrophotometer. Genomic DNA was subsequently dried down and resuspended. Telomere repeat copy number to single gene copy number (T/S) was determined by a modified version of the quantitative real-time PCR telomere assay run on Applied Biosystems 7900HT PCR System (Foster City, CA). Triplicate reactions of each assay were done on each sample. Relative telomere length (RTL) is reported as the exponentiated T/S ratio. The coefficient of variation (CV) for the exponentiated T/S ratio of blinded quality control samples was 23% for T2DM cases and controls and 33% for CVD cases and controls. Within-replicate CVs for participants and blinded QCs were lower within plates, ranging from 10.7 to 19.4%. Further, after excluding QC samples with within-triplicate CVs > 20%, CVs dropped to 14–26%. Finally, non-blinded laboratory QCs were also included across plates and CVs in these samples averaged 11.5%. Overall, these results suggest most variation occurred between batches.
Because the RTL deviated significantly from normality, we took the log to derive a normally-distributed outcome. To control for variation across laboratory batches, we used the Rosner batch-correction method (Rosner, Cook, Portman, Daniels, & Falkner, 2008) as follows. First, we regressed log-RTL on indicators for laboratory batch and potential predictors of RTL that might vary by batch, including participant’s year of birth; father’s age at birth; and age, smoking status (never/past/current), and BMI at time of blood draw. We calculated a correction parameter for each batch by subtracting the average of all batch parameter estimates from the parameter estimate for each individual batch. For each observation assayed within a particular batch, we produced a batch-adjusted log-RTL (henceforth simply log-RTL) by adjusting the log-RTL by subtracting that batch’s correction parameter.
Covariates
To ensure each covariate measured circumstances following PTSD onset and before the blood draw, we used covariates queried on a questionnaire given with the blood draw and on the closest preceding biennial questionnaire (1995), as available. Age at blood draw was determined from self-reported birthdate. BMI was self-reported on the blood draw questionnaire. Self-reported BMI has been validated in the Nurses’ Health studies (Rimm et al., 1990). Past-month smoking was reported on the blood draw questionnaire as: none, 1–4, 5–14, 15–24, 25–34, 35–44, or >=45 cigarettes/day. Smoking history was taken from prior questionnaires to characterize smoking at the blood draw as: current, former, or never. Diet was characterized from responses to a validated food frequency questionnaire (Willett et al., 1988 ) in 1995 using the Alternative Healthy Eating Index (McCullough et al., 2002), which has been associated with significant reductions in chronic disease risk, coded in quintiles. Average past-year alcohol consumption was queried in 1995 and coded as 0–7 days/week. Past-month alcohol consumption (blood draw questionnaire), was queried with options: none, 1–3 drinks/month, 1/week, 2–4/week, 5–6/week, 1/day, 2–3/day, 4–5/day, and 6+/day. Frequency of exercising heavily enough to sweat was queried in 1995 as: <1, 1, 2–3, 4–6, or 7+ times/week. Past-month frequency of physical activity (blood draw questionnaire) included four response options: <once, once, 2–3, 4–6, or 7+ times/week. Current regular antidepressant use (yes/no) was asked on the blood draw questionnaire. High cholesterol, high blood pressure, and doctor’s diagnosis of type 2 diabetes were each queried in 1995 (yes/no). No cases of type 2 diabetes were reported and therefore it was not examined as a covariate.
Statistical analyses
To examine the characteristics of the sample, we calculated means and standard deviations for age and BMI and prevalence of smoking, alcohol consumption, healthy diet, physical activity, antidepressant use, high blood pressure, and high cholesterol by PTSD status. We also examined characteristics of trauma exposure by PTSD status.
To ascertain whether log-RTL was shorter among women with PTSD, we first calculated mean log-RTL by PTSD diagnostic category and then fitted a linear regression model with log-RTL as the dependent variable and PTSD status as the independent variable. Age was only weakly associated with log-RTL in this sample (β=0.003; 95% CI=.005, .010; p=0.52), and a model of RTL with age and age-squared had better fit than a model with age alone according to Akaike information criteria (AIC); thus, we included age and age-squared in all models. To investigate whether lifestyle factors might account for possible shorter telomeres in women with PTSD, we examined the association of each lifestyle factor with log-RTL and examined the association of PTSD with log-RTL in models with and without the lifestyle factor, adjusted for age and age-squared. As our sample size was moderate, to avoid over-fitting we included only lifestyle factors that were either associated with log-RTL at p<0.10 or that altered the association of PTSD with log-RTL by ≥10%. We then fit a model with log-RTL as the dependent variable and PTSD status as the independent variable further adjusted for all lifestyle factors meeting at least one of these criteria. We also examined log-RTL in association with a continuous measure of PTSD symptoms, and examined the impact of lifestyle factors similarly to the models with PTSD diagnostic category.
To understand whether characteristics of trauma exposure accounted for possible associations of PTSD with log-RTL, we examined the association of worst trauma type with log-RTL and the association of PTSD with log-RTL adjusted for type of worst trauma. We further examined whether age at worst trauma was associated with log-RTL. Age at worst trauma and age at blood draw were moderately correlated (Pearson correlation coefficient=0.15). All models adjusted for age at blood draw and age-squared.
To determine whether telomeres were further shortened among women with both PTSD and depression, we created a categorical variable coded as: no PTSD or depression, no PTSD with depression, subclinical PTSD only, subclinical PTSD with depression, PTSD diagnosis only, and PTSD diagnosis with depression, and calculated mean log-RTL for each level of this categorical variable. We modeled log-RTL as the dependent variable with major depression, PTSD diagnosis and their interaction term as independent variables.
We conducted three sensitivity analyses, by: 1) including cases in addition to controls from the diabetes and cardiovascular disease studies; 2) excluding women selected on the basis of childhood abuse (although childhood abuse is not associated with log-RTL in this cohort (Mason, et al., 2015)); 3) excluding women whose worst trauma followed the blood draw but who reported low levels of PTSD symptoms. For all models we used PROC GENMOD in SAS 9.4 (SAS Institute, Cary, NC) with an identity link and a normal distribution to estimate regression coefficients and 95% confidence intervals.
RESULTS
Women with PTSD had slightly higher BMI (28.4 versus 25.1 kg/m2) and were more likely to use antidepressants regularly (52.0 versus 10.7%) at the time of the blood draw compared with women without PTSD (Table 1). For worst trauma type, among women without PTSD, sudden death of a loved one was the most prevalent category (40.0%), and among women with PTSD, other trauma to self (28.0%), rape (24.0%), and interpersonal violence (24.0%) were most prevalent (Table 2).
Table 1.
Characteristics of Nurses’ Health Study II women by PTSD diagnosis (N=116)
| No PTSD (N=25) | Subclinical PTSD† (N=66) |
PTSD diagnosis (N=25) |
||
|---|---|---|---|---|
| Age, years | Mean (SD) | 45.5 (3.6) | 46.6 (3.7) | 46.6 (3.9) |
| BMI, blood draw, kg/m2 | Mean (SD) | 25.1 (4.0) | 26.0 (7.3) | 28.4 (7.6)a |
| Past-month smoking, blood draw, any | % (N) | 14.3 (4) | 9.0 (6) | 16.0 (4) |
| Past-month alcohol consumption, blood draw, none | % (N) | 32.1 (9) | 43.9 (29) | 56.0 (14) |
| Diet, least healthy quintile, 1995 | % (N) | 12.0 (3) | 15.2 (10) | 28.0 (7) |
| Past-month physical activity, blood draw, <1/week | % (N) | 32.1 (9) | 39.4 (26) | 32.0 (8) |
| Regular antidepressant use, blood draw | % (N) | 10.7 (3) | 9.1 (6) | 52.0 (13)b |
| High cholesterol, 1995 | % (N) | 7.1 (1) | 7.6 (5) | 12.0 (3) |
| High blood pressure, 1995 | % (N) | 3.6 (1) | 3.0 (2) | 8.0 (2) |
Respondents were considered to have subclinical PTSD symptoms if they did not meet diagnostic criteria for PTSD and met at least one of re-experiencing, avoidance/numbing, or arousal criteria or had clinically significant impairment.
Mean compared with No PTSD group, chi-square test, p < 0.05
Difference in prevalence across PTSD groups, chi-square test, p < 0.001
Table 2.
Characteristics of trauma exposure by PTSD status
| No PTSD (N=25) |
Subclinical PTSD† (N=66) |
PTSD diagnosis (N=25) |
||
|---|---|---|---|---|
| Age at worst trauma | Mean (SD) | 38.0 (16.2) | 25.8 (17.2) | 22.4 (14.9) |
| Worst trauma, number of years before blood draw | Mean (SD) | 17.0 (14.7) | 19.1 (16.9) | 24.3 (16.8) |
| Worst trauma type | ||||
| Rape | % (N) | 4.0 (1) | 16.7 (11) | 24.0 (6) |
| Interpersonal violence (e.g., assault, partner abuse) | % (N) | 8.0 (2) | 24.2 (16) | 24.0 (6) |
| Other trauma to self | % (N) | 20.0 (5) | 24.2 (16) | 28.0 (7) |
| Sudden death of loved one | % (N) | 40.0 (10) | 19.7 (13) | 4.0 (1) |
| Other trauma to loved one | % (N) | 28.0 (7) | 15.2 (10) | 20.0 (5) |
Respondents were considered to have subclinical PTSD symptoms if they did not meet diagnostic criteria for PTSD and met at least one of re-experiencing, avoidance/numbing, or arousal criteria or had clinically significant impairment.
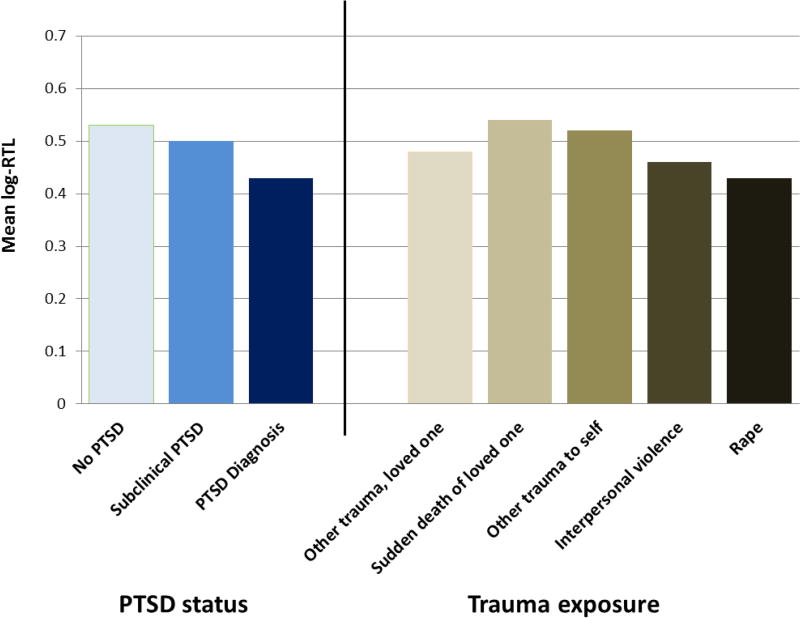
PTSD diagnosis was associated with shorter log-RTL (Figure). After adjusting for age at blood draw, PTSD diagnosis remained associated with shorter log-RTL (Table 3, Model 1a). Subclinical PTSD versus no PTSD was associated with shorter log-RTL, but this difference did not reach statistical significance. In this sample, BMI and smoking, but none of the other health-related covariates, were associated with log-RTL (p<0.10). In age-adjusted models, diet and antidepressant use altered the association of PTSD with log-RTL by ≥10%. We therefore included BMI, smoking, diet and antidepressant use as covariates. Including these lifestyle factors (Table 3, Model 2a) did not substantially attenuate the association between PTSD diagnosis and shorter log-RTL. Results were similar when examining PTSD symptoms as a continuous variable (Table 3, Models 1b–2b).
Figure.
Mean log relative telomere length by PTSD status and trauma exposure
Table 3.
Linear regression of log relative telomere length on PTSD diagnosis (N=116)
| Model 1a: Adjusted for age at blood draw and age2 |
Model 2a: Model 1a further adjusted for BMI, BMI2 and smoking |
Model 3a: Model 2a further adjusted for other behavior/health factors† |
Model 4a: Model 2a further adjusted for worst trauma type‡ |
||
|---|---|---|---|---|---|
|
| |||||
| N | Beta (95% Confidence Interval) | ||||
| PTSD status | |||||
| No PTSD | 25 | Reference | Reference | Reference | Reference |
| Subclinical PTSD | 66 | −.045 (−.115, .024) | −.060 (−.129, .009) | −.058 (−.127, .010) | −.030 (−.105, .040) |
| PTSD diagnosis | 25 | −.112 (−.196, −.028)** | −.104 (−.187, −.021)* | −.121 (−.209, −.032)** | −.092 (−.180, −.040)* |
|
| |||||
| Model 1b: Adjusted for age at blood draw and age2 | Model 2b: Model 1b further adjusted for BMI, BMI2 and smoking | Model 3b: Model 2b further adjusted for behavior/health factors† | Model 4b: Model 2b further adjusted for worst trauma type‡ | ||
|
| |||||
| PTSD symptoms (continuous) | 116 | −.004 (−.006, −.002)** | −.004 (−.006, −.001)** | −.004(−.006, −.001)** | −.003(−.006, −.001)** |
p<0.05
Adjusted for age, BMI, smoking, exercise, diet, alcohol, antidepressant use, high blood pressure, high cholesterol.
Worst trauma type coded in categories as: rape, interpersonal violence, other trauma to self, sudden death of loved one, other trauma to loved one.
Worst trauma type was not associated with log-RTL in a model adjusted for age at blood draw (Wald chi-sqdf=4=6.3, p=0.18). PTSD diagnosis remained associated with log-RTL in models further adjusted for worst trauma type (Table 3, Models 3a and 3b) and also when further adjusted for age at worst trauma (Wald chi-sqdf=1=4.0, p=0.047). To further explore these findings, we restricted to women with subclinical PTSD or PTSD diagnosis and again examined whether trauma type was associated with log-RTL. In this subgroup, type of trauma was not associated with log-RTL (df=4, chi-square=4.21, p=0.38).
PTSD in conjunction with depression was not associated with significantly different log-RTL than PTSD alone (PTSD × depression interaction term, p=0.44, Table 4). To further explore the association of PTSD, depression and log-RTL, we fitted a model of log-RTL with both PTSD and depression as independent variables, adjusted for age and age-squared. PTSD diagnosis was associated with shorter log-RTL (subclinical PTSD, β=−.03, 95% CI −.10, .04, p=0.36; PTSD diagnosis, β= −.08, 95% CI= −.16, −.003, p=0.04) but depression was not statistically significantly associated with log-RTL in this model (depression, β= −.04, 95% CI= −.01, .03, p=0.27).
Table 4.
PTSD, depression and log relative telomere length (N=116)
| Log-RTL | ||
|---|---|---|
|
| ||
| N | Mean (SD) | |
| PTSD / depression status | ||
| No PTSD, no depression | 24 | 0.53 (0.21) |
| No PTSD with depression | 1 | 0.63 (--)† |
| Subclinical PTSD, no depression | 46 | 0.51 (0.14) |
| Subclinical PTSD with depression | 20 | 0.47 (0.13) |
| PTSD diagnosis, no depression | 11 | 0.46 (0.14) |
| PTSD diagnosis with depression | 14 | 0.40 (0.12) |
Standard deviation not estimable
The association between PTSD diagnosis and log-RTL remained statistically significant in sensitivity analyses including cases from the case-control studies (n=175, subclinical PTSD, β=−.04, 95% CI=−.09, .02; PTSD diagnosis, β=−.07, 95% CI=-.13, −.01) and after excluding women selected on the basis of childhood abuse (n=96, subclinical PTSD, β=-.03, 95% CI -.10, .04; PTSD diagnosis, β= -.09, 95% CI= -.16, -.01). In analyses excluding whose worst trauma followed the blood draw and who reported low levels of PTSD symptoms, effect sizes were similar but statistical significance for PTSD diagnosis was slightly less robust (n=97, subclinical PTSD, β=−.03, 95% CI −.12, .05, p=0.45; PTSD diagnosis, β= −.10, 95% CI= −.19, .0002, p=0.05).
DISCUSSION
In this analysis of trauma-exposed women, PTSD diagnosis and PTSD symptoms were associated with shorter telomeres. Prior studies with male combat veterans (Jergović, et al., 2014; Zhang, et al., 2014) have similarly found PTSD associated with shorter telomeres. Our study extends these findings to women in the general population. Moreover, we examined a wide variety of health indicators and health-related behaviors that may account for the PTSD-TL association; we found no evidence that these factors played a role in our finding of shorter telomeres among women with PTSD. This suggests that if PTSD has a causal effect on TL, it may operate through other mechanisms. Only one previous study has examined health behaviors and conditions as factors that may explain the association between PTSD and TL (Ladwig, et al., 2013). This study also found an inverse association of PTSD with TL and found that health behaviors (smoking, BMI, alcohol consumption) and conditions (hypertension) did not account for the association (Ladwig, et al., 2013). Similarly, in the study examining internalizing disorders (including PTSD), smoking, substance dependence, psychiatric medication use, poor physical health, low socioeconomic status and childhood maltreatment did not account for shorter telomeres in participants with internalizing disorders (I Shalev, et al., 2014).
We further found that type of trauma exposure was not associated with TL and accounted for very little of the association of PTSD with TL. Prior studies examining associations of trauma exposure with TL have mostly focused on childhood abuse, with mixed findings. While several have found shorter telomeres associated with a history of childhood abuse (O'Donovan, et al., 2011; I. Shalev et al., 2013; A. R. Tyrka et al., 2010), three of the larger studies found no association (Glass, Parts, Knowles, Aviv, & Spector, 2010; Mason, et al., 2015; Verhoeven, et al., 2015). Only one of these studies also considered PTSD, finding that childhood abuse accounted for the association of PTSD with shorter telomeres (O'Donovan, et al., 2011). A Dutch study of TL before and after military deployment found no difference in change in TL between men who were exposed to high versus low levels of combat trauma (Boks, et al., 2015). This study additionally examined childhood trauma, and found no association of childhood trauma with TL or with change in TL over time. It is possible that the mixed findings in these studies, and our own finding of no association between trauma type and TL, is partly due to imprecise measurement of the extent and severity of trauma exposure across the life course.
Among women with PTSD or subclinical PTSD, we found co-occurring depression was not associated with significantly shorter telomeres. As group sizes were small when examining PTSD with and without depression, a larger study may be needed to identify a potential joint effect of PTSD and depression on TL. In models mutually adjusted for PTSD and depression, PTSD diagnosis -- and not depression -- was significantly associated with shorter telomeres. These results concur with findings that greater overall level of psychopathology may be associated with shorter TL (Bersani et al., 2016; Darrow, et al., 2016).
Our findings are subject to several limitations, including a moderate sample size and assessment of relative versus absolute TL, possibly resulting in greater measurement error. Although TL of women with PTSD were statistically significantly shorter than TL of women without PTSD, confidence intervals overlapped, thus results should be interpreted cautiously. PTSD symptoms and year of onset were assessed retrospectively, which may have introduced error in PTSD assessment. Study strengths include that PTSD cases and controls were sampled from a well-defined longitudinal cohort, and multiple health factors that may account for the association between PTSD and TL were assessed.
Given that health and health-related behaviors did not explain the association of PTSD with TL, it remains possible that PTSD affects TL through more direct biological pathways. Two such pathways are through increased oxidative stress (Richter & von Zglinicki, 2007; Wilson et al., 2013) and reduced telomerase activity. (Epel, et al., 2004) Persons with PTSD have been found to have lower expression of the antioxidative enzymes thioredoxin reductase (TXR) and the superoxide dismutases (SOD) (Zieker et al., 2007). Down-regulation of these genes could impair oxidative defense mechanisms and result in higher concentrations of reactive oxygen species (Zieker, et al., 2007), potentially leading to telomere erosion (Richter & von Zglinicki, 2007; Wilson, et al., 2013). Life stressors and perceived stress have been associated with lower telomerase activity. (Epel, et al., 2004; Schutte & Malouff, 2014b) Moreover, stress management interventions have been associated with increased telomerase activity in the short term (Schutte & Malouff, 2014a) and smaller declines in telomerase activity with ageing. (Ornish et al., 2013) Thus, to the extent there is a causal relationship, PTSD may lead to shorter telomeres via reduced telomerase activity.
Future research with appropriate study designs is needed to strengthen evidence for or against the hypothesis that PTSD causes telomere shortening and that telomere shortening is a mechanism by which PTSD is associated with incident disease. First, if PTSD causally contributes to telomere shortening, one would expect successful PTSD treatment and PTSD remission more broadly would be associated prospectively with smaller declines in TL and possibly increased telomerase activity. Second, prospective studies are needed to determine whether telomere shortening following PTSD onset is a risk factor for subsequent disease incidence. PTSD is an adverse outcome in its own right. However, findings that PTSD may lead to accelerated aging processes suggest PTSD has more far-reaching effects than have previously been appreciated, and have important implications for treatment and prevention of physical health sequelae among individuals with PTSD.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest.
References
- Bersani FS, Lindqvist D, Mellon SH, Epel ES, Yehuda R, Flory J, Henn-Hasse C, Bierer LM, Makotkine I, Abu-Amara D, Coy M, Reus VI, Lin J, Blackburn EH, Marmar C, Wolkowitz OM. Association of dimensional psychological health measures with telomere length in male war veterans. J Affect Disord. 2016;190:537–542. doi: 10.1016/j.jad.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, Keane TM. Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) National Center for Posttraumatic Stress Disorder; 1998. [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, Lerer B. Post-traumatic stress disorder and depression. An analysis of comorbidity. The British Journal of Psychiatry. 1997;170(5):479–482. doi: 10.1192/bjp.170.5.479. [DOI] [PubMed] [Google Scholar]
- Boks MP, van Mierlo HC, Rutten BP, Radstake TR, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JC. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and mortality among US army veterans 30 years after military service. Annals of Epidemiology. 2006;16(4):248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AI. Childhood obesity is associated with shorter leukocyte telomere length. The Journal of Clinical Endocrinology & Metabolism. 2011 doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Felker B, Liu C-F, Yano E, Kirchner J, Chan D, Rubenstein L, Chaney E. Prevalence of Depression–PTSD Comorbidity: Implications for Clinical Practice Guidelines and Primary Care-based Interventions. Journal of General Internal Medicine. 2007;22(6):711–718. doi: 10.1007/s11606-006-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Archives of internal medicine. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189–1195. doi: 10.1093/ntr/ntp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, Wolkowitz OM, Mathews CA. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosomatic medicine. 2016;78(7):776–787. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, De Vivo I. Physical activity, sedentary behavior, and leukocyte telomere length in women. American Journal of Epidemiology. 2012 doi: 10.1093/aje/kwr330. kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I Research Version 2.0) New York: Biometrics Research; 1996. [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post-traumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research. 2007;9(11):1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gander ML, von Kanel R. Myocardial infarction and frequency, outcome, and post-traumatic stress disorder: atherosclerotic mechanisms. European Journal of Cardiovascular Prevention & Rehabilitation. 2006;13(2):165–172. doi: 10.1097/01.hjr.0000214606.60995.46. [DOI] [PubMed] [Google Scholar]
- Gill J, Page G, Sharps P, Campbell J. Experiences of Traumatic Events and Associations with PTSD and Depression Development in Urban Health Care-seeking Women. Journal of Urban Health. 2008;85(5):693–706. doi: 10.1007/s11524-008-9290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biological Psychiatry. 2010;68(6):e21–e22. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KS, Hoerster KD, Yancy WS. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiologic Reviews. 2015;37(1):103–115. doi: 10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience letters. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiology of aging. 2012;33(7):1486, e1483–1488. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depression and Anxiety. 2010;27(12):1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, Horn PS, Nunnink SE, Baker DG. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. Bmc Medicine. 2009;7:8. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergović M, Tomičević M, Vidović A, Bendelja K, Savić A, Vojvoda V, Rac D, Lovrić-Čavar D, Rabatić S, Jovanovic T. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;54:275–283. doi: 10.1016/j.pnpbp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Jodczyk S, Fergusson DM, Horwood LJ, Pearson JF, Kennedy MA. No association between mean telomere length and life stress observed in a 30 year birth cohort. Plos One. 2014;9(5):e97102. doi: 10.1371/journal.pone.0097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler JL, Joshi K, Ma M. Hypertension in Relation to Posttraumatic Stress Disorder and Depression in the US National Comorbidity Survey. Behavioral Medicine. 2009;34(4):125–131. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, Sandler DP. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiology Biomarkers & Prevention. 2009;18(3):816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, De Vivo I, Rich-Edwards J, Smoller JW, Wright RJ, Purcell SM. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses' Health Study II. BMC Psychiatry. 2009;9:29. doi: 10.1186/1471-244X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA psychiatry. 2014;71(1):44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28(1):125–130. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of general psychiatry. 2007;64(1):109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Kulenovic AD, Kucukalic A, Malec D. Changes in plasma lipid concentrations and risk of coronary artery disease in army veterans suffering from chronic posttraumatic stress disorder. Croatian Medical Journal. 2008;49(4):506–514. doi: 10.3325/cmj.2008.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig K-H, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, Codd V, Häfner S, Albrecht E, Illig T. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. Plos One. 2013;8(7):e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, Roberts AL, Koenen KC, Karlson EW. Post-Traumatic Stress Disorder and Risk for Incident Rheumatoid Arthritis. Arthritis care & research. 2016;68(3):292–298. doi: 10.1002/acr.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Révész D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neuroscience & Biobehavioral Reviews. 2015;55(0):333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, Thorp SR, Jeste DV. Is Post-Traumatic Stress Disorder Associated with Premature Senescence? A Review of the Literature. The American Journal of Geriatric Psychiatry. 23(7):709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung F-W, Ku C-S, Kao W-T. Telomere length may be associated with hypertension. Journal of human hypertension. 2008;22(3):230–232. doi: 10.1038/sj.jhh.1002314. [DOI] [PubMed] [Google Scholar]
- Malan S, Hemmings S, Kidd M, Martin L, Seedat S. Investigation of telomere length and psychological stress in rape victims. Depression and Anxiety. 2011;28(12):1081–1085. doi: 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- Mason SM, Prescott J, Tworoger SS, DeVivo I, Rich-Edwards JW. Childhood Physical and Sexual Abuse History and Leukocyte Telomere Length among Women in Middle Adulthood. Plos One. 2015;10(6):e0124493. doi: 10.1371/journal.pone.0124493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. The American Journal of Clinical Nutrition. 2002;76(6):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiology Biomarkers & Prevention. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Friedman MJ, Ruscio AM, Karam EG, Shahly V, Stein DJ, Hill ED, Petukhova M, Alonso J, Andrade LH, Angermeyer MC, Borges G, de Girolamo G, de Graaf R, Demyttenaere K, Florescu SE, Mladenova M, Posada-Villa J, Scott KM, Takeshima T, Kessler RC. Subthreshold Posttraumatic Stress Disorder in the World Health Organization World Mental Health Surveys. Biological Psychiatry. 2015;77(4):375–384. doi: 10.1016/j.biopsych.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjäll K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity. 2008;16(12):2682–2689. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biological Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, Marlin R, Frenda SJ, Magbanua MJM, Daubenmier J, Estay I, Hills NK, Chainani-Wu N, Carroll PR, Blackburn EH. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The lancet oncology. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiology Biomarkers & Prevention. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Ferrara SD, Montisci M, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. International Journal of Cancer. 2011;129(4):983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révész D, Milaneschi Y, Verhoeven JE, Penninx BW. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism. 2014;99(12):4607–4615. doi: 10.1210/jc.2014-1851. [DOI] [PubMed] [Google Scholar]
- Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Experimental gerontology. 2007;42(11):1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic Stress Disorder and Incidence of Type 2 Diabetes Mellitus in a Sample of Women: A 22-Year Longitudinal Study. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Dohrenwend BP, Aiello A, Wright RJ, Maercker A, Galea S, Koenen KC. The stressor criterion for posttraumatic stress disorder: Does it matter? Journal of Clinical Psychiatry. 2012;73(2):264–270. doi: 10.4088/JCP.11m07054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American journal of epidemiology. 2008;167(6):653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- Savela S, Saijonmaa O, Strandberg TE, Koistinen P, Strandberg AY, Tilvis RS, Pitkälä KH, Miettinen TA, Fyhrquist F. Physical activity in midlife and telomere length measured in old age. Experimental gerontology. 2013;48(1):81–84. doi: 10.1016/j.exger.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Savolainen K, Eriksson JG, Kajantie E, Lahti J, Räikkönen K. Telomere length and hypothalamic–pituitary–adrenal axis response to stress in elderly adults. Psychoneuroendocrinology. 2015;53:179–184. doi: 10.1016/j.psyneuen.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology. 2014a;42:45–48. doi: 10.1016/j.psyneuen.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The Relationship Between Perceived Stress and Telomere Length: A Meta-analysis. Stress and health : journal of the International Society for the Investigation of Stress. 2014b doi: 10.1002/smi.2607. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depression and anxiety. 2015;32(4):229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt T, Braithwaite A, Danese A, Fleming N, Goldman-Mellor S, Harrington H, Houts R, Israel S, Poulton R. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Molecular psychiatry. 2014 doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18(5):576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong K-K. Telomere Shortening and Mood Disorders: Preliminary Support for a Chronic Stress Model of Accelerated Aging. Biological Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Solovieff N, Roberts AL, Ratanatharathorn A, Haloosim M, De Vivo I, King AP, Liberzon I, Aiello A, Uddin M, Wildman DE, Galea S, Smoller JW, Purcell SM, Koenen KC. Genetic Association Analysis of 300 Genes Identifies a Risk Haplotype in SLC18A2 for Post-traumatic Stress Disorder in Two Independent Samples. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, McGurn B, Harris SE, Whalley LJ, Deary IJ, Shiels PG. Association between telomere length and heart disease in a narrow age cohort of older people. Experimental gerontology. 2007;42(6):571–573. doi: 10.1016/j.exger.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Saijonmaa O, Tilvis RS, Pitkälä KH, Strandberg AY, Salomaa V, Miettinen TA, Fyhrquist F. Telomere length in old age and cholesterol across the life course. Journal of the American Geriatrics Society. 2011;59(10):1979–1981. doi: 10.1111/j.1532-5415.2011.03610_13.x. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MSV, Roberts AL, Agnew-Blais J, Chen Q, Cerdá M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014492. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Stampfer MJ, Franks PW, Manson JE, Rexrode KM. Healthy lifestyle and leukocyte telomere length in US women. 2012 doi: 10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological Bulletin. 2006;132(6):959. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Research. 2006;66(4):2476–2482. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biological Psychiatry. 2016;79(2):78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A, Andrew T, Gardner J, Kimura M, Oelsner E, Cherkas L, Aviv A, Spector T. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Puterman E, Elzinga B, Penninx BW. The Association of Early and Recent Psychosocial Life Stress With Leukocyte Telomere Length. Psychosomatic Medicine. 2015;77(8):882–891. doi: 10.1097/PSY.0000000000000226. [DOI] [PubMed] [Google Scholar]
- Willett W, Sampson L, Browne M, Stampfer M, Rosner B, Hennekens C, Speizer F. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and Oxidative Stress Are Elevated in the Brain, Blood, and Adrenal Glands during the Progression of Post-Traumatic Stress Disorder in a Predator Exposure Animal Model. Plos One. 2013;8(10):e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LM, van der Harst P, de Boer R, Huzen J, van Gilst W, van Veldhuisen D. Aging, telomeres and heart failure. Heart Failure Reviews. 2010;15(5):479–486. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran S, Snodgrass JG, Maranon DG, Upadhyay C, Granger DA, Bailey SM. Stress and telomere shortening among central Indian conservation refugees. Proceedings of the National Academy of Sciences. 2015;112(9):E928–E936. doi: 10.1073/pnas.1411902112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RYL, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Translational Research. 2010;155(4):166–169. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu X, Benedek D, Fullerton C, Forsten R, Naifeh J, Li X, Li H, Benevides K, Smerin S. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Molecular psychiatry. 2014;19(8):856–857. doi: 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- Zieker J, Zieker D, Jatzko A, Dietzsch J, Nieselt K, Schmitt A, Bertsch T, Fassbender K, Spanagel R, Northoff H, Gebicke-Haerter PJ. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol Psychiatry. 2007;12(2):116–118. doi: 10.1038/sj.mp.4001905. [DOI] [PubMed] [Google Scholar]