Abstract
Background
Lower leisure-time physical activity (LTPA) and higher body mass index (BMI) are independently associated with risk of heart failure (HF). However, it is unclear if this relationship is consistent for both HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF).
Objective
This study sought to quantify dose-response associations between LTPA, BMI, and the risk of different HF subtypes.
Methods
Individual-level data from 3 cohort studies (WHI, MESA, and CHS) were pooled and participants were stratified into guideline-recommended categories of LTPA and BMI. Associations between LTPA, BMI, and risk of overall HF, HFpEF (ejection fraction [EF] ≥45%) and HFrEF (EF <45%) were assessed used multivariable adjusted Cox models and restricted cubic splines.
Results
The study included 51,451 participants with 3,180 HF events (1,252 HFpEF, 914 HFrEF, 1,014 missing EF). In adjusted analysis, there was a dose-dependent association between higher LTPA levels, lower BMI, and overall HF risk. Among HF subtypes, LTPA in any dose range was not associated with HFrEF risk. In contrast, lower levels of LTPA (<500 metabolic equivalents of task [MET]-min/week) were not associated with HFpEF risk, and dose-dependent associations with lower HFpEF risk were observed at higher levels. Compared with no LTPA, higher than twice the guideline-recommended minimum LTPA levels (>1,000 MET-min/week) were associated with an 19% lower risk of HFpEF (HR: 0.81; 95% CI: 0.68 to 0.97). The dose-response relationship for BMI with HFpEF risk was also more consistent than with HFrEF risk, such that increasing BMI above the normal range (≥ 25 kg/m2) was associated with greater increase in risk of HFpEF than HFrEF.
Conclusion
Our study findings demonstrate strong, dose-dependent associations between LTPA levels, BMI, and risk of overall HF. Among HF subtypes, higher LTPA levels and lower BMI were more consistently associated with lower risk of HFpEF compared with HFrEF.
Keywords: body mass index, dose response relationship, heart failure, physical activity
Heart failure (HF) affects approximately 5.8 million people and accounts for an estimated annual health care cost of $34.8 billion (1). Heart failure with preserved ejection fraction (HFpEF) represents a common phenotype that accounts for up to 50% of HF cases and is associated with poor outcomes, similar to heart failure with reduced ejection fraction (HFrEF) (2–4). In contrast to HFrEF, in large randomized trials, several pharmacological therapies have failed to improve clinical outcomes among patients with HFpEF (5–7). Because HFpEF continues to be refractory to available therapies, the focus on primary prevention is a critical part of strategies to control the growing burden of this disease at the population level.
An important step in HFpEF prevention is to identify modifiable risk factors that can be targeted with effective preventive strategies. Lifestyle risk factors, such as physical inactivity and obesity, play an important role in development of HF (8–11). Specifically, in a recent meta-analysis, we observed a linear dose-dependent inverse relationship between leisure time physical activity (LTPA) levels and HF risk; higher levels of LTPA, in excess of the guideline-recommended minimum doses, were associated with lower risk of HF (12). Similarly, a strong dose-dependent association has been reported between higher body mass index (BMI) levels, above the normal range, and risk of HF (13). However, it is unclear if this dose-response relationship is consistent for both HFpEF and HFrEF. Thus, the contribution of different doses of LTPA and levels of BMI to different HF subtypes is not known, and therefore, the optimal target goals that should be achieved for HFpEF prevention remain undefined.
The goal of the present study was to characterize the dose-response relationship between LTPA levels, BMI, and risk of HFpEF and HFrEF using individual-level pooled data from 3 well-characterized prospective cohort studies. On the basis of our previous research showing the unique contributions of low fitness and physical inactivity to diastolic dysfunction and concentric hypertrophy, which are the key intermediate phenotypes in HFpEF development (14), we hypothesized that there would be a stronger inverse dose-response relationship between LTPA and HFpEF compared with HFrEF, such that increasing levels of physical activity (PA) would be associated with greater reduction in risk of HFpEF, as compared with HFrEF. Furthermore, on the basis of prior observations of significant positive associations between BMI and diastolic dysfunction, we also hypothesized that increasing BMI would be more strongly associated with risk of HFpEF versus risk of HFrEF (15, 16).
METHODS
Individual-level data was pooled from 3 large prospective cohort studies that reported quantitative measures of LTPA and BMI at baseline and had HFpEF and HFrEF outcome adjudication on follow-up. These included the Women’s Health Initiative (WHI), the Multiethnic Study of Atherosclerosis (MESA), and the Cardiovascular Health Study (CHS). These 3 observational cohort studies are well established, and descriptions of their design characteristics and HF outcomes were previously published (17–19).
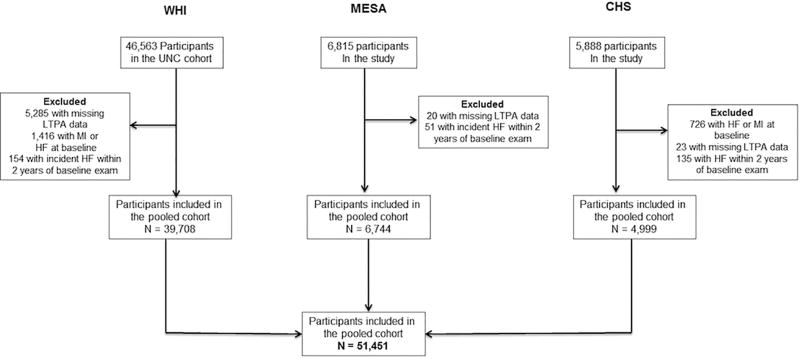
Briefly, CHS is an ongoing, prospective, community-based observational study of cardiovascular disease (CVD) risk in older adults. CHS participants included 5,888 community-dwelling older adults recruited from a random sample of Medicare-eligible older adults in 2 phases, between 1989 and 1990 and between 1992 and 1993, across 4 distinct U.S. communities. MESA is an ongoing, prospective, community-based observational study of CVD risk in multiple ethnicities that included 6,814 participants free of baseline CVD, 45 to 84 years of age, from 6 distinct U.S. communities. WHI is an ongoing observational cohort study of the occurrence and determinants of disease, including CVD, in U.S. post-menopausal women. A subset of this cohort that included participants randomized to the Hormone Trials and all black and Hispanic participants had central adjudication of HF outcome events and HF subtypes on follow-up (WHI-HF cohort, n = 46,563)(20). The present study included all participants from the 3 cohorts who, at baseline, were without known prevalent HF or myocardial infarction, had quantitative measures of LTPA and BMI, and underwent cardiovascular (CV) outcome adjudication on follow-up (Figure 1). The study excluded individuals who developed HF within 2 years of baseline LTPA assessment, to account for reverse causation for subclinical disease at baseline. Individuals with incident HF on follow-up, but missing information on ejection fraction (EF) were excluded from the HF subtype analysis evaluating associations for LTPA and BMI with risks of HFpEF and HFrEF. De-identified participant-level data was obtained from each of the 3 cohort studies after the study protocol was approved by the Institute Review Board at the University of Texas Southwestern Medical Center, Dallas, Texas and the coordinating centers for each of the 3 cohorts.
FIGURE 1. Cohort Derivation for the Pooled Analysis.
The flowchart shows the derivation of the final study population from each of the 3 cohorts: the Women’s Health Initiative (WHI); Multiethnic Study of Atherosclerosis (MESA); and Cardiovascular Health Study (CHS). HF = heart failure; LTPA = leisure time physical activity; MI = myocardial infarction; UNC = University of North Carolina.
STUDY MEASURES: EXPOSURE VARIABLES
Measurement of baseline covariates
In all 3 cohorts, participants underwent comprehensive baseline examinations that included both self-reported and measured baseline risk factors. Details of these prior measurements for each cohort were reported previously (17, 21, 22). Baseline characteristics of interest included in the present analysis were age, sex, ethnicity, annual income, education level, BMI, baseline blood pressure, history of hypertension, diabetes, smoking, and LTPA levels. BMI was calculated at the time of the baseline visit in each of the 3 cohorts as the ratio of weight to height squared (kg/m2). For the present study, BMI was categorized into clinically-meaningful categories, as defined by the National Institutes of Health (<18.5 kg/m2; 18.5 to <25 kg/m2; 25 to <30 kg/m2; 30 to <35 kg/m2; and ≥35 kg/m2 for the underweight, normal weight, overweight, obese class I, and obese class II/III categories, respectively) (23). For other baseline characteristics of interest that were reported differently across the 3 cohorts, standardized categories were used to harmonize data across the cohorts as follows: race/ethnicity (black, white, Hispanics, others); annual income (<$50,000/year, ≥50,000/year); education status (< high school, high school, some college, college +); smoking status (nonsmokers, current smoker, and former smoker); current alcohol use (yes vs. no).
LTPA measurement
Self-reported LTPA was measured at baseline in the included studies using well-validated PA questionnaires, as previously reported (24–29). In WHI, LTPA at baseline was assessed using a self-administered questionnaire which queried on usual frequency (days/week) and duration (20-min increments) of walking outside the home, as well as mild, moderate, and strenuous LTPA (30). Each type of activity was assigned a metabolic equivalent task (MET) intensity score on the basis of its energy cost (31), and PA-related energy expenditure (MET-min/week) was computed as the summed product of frequency, duration, and intensity. In CHS, baseline LTPA was assessed using a modified Minnesota Leisure-Time Activities questionnaire, which evaluated the frequency and duration of 15 different activities during a 2-week period and was used to estimate per week energy expenditure in kilocalories. MET-min/week of LTPA was calculated for CHS participants as follows: total MET-min/week = total kcal per week/(body weight [kg] X 0.0175) (27). In MESA, the Typical Week Physical Activity Survey (adapted from the Cross-Cultural Activity Participation Study (28)) was used to record frequency and duration (per session) of various types and intensities of PA in a typical week at baseline. Minutes of activity were summed for each discrete activity type and multiplied by activity-specific MET levels to determine the total MET-min/week of PA (29, 32). For the present study, the summary variable for total intentional exercise, which focused on the areas of PA recommended by the U.S. Department of Health and Human Services guidelines, was used to determine LTPA levels among the MESA study participants. Examples of different types and intensities of PA (assessed in each of the 3 cohorts) are detailed in the Online Methods.
Although PA ascertainment occurred differently in each of 3 cohorts, summary quantitative estimates of MET-min/week of LTPA calculated for participants of each cohort are comparable, are derived using standardized MET values to account for intensities as a part of PA volume, and were used in previously reported analyses (29, 30, 32–35).
Study measures: outcomes of interest
Primary outcomes of interest in this study were incidence of overall HF and its subtypes, HFpEF and HFrEF. In each of the 3 cohort studies, an expert committee of physicians adjudicated HF incidence on follow-up. In the original WHI cohort, HF events were adjudicated locally or centrally by reviewing medical records for evidence of physician-diagnosed HF. In the subset of the WHI-HF cohort that was included in the present pooled analysis, all self-reported and locally or centrally confirmed HF cases were re-adjudicated at the University of North Carolina. In MESA, criteria for adjudicating HF events were adapted from the WHI (20). In CHS, incident HF was ascertained by participant interview at semiannual study visits, medial record review, and examination of Medicare claims data, and confirmed by an expert adjudication panel. The detailed clinical criteria used to identify HF events in each of the 3 cohorts are described in the Online Methods. Adjudication of HF subtype (HFpEF vs. HFrEF) in each of the 3 cohorts (WHI-HF, MESA, CHS) was on the basis of measured EF at time of HF diagnosis, as determined by echocardiography or other cardiac imaging using the same cutoff of 45% (EF ≥45% vs. <45% for HFpEF and HFrEF, respectively) (36–39).
STATISTICAL ANALYSIS
Individual-level participant data was merged from the 3 cohorts and the pooled study participants were stratified on the basis of their LTPA levels as follows: 1) no LTPA; 2) less than minimum guideline-recommended LTPA (1– <500 MET-min/week); 3) guideline-recommended LTPA (500–1,000 MET-min/week); 4) more than guideline-recommended LTPA (>1,000 MET-min/week) (40). Participant baseline characteristics were compared across the 4 LTPA categories using the Jonckheere-Terpstra test for continuous variables and the Cochran-Armitage trend test for categorical variables. Unadjusted cumulative incidence of HF outcomes across different LPTA and BMI categories were assessed and compared using Kaplan-Meier plots and log-rank tests. To understand the biological relationship between lifestyle factors and risk of HF outcomes independent of other traditional risk factors, multivariable adjusted Cox proportional hazards models were constructed with LTPA and BMI categories as exposure variables and HF outcomes (overall HF, HFpEF, HFrEF) as dependent outcomes of interest. Separate Cox models were constructed for each HF outcome with adjustment for following covariates: Model 1: age; sex; ethnicity; education level; income; BMI; LTPA; and study cohort; Model 2: variables in Model 1 plus history of diabetes; history of hypertension; systolic blood pressure; smoking status; and current alcohol use. The relevant covariates for these Cox models were chosen on the basis of our prior knowledge from published reports about biological factors that may influence the relationship between lifestyle factors and risk of HF. Death was treated as a censoring event for all adjusted analysis. For HF subtype outcome analysis, unclassified incident HF cases were excluded, and the other HF subtype was treated as an additional censoring event. Hazard ratios (HRs) associated with different LTPA and BMI levels were compared by using linear contrast analysis to test the dose-response effect for each HF subtype outcome.
Continuous dose-response relationships between LTPA levels (MET-min/week), BMI (kg/m2), and risk of HFpEF and HFrEF were also assessed using adjusted Cox models and restricted cubic splines. Interaction testing was performed in the most adjusted Cox modes to determine if the associations of LTPA and BMI levels with HF outcomes varied across different baseline characteristics, such as sex, ethnicity, and socioeconomic indexes (education level and annual income) for different HF outcomes using the likelihood ratio test. The variables for interaction testing were chosen on the basis of previously published reports on the impact of different demographic characteristics on the relationship between lifestyle factors and risk of HF (9, 20, 39).
To account for the differences in study cohort, cohort-stratified pooled analysis was performed, evaluating the risk for HF outcomes associated with different LTPA and BMI levels. For this, separate multivariable adjusted Cox models (similar to Model 2 of the primary analysis) were constructed for each cohort to determine the risk of different HF outcomes associated with different LTPA and BMI categories. Cohort-specific HRs for different HF outcomes associated with each LTPA and BMI category were then pooled using the random effects modeling technique, as described by Dersimonian and Laird (41).
Sensitivity analysis was performed to determine the adjusted associations between LTPA, BMI, and risk of HF with missing EF data by constructing multivariable adjusted Cox models as detailed previously (Model 2). Adjusted associations between waist-hip circumference ratio (WHR), a measure of central adiposity, and risk of HF outcomes were also evaluated using multivariable adjusted Cox proportional hazards analysis.
Data analysis was performed using SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina) and STATA 10.0 (STATA Corp, College Station, Texas). All statistical tests performed in this study were 2-sided, and a p value < 0.05 was considered statistically significant.
RESULTS
The present study included 51,451 participants (77.2% from WHI, 13.1% from MESA, 9.7% from CHS) (Figure 1). The baseline characteristics of the study participants stratified by their LTPA levels are compared in Table 1. Participants with higher levels of LTPA were more commonly men, white, and had higher annual income and education levels. Furthermore, the prevalence of traditional CV risk factors, such as hypertension, diabetes mellitus, smoking, and obesity, was significantly lower with higher levels of LTPA. Baseline characteristics of study participants are also compared across different BMI categories in Online Table 1. Participants in the higher BMI groups were younger, had lower levels of LTPA, and had higher prevalence of CV risk factors, such as diabetes and hypertension.
TABLE 1.
Baseline Characteristics of the Study Participants Stratified by Their Baseline PA Levels
| Baseline Characteristics |
No LTPA (N = 10,623) |
1–499 MET- min/week (N = 17,714) |
500–1,000 MET- min/week (N = 9,349) |
>1,000 MET-min/week (N = 13,765) |
pTrend |
|---|---|---|---|---|---|
|
| |||||
| Age, yrs | 62 (56–69) | 63 (57–69) | 64 (57–70) | 64 (55 –70) | <0.0001 |
|
| |||||
| Men | 8.3 | 5.6 | 9.4 | 17.6 | <0.0001 |
|
| |||||
| Race/ethnicity | |||||
| White | 44.3 | 50.0 | 53.8 | 55.4 | <0.0001 |
| Black | 35.3 | 32.8 | 28.8 | 27.0 | <0.0001 |
| Hispanic | 16.7 | 14.3 | 13.8 | 13.6 | <0.0001 |
|
| |||||
| Annual income >$50,000/yr | 21.5 | 23.8 | 28.3 | 33.3 | <0.0001 |
|
| |||||
| Education | |||||
| < High school | 18.4 | 13.5 | 11.1 | 10.1 | <0.0001 |
| High school | 22.1 | 19.9 | 18.4 | 16.1 | <0.0001 |
| Some college | 34.1 | 36.2 | 35.1 | 33.8 | 0.09 |
| College + | 25.4 | 30.4 | 35.3 | 40.0 | <0.0001 |
|
| |||||
| Study cohort | |||||
| MESA | 14.6 | 5.9 | 11.6 | 22.2 | <0.0001 |
| CHS | 8.6 | 8.9 | 9.9 | 11.5 | <0.0001 |
| WHI | 76.8 | 85.2 | 78.5 | 66.3 | <0.0001 |
|
| |||||
| HTN status (yes) | 42.8 | 41.3 | 37.8 | 35.3 | <0.0001 |
|
| |||||
| Systolic BP mm Hg | 129 (117–141) | 129 (118–141) | 127 (116–140) | 126 (115–140) | <0.0001 |
|
| |||||
| DM status (yes) | 11.0 | 10.1 | 8.7 | 7.7 | <0.0001 |
|
| |||||
| BMI, kg/m2 | 29.9 (26.0–34.7) | 28.8 (25.4–33.1) | 27.6 (24.5–31.3) | 26.8 (23.9–30.2) | <0.0001 |
|
| |||||
| BMI categories* | |||||
| Underweight | 0.8 | 0.5 | 0.7 | 0.8 | 0.23 |
| Normal weight | 18.3 | 22.0 | 27.9 | 34.1 | <0.0001 |
| Overweight | 31.6 | 35.3 | 38.3 | 39.0 | <0.0001 |
| Obese class I | 25.8 | 24.4 | 21.5 | 17.8 | <0.0001 |
| Obese class II/III | 23.6 | 17.7 | 11.6 | 8.2 | <0.0001 |
|
| |||||
| Smoking status | |||||
| Former | 35.4 | 35.6 | 38.7 | 41.9 | <0.0001 |
| Current | 13.4 | 11.3 | 9.2 | 8.3 | <0.0001 |
|
| |||||
| Current alcohol use | 20.8 | 26.1 | 32.1 | 35.5 | <0.0001 |
|
| |||||
| PA level (MET-min/week) | 0 | 225 (120–360) | 708 (615–840) | 1,650 (1,260–2,400) | NA |
Data presented as median (interquartile range) for continuous variables and % for categorical variables.
Underweight: <18.5 kg/m2; normal weight: 18.5 to <25 kg/m2; overweight: 25 to <30 kg/m2; obese class I: 30 to <35 kg/m2 obese class II/III ≥35 kg/m2
BMI = body mass index; BP = blood pressure; CHS = Cardiovascular Health Study; DM = diabetes mellitus; HTN = hypertension; LTPA = leisure time physical activity; MESA = Multiethnic Study of Atherosclerosis; MI = myocardial infarction; PA = physical activity; WHI = Women’s Health Initiative; NA = Not Applicable
After 645,515 person-years of follow-up, 3,180 incident HF events were observed, of which 39.4% were HFpEF (n = 1,252), 28.7% were HFrEF (n = 914), and 31.9% were unclassified HF (n = 1,014). Baseline characteristics of the study participants and the follow-up HF events across the individual study cohorts are compared in Online Table 2. The WHI cohort contributed 44.8% of the incident HF events observed in our pooled analysis, whereas CHS and MESA contributed 48.1% and 7.1% of HF events, respectively.
ASSOCIATION BETWEEN LTPA LEVELS AND RISK OF OVERALL HF, HFpEF, AND HFrEF
A consistent, dose-dependent, inverse association was observed between higher LTPA levels and risk of overall HF in both the multivariable adjusted models (Table 2). Compared with no LTPA, low levels of LTPA at less than the guideline-recommended dose (<500 MET-min/week) were associated with a modest, statistically insignificant 6% lower HF risk. The magnitude of inverse association between LTPA and HF risk was stronger at higher levels of LTPA, with 11% and 22% lower HF risk at guideline-recommended (500 to 1,000 MET-min/week) and greater than guideline-recommended (>1,000 MET-min/week) doses, respectively (Table 2, Model 2). Similar findings were also observed in continuous analysis, such that a 1 SD higher LTPA was associated with an 8% lower risk of HF.
Table 2.
Adjusted Associations Between LTPA Levels and Risk of Overall HF and its Subtypes
| Outcome | Adjusted Models |
Adjusted HRs for HF Outcomes* | ||||
|---|---|---|---|---|---|---|
| No LTPA |
1–499 MET- min/week |
500–1,000 MET- min/week |
>1,000 MET- min/week |
per 1 SD higher LTP† |
||
| Overall HF | Model 1 | Ref. | 0.94 (0.85–1.03) | 0.85 (0.75–0.95) | 0.75 (0.68–0.84) | 0.91 (0.87–0.95) |
| Model 2 | Ref. | 0.94 (0.85–1.04) | 0.89 (0.79–0.998) | 0.78 (0.70–0.87) | 0.92 (0.88–0.95) | |
| HFrEF‡ | Model 1 | Ref. | 0.86 (0.72–1.04) | 0.82 (0.66–1.02) | 0.81 (0.67–0.99) | 0.94 (0.88–1.01) |
| Model 2 | Ref. | 0.88 (0.73–1.06) | 0.86 (0.70–1.07) | 0.86 (0.71–1.05) | 0.95 (0.89–1.02) | |
| HFpEF‡ | Model 1 | Ref. | 0.99 (0.84–1.15) | 0.82 (0.68 – 0.996) | 0.78 (0.66 – 0.93) | 0.90 (0.84 – 0.97) |
| Model 2 | Ref | 0.999 (0.86–1.17) | 0.87 (0.72–1.05) | 0.81 (0.68–0.97) | 0.91 (0.85–0.97) | |
Model 1 adjusted for age, sex, ethnicity, income, education, study cohort, and body mass index
Model 2 adjusted for all variables in model 1 + hypertension status, systolic BP, diabetes status, smoking, and alcohol intake
Separate Cox proportional hazard models were created for overall HF, HFpEF and HFrEF outcomes with mortality and other HF type (for HF subtype models) as censoring events
Separate models were also constructed for categorical and continuous measures of LTPA with adjustment for common covariates for each HF outcome
Incident HF patients without data on subtype classification were excluded from the HF subtype analysis
HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; MET = metabolic equivalent. Other abbreviations as in Table 1.
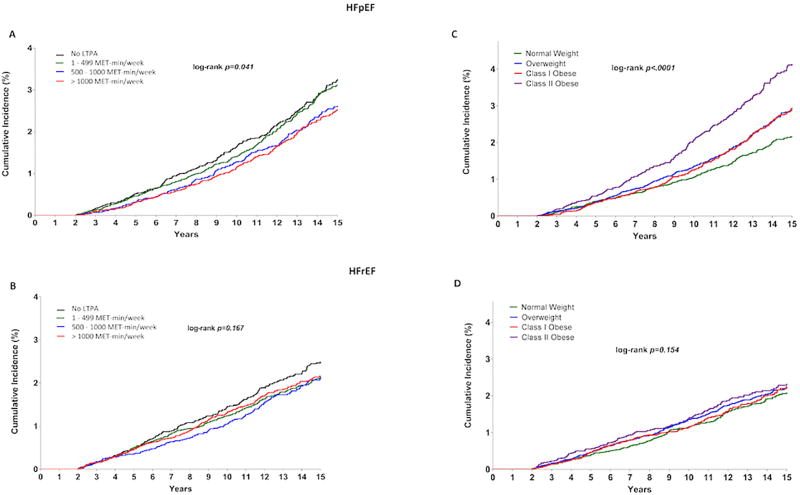
Among HF subtypes, in unadjusted comparisons, the cumulative incidence of HFpEF was significantly lower across higher LTPA categories (plog-rank = 0.04) (Figure 2A). In contrast, the association between higher levels of LTPA and cumulative risk of HFrEF was modest and not statistically significant (plog-rank = 0.167) (Figure 2B). In adjusted analysis, higher LTPA was not associated with risk of HFrEF at any dose range in both multivariable adjusted models (Table 2, Models 1 and 2). In contrast, a significant graded association was observed between higher LTPA levels and risk of HFpEF after adjustment for baseline demographic characteristics and socioeconomic status (Table 2, Model 1). The relationship was slightly attenuated with further adjustment for traditional CV risk factors but stayed significant at higher levels of LTPA above the guideline-recommended dose (Table 2, Model 2). LTPA at levels above 1,000 MET-min/week was associated with 19% lower risk of HFpEF in the most adjusted model. In linear contrast analysis comparing the HRs associated with different LTPA levels for each HF subtype, a significant dose-dependent association was observed between LTPA levels and risk of HFpEF (p trend for HR = 0.006) but not HFrEF (p trend for HR = 0.182).
FIGURE 2. Risk of HF Subtypes by LTPA and BMI Categories.
Kaplan-Meier plots here show the unadjusted cumulative incidence of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction across different LTPA A,B and BMI C,D) groups. BMI = body mass index; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LTPA = leisure time physical activity; MET = metabolic equivalent.
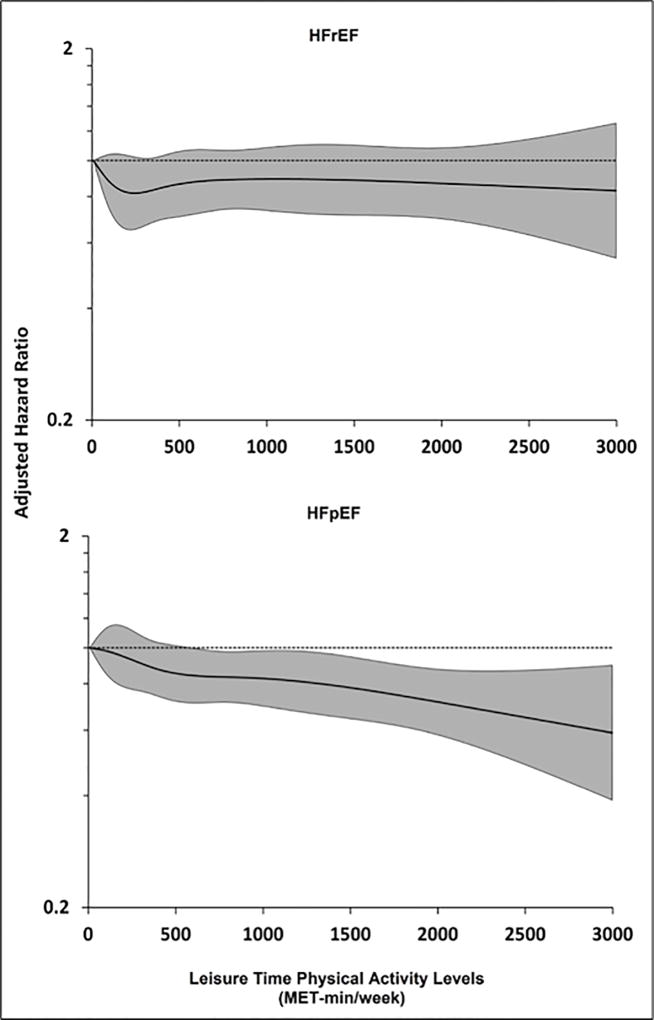
In continuous analysis using restricted cubic splines, the association between LTPA and HFrEF risk was not significant (adjusted HR: 0.95; 95% CI: 0.89 to 1.02) and did not change with increasing LTPA levels (Figure 3, top panel). In contrast, a dose-dependent inverse association was observed between LTPA levels and HFpEF risk (adjusted HR: 0.91; 95% CI: 0.85 to 0.97), with a more linear dose-response relationship observed above the guideline-recommended doses of LTPA (Figure 3, bottom panel). No significant statistical interaction was observed between LTPA levels and sex, ethnicity, education status, or income for the risk of overall HF or HF subtypes (pinteraction > 0.2 for all).
FIGURE 3. Continuous Association Between LTPA Levels and Risk of HF Subtypes.
Restricted cubic splines showing continuous adjusted association between LTPA levels (MET-min/week) and risk of HFrEF (top panel) and HFpEF (bottom panel). The shaded area shows the 95% confidence interval for the hazard ratio point estimates associated with different LTPA levels. MET = metabolic equivalent. Other abbreviations as in Figure 2.
ASSOCIATION BETWEEN BMI AND RISK OF OVERALL HF, HFpEF, AND HFrEF
Compared with participants with normal BMI, those with higher BMI were associated with a graded dose-dependent association with overall HF risk (Table 3). Among HF subtypes, in unadjusted comparisons, the cumulative incidence of HFpEF was significantly higher across higher BMI categories above the normal range (Figure 2C, p log-rank < 0.0001). In contrast, the cumulative incidence of HFrEF across higher BMI categories was not significantly different (Figure 2D, p log-rank = 0.154). Similar findings were also observed in adjusted analysis, such that risk of HFrEF was not significantly different among overweight and obese Class I participants compared with normal weight participants after adjustment for potential confounders, including demographic characteristics and prevalent CV risk factors (Table 3, Model 2). In contrast, there was a significant, dose-dependent association between BMI and HFpEF risk, such that compared with normal weight participants, overweight and obese class I participants had statistically significant 38% and 56% higher risks of HFpEF, respectively. Extremely obese participants with BMI ≥35 kg/m2 had the highest risk for both HFpEF and HFrEF (Table 3, Model 2). In linear contrast analysis comparing the HRs associated with different BMI levels for each HF subtype, a significant dose-dependent association was observed between BMI and risk of HFpEF (p trend for HR < 0.0001), but not HFrEF (p trend for HR = 0.07).
TABLE 3.
Adjusted Associations Between BMI Categories§ and Risk of Overall HF and its Subtypes
| Outcome | Adjusted Models |
Adjusted HRs for HF Outcomes* | |||||
|---|---|---|---|---|---|---|---|
| Underweight | Normal Weight |
Overweight | Obese Class I |
Obese Class II–III |
Per 1 SD higher BMI† |
||
| Overall HF | Model 1 | 1.26 (0.83 – 1.91) | Ref. | 1.22 (1.10 – 1.34) | 1.54 (1.38 – 1.72) | 2.60 (2.30 – 2.93) | 1.37 (1.32 – 1.42) |
| Model 2 | 1.28 (0.84 – 1.94) | Ref. | 1.18 (1.08 – 1.30) | 1.38 (1.24 – 1.54) | 2.19 (1.94 – 2.48) | 1.29 (1.24 – 1.34) | |
| HFrEF‡ | Model 1 | 2.04 (1.11–3.74) | Ref. | 1.07 (0.90–1.27) | 1.36 (1.11–1.65) | 1.87 (1.50–2.35) | 1.19 (1.11–1.28) |
| Model 2 | 2.16 (1.17–3.96) | Ref. | 1.03 (0.87–1.22) | 1.20 (0.98–1.46) | 1.49 (1.18–1.89) | 1.10 (1.01–1.18) | |
| HFpEF‡ | Model 1 | 0.67 (0.25–1.80) | Ref. | 1.41 (1.21–1.65) | 1.74 (1.45–2.08) | 3.28 (2.71–3.97) | 1.47 (1.39–1.55) |
| Model 2 | 0.69 (0.26–1.85) | Ref. | 1.38 (1.18–1.61) | 1.56 (1.30–1.87) | 2.72 (2.24–3.32) | 1.38 (1.30–1.46) | |
Separate Cox proportional hazard models were created for overall HF, HFpEF and HFrEF outcomes with mortality and other HF type (for HF subtype models) as censoring events
Separate models were also constructed for categorical and continuous measures of LPTA with adjustment for common covariates for each HF outcome
Incident HF patients without data on subtype classification were excluded from the HF subtype analysis
Underweight: <18.5 kg/m2; normal weight: 18.5 < 25 kg/m2; overweight: 25 to < 30.0 kg/m2; obese class I: 30.1 to < 35.0 kg/m2 obese class II/III ≥35 kg/m2
Model 1 adjusted for age, sex, ethnicity, income, education, study cohort, and LTPA levels
Model 2 adjusted for all variables in model 1 + HTN status, systolic BP, diabetes status, BMI, smoking, and alcohol intake
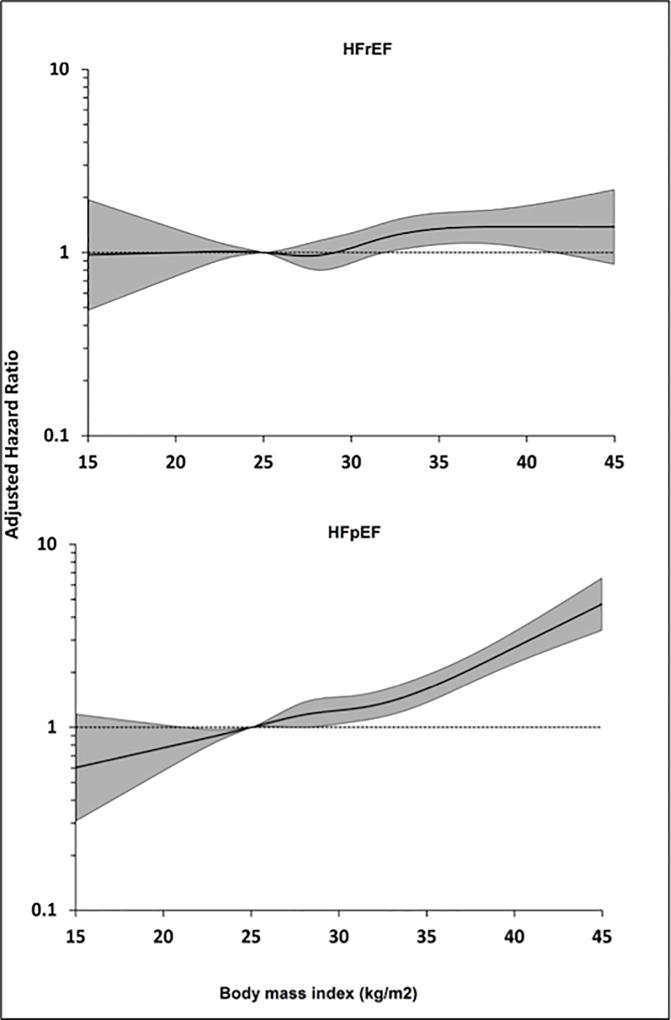
Similar findings were also observed in the continuous analysis using restricted cubic splines with a consistent dose-dependent association between BMI and risk of HFpEF, but not HFrEF (Figure 4). No significant statistical interaction was observed between BMI and ethnicity for the risk of overall HF (pinteraction = 0.17), HFpEF (pinteraction = 0.29), or HFrEF (pinteraction = 0.90). There was a modest, statistically insignificant interaction between BMI and sex for HFrEF (pinteraction = 0.06). No interaction was observed between BMI and sex for HFpEF (pinteraction = 0.92) or overall HF risk (pinteraction = 0.30).
FIGURE 4. Continuous Association Between BMI and Risk of HF Subtypes.
Restricted cubic splines showing continuous adjusted association between BMI (kg/m2) and risk of HFrEF (top panel) and HFpEF (bottom panel). The shaded area shows the 95% confidence interval for the hazard ratio point estimates associated with different LTPA levels. Abbreviations as in Figure 2.
Associations between LTPA levels, BMI, and risk of different HF outcomes in cohort-stratified analysis with pooling of adjusted HRs from each cohort were consistent with that observed in the primary analysis (Online Table 3).
Sensitivity analysis
A sensitivity analysis was performed to evaluate the association between WHR, a measure of central adiposity, and risk of HF outcomes. In adjusted analysis, a significant association between WHR and risk of overall HF, HFpEF, and HFrEF was observed, independent of other risk factors (Online Table 4). Sensitivity analysis was also performed to evaluate the association between LTPA, BMI, and risk of HF with missing EF. Overall, the pattern of associations for HF with missing EF was more like that observed for HFpEF than HFrEF. LPTA was significantly associated with lower risk of HF with missing EF at high levels, in excess of twice the guideline-recommended doses. Furthermore, a dose-dependent association was observed between higher BMI above the normal range and risk of HF with missing EF, independent of other risk factors (Online Table 5).
DISCUSSION
In this large, individual-level pooled analysis, a consistent, graded, dose-response association was observed between LTPA, BMI, and risk of overall HF. Furthermore, the dose-response relationship between LTPA and HFrEF risk was different from that for HFpEF risk. LTPA was not significantly associated with risk of HFrEF at any dose range. In contrast, there was a significant, dose-dependent inverse association between LTPA levels and HFpEF risk. Finally, the dose-response relationship for BMI and HFpEF risk was also more linear and steeper than for HFrEF risk, such that higher BMI above the normal range was associated with greater increase in risk of HFpEF versus HFrEF.
Data on quantitative dose-response associations between LTPA and HF outcomes are sparse. Anderson et al. (42) demonstrated an inverse dose-dependent association between LTPA and risk of HF in a Swedish cohort. Furthermore, they observed a plateau in the risk reduction for HF at doses higher than 3 times the guideline-recommended minimum LTPA levels, likely due to fewer HF events at the very high end of LTPA distribution. More recently, in a study-level meta-analysis of 12 cohort studies with 370,460 participants and 20,203 HF events, we demonstrated a consistent, dose-dependent, inverse association between LTPA levels and HF risk, even at very high levels of PA (12). Our study findings confirm these previously reported observations, and provide further evidence supporting a consistent, dose-dependent association of LTPA for HF prevention. This study also adds significantly to the available published data by characterizing, for the first time, the unique dose-response relationships between LTPA levels and risk of the 2 distinct HF phenotypes, HFpEF and HFrEF.
The observed differences in the dose-response relationship between LTPA and HFrEF versus HFpEF likely reflect differences in the potential mechanisms through which exercise might lower cardiovascular risk. The pattern of association between LTPA levels and HFrEF risk is similar to that reported for PA and coronary heart disease risk (43). It is possible that LTPA may lower HFrEF risk by reducing the atherosclerotic risk factor burden and associated coronary heart disease progression. In contrast, the protective effects of LTPA against HFpEF risk may be more related to the direct effect of LTPA and fitness on the key pathophysiological determinants of HFpEF development, including systemic inflammation, cardiac structure and function, visceral adiposity, and peripheral (skeletal muscle) oxygen extraction and utilization (44–48). Recent studies have demonstrated a significant association of low cardiorespiratory fitness/PA with diastolic dysfunction and decreased left ventricular compliance, 2 important cardiac determinants of HFpEF development, but not systolic function (14, 49). Similarly, studies have also demonstrated exercise training and higher LTPA levels are associated with lower visceral adiposity, lower systemic inflammation, and better skeletal muscle function (50–52). It is plausible that higher levels of LTPA, in excess of the current guideline-recommended doses, may be needed to achieve these pleiotropic effects and lower the risk of HFpEF.
We also observed a distinct dose-response relationship between BMI and risk of HFpEF and HFrEF. Higher BMI levels, above than normal range, were more strongly associated with HFpEF risk in a dose-dependent fashion compared with HFrEF risk. This finding is consistent with the findings from the Framingham Heart Study that identified BMI as a stronger predictor for HFpEF compared with HFrEF (53). Furthermore, our results are also supported by observations from longitudinal and cross-sectional cohort studies that demonstrate a strong association of general and central adiposity with measures of left ventricular diastolic dysfunction. (54–56) In contrast, the PREVEND study investigators reported similar associations between BMI and the risk of HFpEF versus HFrEF (57). There are several methodological differences between our pooled analysis and the PREVEND study that may underlie this discrepancy in study findings. These include a significantly lower number of HFpEF events, use of a higher EF cutoff for HFpEF, and lack of adjustment for PA levels in the adjusted analysis in the PREVEND study compared with our pooled analysis.
Our study findings may have important clinical and public health implications. Although HFpEF and HFrEF represent 2 distinct disease processes that have different underlying pathophysiological mechanisms and management strategies, the current guidelines for HF prevention do not have specific recommendations for lifestyle modification targeting HFpEF and HFrEF (58). Findings from our study highlight the stronger contribution of lifestyle risk factors to HFpEF risk versus HFrEF risk and suggest that the LTPA and BMI goals that must be targeted to prevent HFpEF and HFrEF may be different. Randomized controlled prevention studies are needed to further evaluate and more adequately test this hypothesis than can be done using observational cohort data, as in the present study.
Recent studies have demonstrated a temporal increase in prevalence of HFpEF relative to HFrEF and it is estimated that by 2020, up to two-thirds of hospitalized HF patients will have HFpEF (4, 59). Furthermore, evidence-based management of HFpEF patients continues to be a challenge, with several pharmacological therapies failing to improve clinical outcomes in randomized controlled trials. This scenario highlights the need for identification of additional approaches that can then be evaluated in randomized clinical trials to determine their efficacy and safety in treatment and prevention of HF subtypes. Recent studies have reported significant improvement in exercise tolerance and quality of life among HFpEF patients with nonpharmacological interventions, such as exercise training and maintenance of optimal weight (60, 61). Our study findings provide additional suggestive evidence that lifestyle interventions aiming to increase LTPA and achieve more optimal BMI goals need to be properly evaluated for use as preventive therapies for HFpEF in the at-risk population.
Several limitations to our study are noteworthy. First, owing to the observational nature of this study, there is a potential for residual or unmeasured confounding and a casual association between PA, BMI, and HF outcomes cannot be established. However, several factors, including the presence of consistent dose-response relationship for overall HF and HFpEF outcomes, biological plausibility of the observed associations, temporality in the relationship, and consistency with the previously reported associations between PA, BMI, and overall HF, enhance confidence in the internal validity of our results. Second, measurement errors in body weight, height, and inaccuracies in the self-reported PA levels might have affected our study results. However, given that the prospective study designs ensured that the PA and BMI exposures were assessed prior to ascertainment of HF cases, measurement errors would most likely be non-differential with respect to the HF subtypes, and tend to bias observed associations toward the null. It is unlikely that measurement error accounts substantially for the dose–response relationships observed in the present study. Third, we only focused on LTPA in our study, and did not evaluate occupational or total PA association with HF outcomes. This strategy was chosen a priori to allow for interpretation of our study findings in the context of the guideline recommendations on LTPA. Fourth, EF information was missing in 32% of the incident HF cases, which may have biased the results of association with HF subtypes. Fifth, we cannot completely exclude the potential for reverse causation, despite excluding individuals who developed HF within the first 2 years of the baseline examination. Finally, the high proportion of women in the pooled study population could limit the generalizability of the study findings in men. However, we did not observe any significant statistical interaction between sex and PA or BMI levels for the HF outcomes.
CONCLUSIONS
we observed a dose-response association between LTPA, BMI, and risk of HF, with a more consistent, dose-dependent association between PA, BMI, and risk of HFpEF compared with HFrEF. These findings suggest the possible preventive role of lifestyle patterns for HFpEF in the community. In addition, these findings could also have implications for future guidelines aimed at the prevention of HFpEF in the general population. Adequately designed randomized prevention trials are needed to further evaluate this hypothesis and determine its implications for clinical and public health practice.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Significant associations between lower levels of LTPA and higher BMI with the risk of developing HF are more consistent for patients with preserved than reduced left ventricular EFs.
TRANSLATIONAL OUTLOOK
Prospective trials are needed to assess the impact of increasing PA and weight reduction on the incidence of the 2 types of HF, and to explore the mechanisms responsible for the differences in impact.
Acknowledgments
The authors would like to thank the investigators of Women’s Health Initiative: Program Office (NHLBI): Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center (Fred Hutchinson Cancer Research Center): Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School) JoAnn E. Manson; (MedStar Health Research Institute/Howard University) Barbara V. Howard; (Stanford Prevention Research Center) Marcia L. Stefanick; (The Ohio State University) Rebecca Jackson; (University of Arizona) Cynthia A. Thomson; (University at Buffalo) Jean Wactawski-Wende; (University of Florida) Marian Limacher; (University of Iowa) Robert Wallace; (University of Pittsburgh) Lewis Kuller; (Wake Forest University School of Medicine) Sally Shumaker Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine) Sally Shumaker.
The authors would also like to thank the investigators of the Cardiovascular Health Study (CHS). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The authors would also like to thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: Dr. Berry receives funding from the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center, and 14SFRN20740000 from the American Heart Association prevention network. This project was done in collaboration with the American Heart Association SFRN centers for prevention at UTSW, Dallas and Northwestern University, Chicago. The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources (NCRR). The Women’s Health Initiative program was supported by the NHLBI, National Institutes of Health (NIH), U.S. Department of Health and Human Services fund through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CVD
cardiovascular disease
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- LTPA
leisure time physical activity
- MET
metabolic equivalent of task
- PA
physical activity
Footnotes
Disclosures: All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:354–65. doi: 10.1007/s11897-014-0223-7. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta-analysis. J Am Coll Cardiol. 2011;57:1676–86. doi: 10.1016/j.jacc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 8.Pandey A, Berry JD, Lavie CJ. Cardiometabolic Disease Leading to Heart Failure: Better Fat and Fit Than Lean and Lazy. Curr Heart Fail Rep. 2015;12:302–8. doi: 10.1007/s11897-015-0265-5. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 11.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, Garg S, Khunger M, et al. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132:1786–94. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Sen A, Norat T, et al. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation. 2016;133:639–49. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 14.Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail. 2014;2:238–46. doi: 10.1016/j.jchf.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2:489–99. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Eaton CB, Pettinger M, Rossouw J, et al. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circulation Heart failure. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 22.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 23.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 24.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–8. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 27.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O'Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–86. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 28.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8:805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 29.McAuley PA, Chen H, Lee DC, Artero EG, Bluemke DA, Burke GL. Physical activity, measures of obesity, and cardiometabolic risk: the Multi-Ethnic Study of Atherosclerosis (MESA) J Phys Act Health. 2014;11:831–7. doi: 10.1123/jpah.2012-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manson JE, Greenland P, LaCroix AZ, et al. Walking Compared with Vigorous Exercise for the Prevention of Cardiovascular Events in Women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Bapat A, Zhang Y, Post WS, et al. Relation of Physical Activity and Incident Atrial Fibrillation (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2015;116:883–8. doi: 10.1016/j.amjcard.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–7. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel K, Sui X, Zhang Y, et al. Prevention of heart failure in older adults may require higher levels of physical activity than needed for other cardiovascular events. Int J Cardiol. 2013;168:1905–9. doi: 10.1016/j.ijcard.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 37.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–8. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 38.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 39.Silverman MG, Patel B, Blankstein R, et al. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2016;117:1474–81. doi: 10.1016/j.amjcard.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241–6. [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Andersen K, Mariosa D, Adami HO, et al. Dose-response relationship of total and leisure time physical activity to risk of heart failure: a prospective cohort study. Circ Heart Fail. 2014;7:701–8. doi: 10.1161/CIRCHEARTFAILURE.113.001010. [DOI] [PubMed] [Google Scholar]
- 43.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–95. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey A, Patel M, Gao A, et al. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–297 e1. doi: 10.1016/j.ahj.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah RV, Murthy VL, Colangelo LA, et al. Association of Fitness in Young Adulthood With Survival and Cardiovascular Risk: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med. 2016;176:87–95. doi: 10.1001/jamainternmed.2015.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. Journal of the American College of Cardiology. 2016;68:200–3. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 2015;119:739–44. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhella PS, Hastings JL, Fujimoto N, et al. Impact of lifelong exercise "dose" on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–66. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyberg M, Gliemann L, Hellsten Y. Vascular function in health, hypertension, and diabetes: effect of physical activity on skeletal muscle microcirculation. Scand J Med Sci Sports. 2015;25(Suppl 4):60–73. doi: 10.1111/sms.12591. [DOI] [PubMed] [Google Scholar]
- 51.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Medicine and science in sports and exercise. 2008;40:1863–72. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 53.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canepa M, Strait JB, Milaneschi Y, et al. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutr Metab Cardiovasc Dis. 2013;23:1263–70. doi: 10.1016/j.numecd.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults) JACC Heart Fail. 2014;2:500–8. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai CC, Sun D, Cen R, et al. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64:1580–7. doi: 10.1016/j.jacc.2014.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 58.Heart Failure Society of A. Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 60.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.