Abstract
Snake venoms are sources of molecules with proven and potential therapeutic applications. However, most activities assayed in venoms (or their components) are of hemorrhagic, hypotensive, edematogenic, neurotoxic or myotoxic natures. Thus, other relevant activities might remain unknown. Using functional genomics coupled to the connectivity map (C-map) approach, we undertook a wide range indirect search for biological activities within the venom of the South American pit viper Bothrops jararaca. For that effect, venom was incubated with human breast adenocarcinoma cell line (MCF7) followed by RNA extraction and gene expression analysis. A list of 90 differentially expressed genes was submitted to biosimilar drug discovery based on pattern recognition. Among the 100 highest-ranked positively correlated drugs, only the antihypertensive, antimicrobial (both antibiotic and antiparasitic), and antitumor classes had been previously reported for B. jararaca venom. The majority of drug classes identified were related to (1) antimicrobial activity; (2) treatment of neuropsychiatric illnesses (Parkinson’s disease, schizophrenia, depression, and epilepsy); (3) treatment of cardiovascular diseases, and (4) anti-inflammatory action. The C-map results also indicated that B. jararaca venom may have components that target G-protein-coupled receptors (muscarinic, serotonergic, histaminergic, dopaminergic, GABA, and adrenergic) and ion channels. Although validation experiments are still necessary, the C-map correlation to drugs with activities previously linked to snake venoms supports the efficacy of this strategy as a broad-spectrum approach for biological activity screening, and rekindles the snake venom-based search for new therapeutic agents.
Keywords: Bothrops jararaca, therapeutic potential, connectivity map, drug discovery, biosimilar drugs
1. Introduction
The development of therapeutic drugs such as the antihypertensive Captopril® [1,2] and the anticoagulant Exanta® (also known as ximelagatran) [3] can be traced back to the study of isolated snake venom components and their biological roles during envenomation. Over the years, venoms, and fractions thereof, have displayed several biological activities/applications, including antibacterial [4,5,6,7,8,9,10], antiprotozoarian [7,11,12,13,14,15,16], antimeasles [17], antiviral (human immunodeficiency virus) [18,19], analgesic [20,21,22,23,24], and for the treatment of multiple sclerosis [25]. It is important to note that some of those aforementioned activities can be related not only to medium to high abundance specific venom toxins but also to low abundance components and, eventually, to their synergistic effects. Also, secondary effects generated by venom components should be considered; such is the case for the activation of inflammation and apoptosis pathways through the action of DAMPs (damage-associated molecular patterns), released after tissue injuries generated by the snake venom/snake venom fraction being assayed [26]. For instance, DAMPs released in the wound exudate after viperid envenomation contribute to vascular permeability mediated by TLR4 (toll-like receptor 4) [27].
The use of functional genomics (microarray techniques) to analyze the subtoxic effects, through gene expression analysis, on cell cultures treated with snake venoms and/or their components has been successfully demonstrated [28,29]. However, it is still challenging to associate signaling pathways identified through functional genomics to the pathophysiology of snakebite (assessed through well-established biochemical and biological assays, screening for hemorrhagic, hypotensive, edematogenic, neurotoxic, and myotoxic activities) [30]. Although these assays are useful in reproducing some of the effects of snakebite envenoming, activities other than those traditionally associated with snake venoms could remain unknown. Hence, without a priori knowledge, it is no simple task to identify potentially novel therapeutic activities derived from snake venoms and/or their components.
An alternative “blind” biological activity screening approach is to use the C-map (connectivity map) platform (https://portals.broadinstitute.org/cmap/). C-map consists in a public database of gene expression patterns generated from the treatment of known cell lineages with 1309 small molecules and drugs, whose pharmacological properties are well characterized [31,32]. Thus, the biological activity of the sample tested can be indirectly inferred by matching the experimental list of differentially expressed genes to the gene expression patterns present in the C-map database. A proof-of-concept for the application of C-map approach in Toxinololgy was demonstrated by treating MCF7 (Michigan cancer foundation 7) cells with Heloderma suspectum (Gila monster) venom or the anti-diabetic drug Byetta (developed from a peptide isolated from that same venom). As predicted, C-map analysis of differentially expressed genes in either condition displayed high positive correlation with different anti-diabetes drugs [33].
Thus, to test the feasibility of C-map analysis for biological activity screening in snake venoms, we chose the venom of the South American pit viper Bothrops jararaca, one of the best characterized venoms by proteomic approaches [34]. Although this venom is highly diverse, few protein classes account for around 94% of its composition [34] (Table 1). Consequently, the less abundant proteins such as hyaluronidases, cysteine-rich secretory proteins, growth factors, nucleotidases, among others, are underexplored [35,36], resulting in a lack of knowledge about their individual contributions to the snake envenoming pathology. Boldrini-França and colleagues [37] recently emphasized the importance of studying and characterizing minor components from snake venoms, since these can display different potential therapeutical applications, such as: antiparasitic, antitumor, neuroprotection, and ischemic tissue protection.
Table 1.
Summary information on Bothrops jararaca venom components.
| Protein Class | Associated Activities | Molecular Mass (kDa) | Relative Abundance (%) a |
|---|---|---|---|
| Metalloendopeptidase | Degrades extracelular matrix and coagulation cascade components leading to hemorrhage, edema, inflammation, and necrosis [38,39,40] | 20–100 | 33.6 |
| Serine endopeptidase | Acts on platelet aggregation, blood coagulation, fibrinolysis, complement system, blood pressure, and the nervous system [41,42,43] | 20–70 | 22.8 |
| C-type lectin/C-type lectin-like | Anticoagulant, procoagulant, agonist/antagonist of platelet activation [44] | 26–124 | 18.2 |
| Cysteine-rich secretory protein | Induces inflammatory response and affects the complement system (anaphylatoxins generation) [45,46] | 25 | 8.2 |
| Phospholipase A2 | Miotoxicity, neurotoxicity, anticoagulant effects [41,47] | 12–15 | 6.3 |
| l-amino acid oxidase | Agonist and antagonist of platelet aggregation; induces apoptosis [48] | 110–150 | 5.0 |
| Snake venom vascular endothelial growth factor | Increases vascular permeability [49,50] | 30 | 1.4 |
| Bradykinin-potentiating- and C-type-natriuretic peptides | Vasodilatation by inhibition of angiotensin-converting enzyme [1,51] | <2.5 | 1.3 |
| Phosphodiesterase | Pyrimidine and purine release, possibly contributing to the increase of vascular permeability [52,53] | 100–130 | <1.0 |
| Hyaluronidase | Degrades the hyaluronic acid present in the extracellular matrix, facilitating toxin diffusion [54] | 30–80 | <1.0 |
| Ecto-5′-nucleotidase | Pyrimidine and purines release, possibly contributing to the increase of vascular permeability [52] | 74 | <1.0 |
| Metalloendopeptidase inhibitor | Inhibits enzymatic and hemorrhagic activity of snake venom metalloendopeptidases; abundantly found in the snake’s plasma (protective mechanism) [55] | 46 | <1.0 |
| Disintegrin | Inhibits platelet aggregation [56] | 4–15 | <1.0 |
| Cobra venom factor b | Activates the complement cascade [57] | 149 | <1.0 |
| Three-finger toxin b | Neurotoxicity and cardiotoxicity effects by targeting nicotinic and muscarinic acetylcholinesterase receptors, beta-adrenergic receptors, and L-type calcium channels [58,59] | 6–8 | <1 |
In this work, we have analyzed the gene expression of MCF7 cells treated with B. jararaca venom and used connectivity mapping to infer novel (therapeutic) activities potentially present in this biological sample. The majority of biosimilar drugs inferred were related to antimicrobial and anti-inflammatory activities, as well as to the treatment of neuropsychiatric and cardiovascular diseases. In short, our data rekindle the snake venom-based search for new therapeutic agents.
2. Results and Discussion
2.1. Gene Expression Analysis
MCF7 cells were used in this work since most of the C-map database information relies on assays using this cell type, due to its extensive molecular characterization and ubiquitous use as a reference cell line [32]. However, since MCF7 cells are not natural targets for snake venom components, it was not the focus of this study to make detailed associations between differentially expressed genes and snakebite envenoming. More importantly, our goal was to submit the list of up- and down-regulated genes to C-map analysis, in order to screen for a panel of biosimilar drug activities related to B. jararaca venom. Nonetheless, we will highlight some of the differentially expressed genes and their possible correlations with snake venom toxins.
B. jararaca venom induced (p-value < 0.01) the differential expression of 90 genes (74 up- and 16 down-regulated) in MCF7 cells. We only considered up- or down-regulated genes those displaying a log2 of fold-change equal or greater than 0.58 (fold change ≥1.50) or −0.58 (fold change ≤0.67), respectively, when compared to expression in the untreated cells (control). The up- and down-regulated genes are shown as supplementary material (Tables S1 and S2, respectively) and the data used to generate these tables are supplied in Tables S5 and S6.
The cytochrome P450 family, which is represented by heme-thiolate proteins [60], displayed the highest differentially expressed gene. The CYP1A1 (cytochrome P450, family 1, subfamily A, polypeptide 1) gene had a 29.6-fold increase in expression, compared to control, when MCF7 cells were treated with venom (Table S1). Among the up-regulated genes, we also identified another member of this family, CYP1B1, with 3.4-fold increase. CYP1A1 and CYP1B1 genes are involved in the metabolism of arachidonic acid generating ROS (reactive oxygen species), which is one of the triggers to initiate the apoptosis process [61]. Even though the cytochrome P450 main function is to metabolize drugs and synthesize lipids such as cholesterol and steroids [62], its high expression in MCF7 cells treated with B. jararaca venom could also be influenced by three venom components activities through: (i) indirect involvement in the metabolism of arachidonic acid [63] eventually released after PLA2 (phospholipase A2) metabolizes phospholipids [64]; (ii) involvement in the metabolism of arachidonic acid released by the action of bradykinin, which would be possible due to the action of BPPs (bradykinin-potentiating peptides) present in snake venoms [65]; and (iii) use of hydrogen peroxide, released by the action of venom LAAO (l-amino acid oxidase), as an oxygen donor [60]. Those activities may contribute to activation of apoptosis- and inflammatory-related pathways through the generation of ROS. In this regard, the venom from another Viperidae, Echis carinatus, induced an overexpression of genes associated to ROS pathways, including the cytochrome P450 enzymes, in HUVECs (human umbilical vein endothelial cells) [66]. Additionally, B. jararaca and Crotalus atrox venoms induced a significant increase in the expression of genes related to apoptosis and inflammatory pathways in HUVECs [28]. Interestingly, these authors also showed that the proteolytic activity of jararhagin, the major hemorrhagic metalloendopeptidase from B. jararaca venom, is mandatory for the generation of an inflammatory and pro-apoptotic response in human fibroblasts [29].
The presence of oxidative stress in MCF7 cells treated with B. jararaca venom is also supported by the significantly higher expression of HMOX1 (heme oxigenase 1) (Table S1), which is an enzyme involved in antioxidant response [67]. HMOX1 degrades heme releasing antioxidant agents such as carbon monoxide and biliverdin (which is further converted to the antioxidant bilirubin) [68,69]. Thus, the higher expression of HMOX1 may represent a response to the oxidative stress induced by B. jararaca venom.
Finally, Sunitha and co-workers [26] summarized experimental evidence from the literature for oxidative stress and inflammation induced by viper bites, as well as the apparent involvement of DAMPs, generated after SVMP (snake venom metalloendopeptidase) and PLA2 activities, in these processes. Recently, it has been confirmed that at least part of the inflammatory process generated after viper bites is dependent on the activation of TLR4 pathway by DAMPs [27].
Overall, it is possible that B. jararaca venom induces apoptosis and inflammation through different pathways. The apoptotic feature of snake venoms is likely related to secondary molecules such as H2O2 released after LAAO activity and NO (nitric oxide) production. Snake venoms such as B. jararaca and B. asper are able to induce the release of inflammatory mediators like NO [70,71,72]. Although MCF7 cells do not possess the major molecular targets of snake venoms, and do not produce cytokines, it has been demonstrated that breast cancer cells, including MCF7, express inducible NO synthase [73,74,75].
2.2. Connectivity Map Analysis
We submitted the MCF7/B. jararaca venom genomic signature (list of up- and down-regulated genes following MCF7 cells treatment with venom) to the C-map algorithm for comparison with the gene-expression profiles (signatures) generated by the treatment of different cell lineages with drugs or small molecules, also called perturbagens. In short, the algorithm returns a list of perturbagens (compounds) with score values ranging from +1.000 to −1.000, encompassing the most positively- (agonistic effect) to the most negatively-(antagonistic effect) correlated perturbagens. The C-map score is calculated by a combination of the up and down scores (which represent the absolute enrichment of the list of up- and down-regulated genes, respectively) submitted to the algorithm when compared to the signatures induced by the perturbagen. The C-map score reflects how well the genomic signature induced by the assayed sample correlates with the perturbagens’ genomic signatures deposited in the database. In the original publication [31], no statistical treatment has been envisaged following the C-map score calculation. Therefore, to ensure low false discovery rates, a reasonable alternative could be to consider only the highest C-map score values (e.g., >+0.900 or <−0.900). However, when looking at the data from the literature where, following C-map analysis, biological validation assays have been performed, C-map score values for confirmed hits were as low as 0.530 [31] and −0.777 [76]. The present work established an arbitrary C-map score threshold of 0.600. On one hand, we acknowledge that, at some instances, this could eventually generate a more speculative discussion. On the other hand, our C-map results (Table S3) displayed positive hits to most of the published biological activities (related to possible therapeutical applications) directly associated to different snake venoms (Table 2), indicating that, as expected, the biological significance of the results has not been impaired by a less stringent cut-off value.
Table 2.
Hypothetical activities that could lead to therapeutical applications (identified by the present work) which have already been reported for snake venoms (or fractions thereof).
| Activity | Venom Source | Reference |
|---|---|---|
| Antibacterial | Bothrops jararaca; B. asper; B. alternatus; B. atrox; B. pirajai: Bothropoides lutzi | [4,8,9,10,77,78] |
| Anti-parasatic (trypano-, leishmani-, and plasmodicidal) | B.jararaca; B. moojeni; Crotalus adamanteus; B. jararacussu; B. asper; B. pirajai; C. durissus collilineatus; B. marajoensis; B. lutzi; C. d. cumanensis | [4,11,12,13,14,16,77,78,79,80,81,82,83,84,85] |
| Antihypertensive | B. jararaca | [1,2,86] |
| Antitumor | B. jararaca; Ophiophagus hannah; Agkistrodon acutus; Bungarus fasciatus; B. atrox; B. leucurus; C. atrox; Lachesis muta; A. contortrix laticinctus; A. halys; A. halys pallas; B. moojeni; B. pirajai; Calloselasma rhodostoma | [87,88,89,90,91] |
| Antiparkinsonian | B. atrox | [92] |
| Anti-inflammatory and/or analgesic | Naja naja; N. n. atra; C. d. terrificus; O. hannah | [20,21,22,23,24,93,94] |
| Antidiabetic | C. adamanteus; C. vegrandis; Bitis nasico; C. d. cascavella; C. d. terrificus; N. kaouthia; C. d. collilineatus | [95,96,97,98,99,100,101] |
Considering only genomic signatures generated by MCF7 cells treated with known drugs, we identified 792 positive correlations, sometimes also described as “agonist-related” activities (Table S7). The top-100 positively correlated drugs are shown in Table S3, and some of them will be discussed below. Additionally, we have rearranged the data from Table S3 according to the major findings and their applications: antimicrobial, anti-inflammatory, and treatment of neuropsychiatric or cardiovascular disorders (Table 3, Table 4, Table 5 and Table 6). The top-20 negatively correlated signatures (“antagonist-related”) are shown in Table S4.
Table 3.
C-map hits for antimicrobial drugs, following MCF7 cells incubation with Bothrops jararaca venom.
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Primaquine | 0.9 × 104 | 0.915 | 0.429 | −0.517 | Antiparasite (antimalarian activity) |
| Tanespimycin | 0.1 × 104 | 0.814 | 0.161 | −0.681 | Antineoplastic Antibiotic |
| Cefalonium | 0.9 × 104 | 0.775 | 0.250 | −0.551 | Antibiotic |
| Chlorhexidine | 0.8 × 104 | 0.743 | 0.102 | −0.667 | Antibiotic |
| Novobiocin | 1.0 × 105 | 0.737 | 0.056 | −0.706 | Antibiotic |
| Clioquinol | 1.3 × 104 | 0.737 | 0.366 | −0.396 | Antifungal and antiprotozoal |
| Erythromycin | 0.5 × 104 | 0.721 | 0.119 | −0.627 | Antibiotic |
| Tetracycline | 0.8 × 104 | 0.710 | 0.211 | −0.523 | Antibiotic |
| Piperacillin | 0.7 × 104 | 0.677 | 0.130 | −0.571 | Antibiotic |
| Ciclacillin | 1.2 × 104 | 0.675 | 0.069 | -0.629 | Antibiotic |
| Halofantrine | 0.7 × 104 | 0.675 | 0.146 | −0.552 | Antimalarial |
| Colistin | 0.3 × 104 | 0.665 | 0.167 | −0.521 | Antibiotic |
| Cefoxitin | 0.9 × 104 | 0.662 | 0.260 | −0.424 | Antibiotic |
| Minocycline | 1.1 × 104 | 0.653 | 0.143 | −0.532 | Antibiotic |
| Azlocillin | 0.8 × 104 | 0.649 | 0.099 | −0.572 | Antibiotic |
| Vancomycin | 0.3 × 104 | 0.646 | 0.096 | −0.572 | Antibiotic |
| Sulfamonomethoxine | 1.4 × 104 | 0.628 | 0.095 | −0.555 | Antibiotic |
| Dicloxacillin | 0.8 × 104 | 0.627 | 0.097 | −0.551 | Antibiotic |
| Hycanthone | 1.1 × 104 | 0.619 | 0.137 | −0.503 | Antischistosomal |
| Ribostamycin | 0.7 × 104 | 0.605 | 0.126 | −0.500 | Antibiotic |
a Values between +1 and −1 represent the relative strength of a given signature in an instance from the total set of calculated instances; b values between +1 and −1 represent the absolute enrichment of an up tag-list in a given instance; c values between +1 and −1 represent the absolute enrichment of a down tag-list in a given instance.
Table 4.
C-map hits for neuropsychiatric illnesses treatment drugs, following MCF7 cells incubation with Bothrops jararaca venom.
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Carbamazepine | 1.0 × 102 | 0.803 | 0.219 | −0.611 | Anticonvulsant (epilepsy and nerve pain treatment) |
| Thioridazine | 1.0 × 104 | 0.802 | 0.241 | −0.589 | Antipsychotic (schizophrenia treatment) |
| Prochlorperazine | 1.0 × 104 | 0.787 | 0.218 | −0.596 | Antipsychotic (schizophrenia, nonpsychotic anxiety treatment) |
| Perphenazine | 1.0 × 104 | 0.785 | 0.260 | −0.552 | Antipsychotic (schizophrenia treatment) |
| Metixene | 1.2 × 104 | 0.778 | 0.302 | −0.503 | Antiparkinsonian |
| Pirlindole | 1.2 × 104 | 0.740 | 0.140 | −0.625 | Antidepressant |
| Mianserin | 1.3 × 104 | 0.726 | 0.226 | −0.525 | Antidepressant |
| Lisuride | 1.2 × 104 | 0.722 | 0.242 | −0.505 | Antiparkinsonian |
| Mesoridazine | 0.7 × 104 | 0.721 | 0.109 | −0.637 | Antipsychotic (schizophrenia treatment) |
| Clozapine | 1.0 × 104 | 0.712 | 0.109 | −0.627 | Antipsychotic (treatment-resistant schizophrenia) |
| Trimethadione | 2.8 × 104 | 0.681 | 0.117 | −0.587 | Anticonvulsant (seizures treatment) |
| Zuclopenthixol | 0.9 × 104 | 0.673 | 0.192 | −0.505 | Antipsychotic (schizophrenia treatment) |
| Haloperidol | 1.0 × 104 | 0.669 | 0.057 | −0.635 | Antipsychotic (schizophrenia and Huntington’s disease treatment) |
| Thioproperazine | 0.6 × 104 | 0.658 | 0.160 | −0.520 | Antipsychotic (schizophrenia treatment) |
| Diclofenamide | 1.3 × 104 | 0.631 | 0.141 | −0.511 | Anticonvulsant (antiglaucoma, antiepileptic) |
| Levomepromazine | 0.9 × 104 | 0.627 | 0.214 | −0.434 | Antipsychotic (schizophrenia, anxiety treatment) |
| Fluphenazine | 1.0 × 104 | 0.623 | 0.284 | −0.361 | Antipsychotic (psychotic disorders treatment) |
| Valproic Acid | 5.0 × 104 | 0.622 | 0.174 | −0.469 | Anticonvulsant (antiepileptic) |
| Paroxetine | 0.1 × 104 | 0.604 | 0.103 | −0.522 | Antidepressant |
a Values between +1 and −1 represent the relative strength of a given signature in an instance from the total set of calculated instances; b values between +1 and −1 represent the absolute enrichment of an up tag-list in a given instance; c values between +1 and −1 represent the absolute enrichment of a down tag-list in a given instance.
Table 5.
C-map hits for cardiovascular disorders treatment drugs, following MCF7 cells incubation with Bothrops jararaca venom.
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug Type |
|---|---|---|---|---|---|
| Clopamide | 1.2 × 104 | 0.771 | 0.126 | −0.671 | Antihypertensive |
| Dobutamine | 1.2 × 104 | 0.714 | 0.126 | −0.612 | Treatment of heart failure and cardiogenic shock |
| Amrinone | 2.1 × 104 | 0.709 | 0.137 | −0.596 | Vasodilator |
| Quinidine | 1.1 × 104 | 0.702 | 0.112 | −0.614 | Arrhythmias |
| Sotalol | 1.3 × 104 | 0.679 | 0.121 | −0.582 | Arrhythmias |
| Metolazone | 1.1 × 104 | 0.673 | 0.115 | −0.581 | Antihypertensive |
| Papaverine | 1.1 × 104 | 0.652 | 0.133 | −0.542 | Vasodilator |
| Phenoxybenzamine | 1.2 × 104 | 0.637 | 0.247 | −0.413 | Antihypertensive |
| Midodrine | 1.4 × 104 | 0.625 | 0.155 | −0.492 | Antihypotensive |
| Isoprenaline | 1.6 × 104 | 0.617 | 0.148 | −0.490 | Bradycardia |
| Minoxidil | 1.9 × 104 | 0.608 | 0.092 | −0.537 | Vasodilator |
| Moracizine | 0.9 × 104 | 0.607 | 0.198 | −0.430 | Arrhythmias |
| Hydroflumethiazide | 1.2 × 104 | 0.604 | 0.131 | −0.494 | Antihypertensive |
| Tocainide | 1.7 × 104 | 0.602 | 0.150 | −0.472 | Arrhythmias |
| Practolol | 1.5 × 104 | 0.602 | 0.152 | −0.471 | Arrhythmias |
a Values between +1 and −1 represent the relative strength of a given signature in an instance from the total set of calculated instances; b values between +1 and −1 represent the absolute enrichment of an up tag-list in a given instance; c values between +1 and −1 represent the absolute enrichment of a down tag-list in a given instance.
Table 6.
C-map hits for anti-inflammatory drugs, following MCF7 cells incubation with Bothrops jararaca venom.
| C-Map Name | Dose (nM) | Score a | Up b | Down c | Drug type |
|---|---|---|---|---|---|
| Sulindac | 1.1 × 104 | 0.854 | 0.330 | −0.553 | Anti-inflammatory |
| Thalidomide | 1.0 × 105 | 0.752 | 0.145 | −0.632 | Anti-inflammatory |
| Oxyphenbutazone | 1.2 × 104 | 0.732 | 0.258 | −0.499 | Anti-inflammatory |
| Tenoxicam | 1.2 × 104 | 0.710 | 0.107 | −0.627 | Anti-inflammatory |
| Epirizole | 1.7 × 104 | 0.700 | 0.152 | −0.572 | Anti-inflammatory |
| Indoprofen | 1.4 × 104 | 0.672 | 0.071 | −0.624 | Anti-inflammatory and analgesic |
| Budesonide | 0.9 × 104 | 0.665 | 0.126 | −0.562 | Anti-inflammatory (Crohn’s Disease Treatment) |
| Methylprednisolone | 1.1 × 104 | 0.663 | 0.142 | −0.544 | Anti-inflammatory |
| Mefenamic Acid | 1.7 × 104 | 0.645 | 0.104 | −0.563 | Anti-inflammatory |
| Felbinac | 1.9 × 104 | 0.642 | 0.151 | −0.513 | Anti-inflammatory (analgesic and antipyretic) |
| Acemetacin | 1.0 × 104 | 0.627 | 0.116 | −0.532 | Anti-inflammatory |
a Values between +1 and −1 represent the relative strength of a given signature in an instance from the total set of calculated instances; b values between +1 and −1 represent the absolute enrichment of an up tag-list in a given instance; c values between +1 and −1 represent the absolute enrichment of a down tag-list in a given instance.
2.2.1. Major Drug Classes Positively Correlated to Venom through C-Map Analysis
Antimicrobial Activity
Our biosimilar drug discovery study revealed 20 antimicrobial molecules (Table 3), of which 16 were antibiotics and 4 were antiparasitics (antimalarial, antifungal/antiprotozoal, and antischistosomal).
Antibiotic activity has already been reported for B. jararaca venom against Gram-negative and Gram-positive bacteria [4], as well as in other venoms from the Bothrops genus [8,9,10]. Additionally, all these studies have associated the antibiotic activity of snake venoms to LAAO or PLA2, even though their mechanism of action remains unclear. Both enzymes, isolated from different snake venoms (including B. jararaca’s) are also frequently associated with anti-parasitic action, such as trypanocidal and leishmanicidal [4,11,13,14,77,79,80].
The second highest positively-correlated drug identified through C-map was primaquine, the only antimalarial drug available to treat malaria relapse caused by Plasmodium vivax [102,103]. This parasite presents a dormant stage (hypnozoite), which remains in the liver, creating a persistent reservoir of infection by subsequently reactivating blood-stage infections [104]. Although primaquine is the current treatment against hypnozoite forms of P. vivax, the drug has limited therapeutic efficacy [105] and is toxic to glucose-6-phosphate dehydrogenase deficient patients, due to the risk of hemolytic anemia [106]. Also, studies have indicated that some hypnozoites may be resistant to primaquine [107]. Thus, the development of more effective antimalarial treatments against hypnozoite stages of P. vivax is highly desirable [105]. Furthermore, halofantrine, another antimalarial which acts similarly to chloroquine by forming toxic complexes with ferritoporphyrin IX, thereby damaging the membrane of the parasite [108,109,110], was also inferred by our C-map data (Table 3).
Although anti-parasitic (Leishmania amazonensis, L. chagas, L. infantum, L. major, Trypanosoma cruzi, and Plasmodium falciparum) activities have already been reported for venoms (and fractions thereof) from different Bothrops genus snakes [14,15,16,78,79,81,82,83], an anti-Plasmodium activity had not yet been described specifically for B. jararaca venom. However, isolated PLA2 from snake venoms belonging to different genera, including the genus Bothrops, displayed anti-Plasmodium activity [12,16,84,85]. It is noteworthy that primaquine induces the expression of CYP1A1 [111], which was the most up-regulated gene identified in this study.
The potential antimalarial activity herein identified may also reflect an effect of HMOX-1, coded by the third most up-regulated gene identified in this work, through heme catabolism (Table S1). HMOX-1 is able to prevent apoptosis through TNF (tumor necrosis factor) pathway in Plasmodium-infected hepatocytes [67]. Studies have indicated that heme might have an important role in Plasmodium survival, especially in the mosquito and in the liver stages of infection, since the parasite is able to synthesize heme, in addition to its capability to obtain heme from the infected erythrocyte [112,113]. Furthermore, carbon monoxide released as a consequence of HMOX-1 enzymatic activity precludes the start of cerebral malaria through binding to hemoglobin released from the cells, thus preventing heme release [114,115].
In summary, some findings of this work corroborate the presence of antimalarial component(s) in B. jararaca venom. They consist of (i) the up-regulation of HMOX1 gene (Table S1) and (ii) the C-map analysis that led to the biosimilar drug discovery of the antimalarials primaquine and halofantrine (Table S3).
Neuropsychiatric Illnesses
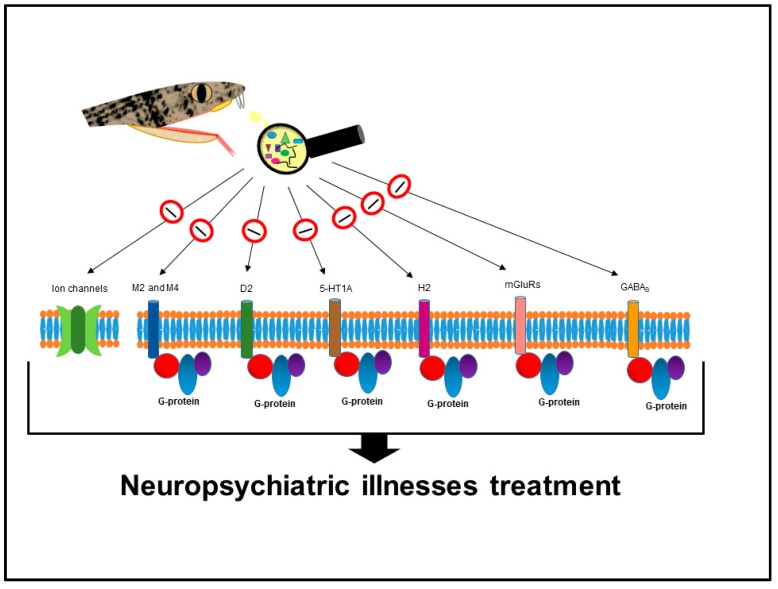
C-map analysis associated B. jararaca venom to 19 drugs used in the treatment of neuropsychiatric disorders; among those, ten antipsychotics, three antidepressants, four anticonvulsants, and two antiparkinsonian drugs (Table 4). These compounds, especially the antipsychotics, usually act on muscarinic, adrenergic, dopaminergic, serotonergic, and/or histaminergic postsynaptic receptors [116,117,118,119,120]. The aforementioned receptors belong to the GPCR (G-protein-coupled receptor) family [121] and they are involved in different cell signal transduction pathways induced by hormones and neurotransmitters [122]. Additionally, the metabotropic glutamate and GABAB (gamma-aminobutyric acid, class B) receptors are also described as potential targets for treatment of multiple disorders related to the CNS (central nervous system), such as depression, anxiety disorders, schizophrenia, epilepsy, Alzheimer’s, and Parkinson’s diseases [123,124,125,126].
The potential of snake venom components to treat CNS disorders [127] may be partially explained by the presence of neurotoxins that target muscarinic receptors [128,129,130,131] and/or other families of G-protein-coupled receptors [132,133,134,135]. Three finger toxins are widely described in venoms of members of the Elapidae family; they act on a great variety of targets, including: (i) muscle nicotinic acetylcholine receptor; (ii) neuronal nicotinic receptor; (iii) muscarinic receptor (agonist or antagonist); (iv) acetylcholinesterase (inhibitor); (v) calcium channel; (vi) potassium channel-interacting protein; and (vii) β1- and β2-adrenergic receptors [58]. Although 3FTX are primarily described for Elapidae venoms, they were recently identified, albeit in low abundance, in the venom of B. jararaca (Viperidae family) [34]. Thus, it is possible that 3FTX are responsible, at least partially, for the potential of a B. jararaca venom isolated component to treat CNS disorders. CRISPs (cysteine-rich secretory proteins) present in Viperidae venoms, including B. jararaca, may also contribute to that effect once they target different types of ion channels as well as nicotinic acetylcholine receptors [45,136].
Different drug classes to treat neuropsychiatric illnesses have been associated to the venom; their respective targets are illustrated in Figure 1.
Figure 1.
G protein–coupled receptors and ion channels potentially targeted by B. jararaca venom components, as hypothesized by C-map analysis. M2 and M4—subtypes 2 and 4 of muscarinic receptors; D2—subtype 2 of dopaminergic receptor; 5-HT1A—subtype 1A of 5-hydroxytryptamine serotonergic receptor; H2—subtype 2 of histaminic receptor; mGluRs—metabotropic glutamate receptors; GABAB—subtype B of gamma-aminobutyric acid receptor.
Antipsychotics: Antipsychotics are commonly used to treat schizophrenia primarily through dopamine receptors (especially D2) inhibition [137]. However, they also display varied affinities for serotonin, cholinergic, adrenergic, and histamine receptors [138,139]. The antipsychotics are classified in two categories, typical and atypical. Members of the former category induce high EPS (extrapyramidal side effects) such as acute dystonia, akathisia, parkinsonism, and tardive dyskinesia [140] whereas the atypical ones cause fewer EPS [141]. Clozapine was the only atypical antipsychotic drug identified in the present work. This drug is characterized by a low affinity to dopamine receptors but high affinity for 5HT2 (5-hydroxytryptamine, type 2) serotonin receptor [141,142]. Although clozapine is not the first drug of choice against schizophrenia, it is frequently used to treat drug resistance cases, when the typical antipsychotics have not worked [143,144].
Anticonvulsants: Anticonvulsants are used to treat epilepsy and seizures. Epilepsy is a multifactor neurological disorder characterized by a dysfunction in the speed and intensity of the electrical neuronal discharges leading to unprovoked seizures. Antiepileptic drugs can act in distinct manners: (i) by blocking ion channels, such as voltage activated sodium and T-type calcium channels, and/or excitatory amino acids receptors; (ii) by improving the GABA activity as a brain inhibitor [145]. We identified anticonvulsant drugs that target all those pathways: calcium channels (trimethadione), sodium channels (carbamazepine), and GABA (valproic acid). Additionally, we identified a carbonic anhydrase inhibitor (diclofenamide), which is primarily used to treat glaucoma [146]; however, it might be also used to treat epilepsy since the inhibition of carbonic anhydrase, and the consequent increase in brain CO2 level, is a known indirect pathway for epilepsy treatment [147].
Antidepressants: Symptoms of depression are common in medically sick people. However, only a few patients actually undergo a major depressive disorder. This disorder is characterized by disturbances in mood, appetite, and sleep as well as psychomotor compromise, fatigue, and suicidal thoughts, among others [148]. Dysfunctions of norepinephrine and serotonin neurotransmission are frequent in depression and anxiety disorders, which may be explained by the involvement of this neurotransmitter systems in the modulation of other neurobiological systems compromised by this illness [149]. Thus, the antidepressant drugs usually have potent effects on central noradrenergic and serotonergic systems and, in the case of the monoamine oxidase inhibitors, dopaminergic systems as well [150]. Regarding the antidepressants identified in this work, they act by inhibiting α2-adrenoceptor receptor (mianserin), serotonin (5-HT) reuptake (paroxetine), and monoamine oxidase A (pirlindole).
Parkinson’s Disease Treatment: Parkinson’s is a neurodegenerative disorder characterized by a progressive death of dopamine neurons leading to motor disturbances such as muscular rigidity, bradykinesia, and tremor [151,152]. The majority of antiparkinsonian drugs target serotonergic (5-HT1A) and dopaminergic (D2) receptors [153]; such is the case for lisuride, herein identified (Table 4). On the other hand, metixene, also identified in this work, is an anticholinergic drug [154]. As mentioned above (Section 2.2.1—Neuropsychiatric Illnesses) some neurotoxins have affinity for the muscarinic receptors [58]; this might contribute for the potential presence of antiparkinsonian activity in B. jararaca venom. Additionally, it has been shown that a tripeptide (Glu-Val-Trp) isolated from the venom of Bothrops atrox has the potential to decrease apoptosis in a classic model of Parkinson’s disease [92]. Considering that the compositions of B. atrox and B. jararaca venoms are related [155], the presence of this peptide and its neuroprotective activity in the venom of B. jararaca should be further investigated.
On the other hand, Parkinson’s disease patients typically display an accumulation of phosphorylated extracellular protein aggregates. Thus, some authors have suggested that a snake venom metalloendopeptidase, displaying a basic isoelectric point, should be able to cleave these highly phosphorylated protein aggregates, helping to slow the progression of the disease [156].
Cardiovascular Related Disorders
C-map analysis ascribed, with high positive correlation scores, antihypertensive and vasodilator activities amongst seven different drugs (Table 5). Those activities are usually associated to BPPs [1], which act by blocking the ACE (angiotensin-converting enzyme) [157,158], and had their structure used as a scaffold for development of the anti-hypertensive drug Captopril [2]. Although the hypotensive activity of BPPs is generally associated to ACE inhibition [157,158], BPP 5a from B. jararaca venom induced hypotension through muscarinic and bradykinin receptors [86], both present in MCF7 cells [159,160]. Thus, at least part of the antihypertensive activity, indirectly identified through C-map, might be related to BPP 5a. On the other hand, the antihypertensive drugs identified through C-map belong to the alpha-adrenergic blocker (phenoxybenzamine), thiazide diuretic (hydroflumethiazide), and thiazide-like diuretic (clopamide and metolazone) classes [161].
We also identified beta-1 and/or beta-2 blockers drugs (practolol and sotalol, Table 5), that are usually used to treat arrhythmias. These results suggest that B. jararaca venom could be a source of molecules acting on beta adrenergic receptors, similarly to beta-cardiotoxin, from Ophiophagus hannah venom, which blocks both beta-1 and beta-2 receptors [162]. Interestingly, we also identified beta-1 and beta-2 agonist drugs to treat heart failure/cardiogenic shock and bradycardia, respectively (Table 5).
It is important to stress that there are other snake venoms compounds such as natriuretic peptides, L-type calcium channels blockers, sarafatoxins, and vascular endothelial growth factors that display cardiovascular effects (reviewed in [163,164,165,166]). Two recent works have demonstrated the vasorelaxant effect (which is likely due to the inducing of NO production) of Montivipera bornmuelleri [167] and Crotalus durissus cascavella [168] venoms, indicating their therapeutic potential in the treatment of cardiovascular diseases such as hypertension.
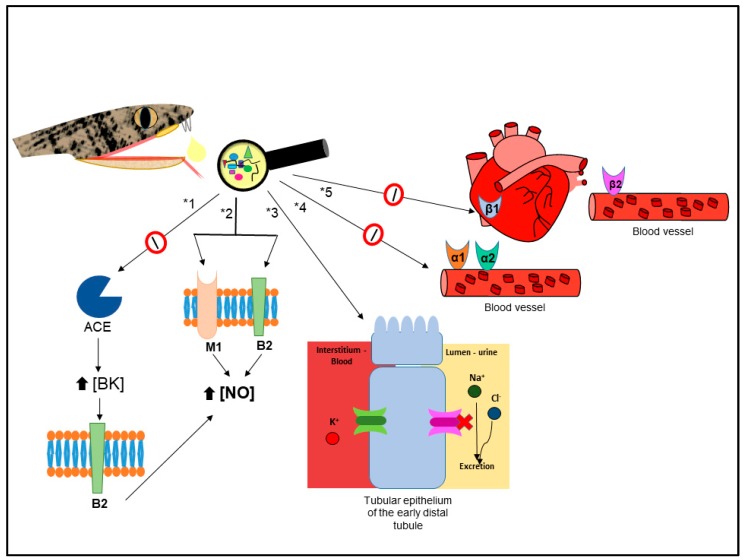
All considered, it is possible that the known antihypertensive activity of B. jararaca venom, as well as its potential to treat other cardiovascular related disorders, is more complex than the actual perception, being related to different molecules and/or mechanisms of action, as briefly proposed in Figure 2.
Figure 2.
Schematic representation of established and hypothesized (this work) mechanisms of action that contribute to the overall antihypertensive effect of B. jararaca venom. ACE—Angiotensin-converting enzyme; BK—bradykinin; B2—subtype 2 of bradykinin receptor; M1–subtype 1 of muscarinic receptor; NO–nitric oxide; α1—subtype 1 of the α-adrenergic receptor; α2–subtype 2 of the α-adrenergic receptor; β1—subtype 1 of the β-adrenergic receptor; β2—subtype 2 of the β-adrenergic receptor; *1—antihypertensive pathway based on [2]; *2—antihypertensive pathway based on [118]; *3 to *5—hypotheses, raised after C-map analysis, suggesting that B. jararaca venom may present: (*3) components acting similarly to thiazide/thiazide-like molecules; (*4) α-adrenergic receptor blockers; and (*5) components inhibiting both β1 and β2-adrenergic receptors contributing to the antihypertensive effect.
Anti-Inflammatory
The anti-inflammatory drug Sulindac displayed the third highest positive correlation with B. jararaca venom effects (Table 6). Although this activity was indirectly identified 11 times among the top-100 drugs, its presence in snake venoms is unexpected, since snake venom toxins usually have pro-inflammatory effects [26,29,169,170,171,172,173]. However, this activity was recently reported for a cytotoxic protein present in the venom of Naja naja [93], as well as for a known analgesic peptide isolated from Naja naja atra venom [94]. A possible explanation would be an indirect action of B. jararaca venom inducing the overexpression of HMOX1, which is able to degrade proinflammatory free heme, generating carbon monoxide, iron, and biliverdin [68]. Additionally, both carbon monoxide and biliverdin, as well as its final product bilirubin, have already been described as anti-inflammatory agents [174,175,176,177,178,179].
Other Relevant Potential Applications
Novel Anticancer Drugs: Antitumor activity, herein associated to three drugs against different tumor cell lineages (Table S3), has previously been reported for B. jararaca venom [87]. The antitumor activity of snake venoms may be partially due to LAAO activity. Costa and colleagues recently published a review highlighting the antitumor potential of LAAO [88]. It is hypothesized that LAAO binds preferentially to the tumor cell surface, catalyzes the release of H2O2 which, once accumulated, induces oxidative stress leading to apoptosis [89]. Recently, Fung and co-workers [90] investigated the molecular mechanisms of antitumor effect of LAAO from Ophiophagus hannah through gene expression analysis of MCF7 cells. They also observed a significant increase in expression of CYP1A1 and, to a lesser extent, of CYP1B1. The authors suggested that both the direct cytotoxic effect of H2O2 released by LAAO and the oxidative stress are likely the major leading causes of apoptosis and cell death. Nevertheless, another work [91] observed that rusvinoxidase (LAAO from Russell’s viper venom) induced apoptosis in MCF7 cells through both extrinsic and intrinsic pathways, which supports the hypothesis for different pathways leading to apoptosis in tumor cells. Although LAAO is probably a key player in the antitumor effect of snake venoms, other components such as SVMPs, disintegrins, PLA2, and C-type lectin/lectin-like proteins are known to have antiangiogenic properties and may also influence the overall antitumor activity [180,181,182,183].
Diabetes Treatment: Through C-map analysis, we identified three drugs to treat type II diabetes mellitus (Table S3). Tolbutamide belongs to the sulfonylureas antidiabetic drug class and acts by stimulating β cells of the pancreas to release insulin through the inhibition of a potent potassium channel on the β cells membrane [184]. Furthermore, troglitazone and rosiglitazone belong to the thiazolidinedione drug class which acts as an agonist of peroxisome proliferator-activated receptors (specifically PPARγ). This class of antidiabetic drug influences free fatty acid flux, thus reducing insulin resistance and blood glucose levels [185].
“Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both” [186]. Insulin secretion is modulated by the action of different hormonal and neural stimuli [95,187,188], such as through the activation of G-protein-coupled receptors [189], but also through modulation of ion channel activity [190,191].
It is well known that toxins from venomous animals are able to target a great diversity of G-protein-coupled receptors, such as glucagon receptor family as well as affect membrane excitability through ionic channels modulation [95,134,192]. Thus, the identification of antidiabetic activity was not surprising since insulinotropic properties of snake venoms have already been reported for some components such as PLA2, serine endopeptidases, disintegrins [96], crotamine [97,193], and cardiotoxin [95]. In the case of PLA2, the increase in insulin secretion is likely related to cytosolic Ca2+ [98,99,100]. On the other hand, crotamine and cardiotoxin act on potassium and sodium ion channels, respectively [95,101,193]. It is noteworthy that Byetta®, a commercial antidiabetic drug, is a glucagon-like peptide-1 receptor agonist synthesized based on the peptide exendin-4, isolated from the saliva/venom of the Gila monster (Heloderma suspecturn) [194,195]. The potential to treat type II diabetes has also been described for components of wasp [196,197], scorpion [198,199], spider [200], and bee [201] venoms.
Gastroesophageal Reflux Disease Treatment: Gastroesophageal reflux is characterized by movement of harmful gastroduodenal contents such as gastric and bile acids into the esophagus [202]. GERD (gastroesophageal reflux disease) is a condition that causes either esophageal mucosal break, or annoying symptoms such as heartburn and regurgitation, or both [203,204]. GERD is usually treated by: (i) altering gastric contents by neutralization of acid; (ii) augmenting the antireflux barrier; (iii) improving of mucosal defense mechanisms; (iv) blocking esophageal nociceptors; or (v) modulating afferent signals and their interpretation in the brain cortex [202]. In this work, we indirectly identified one of those treatment pathways: alteration of gastric contents by neutralization of acid.
The drug lansoprazole, which showed the highest positive correlation with B. jararaca venom, is a proton pump inhibitor that treats GERD by blocking the gastric acid secretion [205]. However, the identification of lansoprazole may be correlated to its ability to induce the expression of CYP1A1, observed in hepatoma cell line HepG2 [206] and hepatocytes [207]. This ability has already been ascribed to primaquine [111], which was the second highest correlated drug identified (Table S3).
Moreover, H2 (histamine, type 2) receptor antagonists such as ranitidine, another drug related to the venom by C-map, can neutralize the gastric acid secretion dependent of histamine binding to H2 [208]. It has already been shown that the venom of Bothrops moojeni induces edema through the binding of histamine, released by the degranulation of mast cells, to H2 receptor [209]. Nevertheless, as far as we know, no compound with H2 antagonist properties has been described in snake venoms so far.
Antihistamines: We identified seven antihistamine drugs (meclozine, chlorphenamine, clemizole, carbinoxamine, ketotifen, mebhydrolin, and diphenhydramine) with good positive correlation with B. jararaca venom (Table S3). All these drugs display an antagonist effect on histamine receptor (H1) [210] but some of them (meclizine and mebhydrolin) have an additional anticholinergic effect. The binding of histamine to H1 receptor induces a proinflammatory response leading to many effects associated with anaphylaxis and other allergic diseases [211] such as asthma, bronchospasm, and mucosal edema [212]. The antihistaminic activity is unexpected for B. jararaca venom since it contains molecules (e.g., PLA2 and SVMPs) that are able to induce histamine release through mastocyte degranulation [213,214,215,216], leading to increased vasodilation and vascular permeability. However, considering that snake venoms can display ambivalent actions such as pro- and anti-coagulant effects or possess both agonists and antagonists of platelet aggregation [41,217], we could hypothesize that snake venoms could display antihistamine activity. It is noteworthy that MCF7 cells express both histamine H1 and H2 receptors [218].
2.2.2. Major Drug Classes Negatively Correlated to Venom through C-Map Analysis
As previously mentioned, we have also generated a list of negatively correlated genomic signatures following MCF7 cells treatment with the venom (Tables S4 and S7). Although the interpretation of these results is not self-evident, we will comment on some of the hits obtained. For instance, oxymetazoline is a decongestant which acts as an alpha adrenergic agonist [219]. Since there was a negative correlation to venom, one could expect the presence of adrenergic antagonists (blockers). This is consistent with the data discussed in Section 2.2.1—Cardivascular Related Disorders, linking the venom to antihypertensive compounds. Another high-ranking hit was Trapidil, a PDGF (platelet-derived growth factor) antagonist. Although we could not find in the literature a PDGF agonist related to snake venoms, it has been shown that aggretin (a C-type lectin from Calloselasma rhodostoma venom [220]) phosphorylates PDGF receptor beta, leading eventually to PDGF-BB production [221]. Three anti-inflammatory- and one antihistaminic-related drugs could represent the known pro-inflammatory and histamine release activities related to bothropic venoms, which were discussed above (Section 2.2.1—Anti-Inflammatory and Section 2.2.1—Other Relevant Potential Applications: Antihistamines).
3. Conclusions
We aimed the exploration of novel potential therapeutic activities in B. jararaca venom through gene expression analysis allied to biological screening using connectivity mapping. The identification of drugs with activities (e.g., antihypertensive, antimicrobial, and antitumoral) previously reported for high abundance components of snake venoms, especially in B. jararaca, supported the efficacy of C-map as an unbiased exploratory approach for biological activity screening, and rekindles the snake venom-based search for new therapeutic agents. Moreover, this work indicated the existence of active venom components that could potentially be used in the treatment of other disorders (e.g., schizophrenia, depression, epilepsy, and gastroesophageal disease). However, those “newfound” activities should be assayed for in vitro and in vivo (eventually) confirmations, followed by venom fractionation in order to determine the molecular species associated to them. Furthermore, venom prefractionation could be performed and individual fractions submitted to C-map analysis; one such approach would be to assay the complex B. jararaca venom peptidome, recently described in the literature [34]. This peptidome is composed of hundreds of relatively short peptides (9 to 10 amino acids long, on average) that could prove a rich bioactive peptide library. In summary, the present work paves the way for further studies exploring the therapeutic potential of snake venoms by providing a rich set of novel activities to be assayed beyond the classical ones (e.g., hemorrhage, myotoxicity, hypotension).
4. Materials and Methods
4.1. Venom
Lyophilized venom, a pool from several juvenile/adult, male/female Bothrops jararaca specimens, was kindly provided by Instituto Butantan (São Paulo, Brazil). The access to Brazilian fauna genetic heritage was issued by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under license number 010578/2014-5.
4.2. Tissue Culture
MCF7 cells were obtained from HTB022TMAmerican Type Culture Collection, Manassas, VA and grown in Dulbecco’s modified Eagle medium containing 0.01 mg/mL bovine insulin and 10% fetal bovine serum. MCF7 cells were passed and grown to 80% confluence in medium.
4.3. MCF7 Cells Treatment with B. jararaca Venom
Initially, one milligram of B. jararaca venom was dissolved in 1 mL of MCF7 medium. Based on previous results with HUVECs [29], four different concentrations (1, 2, 5, and 10 μg/mL) were tested. We then chose the highest concentration (5 μg/mL) at which no overt phenotypic changes were observed in the MCF7 cells, and added 1mL of this solution to each well on a six-well plate (85.20 mm × 127.80 mm). After that, the cells were incubated for 6 h at 37 °C. A plate containing only cells in 1 mL of media was assayed as control. All experiments were performed in duplicate.
4.4. Gene Expression Analysis
The total RNA was extracted from the cells using the RNeasy mini kit (Quiagen, Hilden, Germany, cat. no. 74104) following the manufacturer’s instructions. The sense strand DNA was generated from cRNA, fragmented, and labeled for hybridization to HuGene ST 2.0 array (Affimetrix, lot. 4265888, Ref. 902112, Thermo Fisher Scientific, Waltham, MA, USA). The samples were hybridized to the chips overnight and washed and stained using Affymetrix’s Fluidics Station 450 (P/N 00-0079, Affymetrix, Santa Clara, CA, USA) and the GeneChip Hybridization, Wash and Stain kit (P/N 900720, Affymetrix) following the manufacturer’s instructions. The chips were scanned using Affymetrix’s GeneChip Scanner 3000 7G (p/n 00-0213, Affymetrix, Santa Clara, CA, USA). Four chips were run for the two experimental groups (venom and control) assayed in duplicate, as aforementioned.
4.5. Bioinformatics Analysis
The gene expression analysis, to determine changes in transcripts following MCF7 cell treatment with B. jararaca in comparison to untreated cells, was carried out as previously described [33]. Furthermore, we also used the C-map software build 02 (https://portals.broadinstitute.org/cmap/) to query the probe sets of significantly differentially expressed genes with the perturbagen signatures present in the C-map database. Initially, we converted the probe sets from HuGene ST 2.0 to HGU133A dataset, which is compatible with the C-map database, using the Affymetrix tool that provided a best match between the two chip types.
Afterwards, the algorithm returned a ranked list of all perturbagens found in the C-map database along with scores indicating their relation to the venom. The top-100 positively correlated drugs identified through C-map were submitted to the website Drugbank, available on query (https://www.drugbank.ca/, (accessed on 12 June 2017) [222], to retrieve information about their mechanisms of action. The same was done to the top-20 negatively correlated drugs.
Acknowledgments
This study was supported by PAPES VI/FIOCRUZ grant number 407611/2012-6 and CAPES grant AUXPE 1214/2011. At the time this work was conducted, C.A.N. was a Ph.D. student enrolled in the Cellular and Molecular Biology Graduate Program (Oswaldo Cruz Foundation, FIOCRUZ, Brazil) with a fellowship from CAPES (AUXPE 1214/2011). During that time, she was awarded a 4-month period sandwich fellowship from CAPES (grant number BEX 2832/15-1) to perform experiments at the University of Virginia, USA. A.G.d.C.N-F is a CNPq fellow (311539/2015-7).
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6651/10/2/69/s1, Table S1: Up-regulated MCF7 genes after Bothrops jararaca venom treatment, Table S2: Down-regulated MCF7 genes after Bothrops jararaca venom treatment, Table S3: Top-100 positively correlated C-map hits for drug-related activities potentially present in Bothrops jararaca venom, Table S4: Top-20 negatively correlated C-map hits for drug-related activities potentially present in Bothrops jararaca venom, Table S5: Gene expression profiles induced by Bothrops venom on MCF7 cells, Table S6: Full signal intensities obtained by Bothrops jararaca venom and control (only MCF7 cells), and Table S7: Full C-map hits for drug-related activities identified for Bothrops jararaca venom (only MCF7 cells).
Author Contributions
J.W.F., R.H.V., and C.A.N. conceived and designed the experiments; C.A.N. and A.P. performed the experiments; C.A.N., R.H.V., and A.G.d.C.N-F. analyzed the data; Y.B. contributed reagents/materials/analysis tools; C.A.N. and R.H.V. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This work has applied connectivity mapping for a biological activity screen in snake venom, leading to the discovery of several new activities with possible therapeutical applications.
References
- 1.Ferreira S.H., Bartelt D.C., Greene L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2593. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 2.Cushman D.W., Cheung H.S., Sabo E.F., Ondetti M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16:5484–5491. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- 3.Hrebickova L., Nawarskas J.J., Anderson J.R. Ximelagatran: A new oral anticoagulant. Heart Dis. 2003;5:397–408. doi: 10.1097/01.hdx.0000099777.39577.e8. [DOI] [PubMed] [Google Scholar]
- 4.Ciscotto P., Machado de Avila R.A., Coelho E.A., Oliveira J., Diniz C.G., Farias L.M., de Carvalho M.A., Maria W.S., Sanchez E.F., Borges A., et al. Antigenic, microbicidal and antiparasitic properties of an L-amino acid oxidase isolated from Bothrops jararaca snake venom. Toxicon. 2009;53:330–341. doi: 10.1016/j.toxicon.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Bustillo S., Leiva L.C., Merino L., Acosta O., Joffé E.B.d.K., Gorodner J.O. Antimicrobial activity of Bothrops alternatus venom from the Northeast of Argentine. Rev. Latinoam. Microbiol. 2008;50:79–82. [Google Scholar]
- 6.Torres A.F.C., Dantas R.T., Menezes R.R.P.P.B., Toyama M.H., Oliveira M.F., Nogueira N.A.P., Oliveira M.R., Monteiro H.S.A., Martins A.M.C. Antimicrobial activity of an l-amino acid oxidase isolated from Bothrops leucurus snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010;16:614–622. doi: 10.1590/S1678-91992010000400012. [DOI] [Google Scholar]
- 7.De Melo Alves Paiva R., de Freitas Figueiredo R., Antonucci G.A., Paiva H.H., de Lourdes Pires Bianchi M., Rodrigues K.C., Lucarini R., Caetano R.C., Linhari Rodrigues Pietro R.C., Gomes Martins C.H., et al. Cell cycle arrest evidence, parasiticidal and bactericidal properties induced by l-amino acid oxidase from Bothrops atrox snake venom. Biochimie. 2011;93:941–947. doi: 10.1016/j.biochi.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Paramo L., Lomonte B., Pizarro-Cerda J., Bengoechea J.A., Gorvel J.P., Moreno E. Bactericidal activity of Lys49 and Asp49 myotoxic phospholipases A2 from Bothrops asper snake venom—Synthetic Lys49 myotoxin II-(115-129)-peptide identifies its bactericidal region. Eur. J. Biochem. 1998;253:452–461. doi: 10.1046/j.1432-1327.1998.2530452.x. [DOI] [PubMed] [Google Scholar]
- 9.Stabeli R.G., Marcussi S., Carlos G.B., Pietro R.C., Selistre-de-Araujo H.S., Giglio J.R., Oliveira E.B., Soares A.M. Platelet aggregation and antibacterial effects of an l-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorg. Med. Chem. 2004;12:2881–2886. doi: 10.1016/j.bmc.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria C., Larios S., Quiros S., Pizarro-Cerda J., Gorvel J.P., Lomonte B., Moreno E. Bactericidal and antiendotoxic properties of short cationic peptides derived from a snake venom Lys49 phospholipase A2. Antimicrob. Agents Chemother. 2005;49:1340–1345. doi: 10.1128/AAC.49.4.1340-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempone A.G., Andrade H.F., Jr., Spencer P.J., Lourenco C.O., Rogero J.R., Nascimento N. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its L-amino acid oxidase. Biochem. Biophys. Res. Commun. 2001;280:620–624. doi: 10.1006/bbrc.2000.4175. [DOI] [PubMed] [Google Scholar]
- 12.Zieler H., Keister D.B., Dvorak J.A., Ribeiro J.M. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J. Exp. Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]
- 13.Franca S.C., Kashima S., Roberto P.G., Marins M., Ticli F.K., Pereira J.O., Astolfi-Filho S., Stabeli R.G., Magro A.J., Fontes M.R., et al. Molecular approaches for structural characterization of Bothrops L-amino acid oxidases with antiprotozoal activity: CDNA cloning, comparative sequence analysis, and molecular modeling. Biochem. Biophys. Res. Commun. 2007;355:302–306. doi: 10.1016/j.bbrc.2006.12.217. [DOI] [PubMed] [Google Scholar]
- 14.Deolindo P., Teixeira-Ferreira A.S., Melo E.J., Arnholdt A.C., Souza W., Alves E.W., DaMatta R.A. Programmed cell death in Trypanosoma cruzi induced by Bothrops jararaca venom. Mem. Inst. Oswaldo Cruz. 2005;100:33–38. doi: 10.1590/S0074-02762005000100006. [DOI] [PubMed] [Google Scholar]
- 15.Deolindo P., Teixeira-Ferreira A.S., DaMatta R.A., Alves E.W. L-amino acid oxidase activity present in fractions of Bothrops jararaca venom is responsible for the induction of programmed cell death in Trypanosoma cruzi. Toxicon. 2010;56:944–955. doi: 10.1016/j.toxicon.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Castillo J.C., Vargas L.J., Segura C., Gutierrez J.M., Perez J.C. In vitro antiplasmodial activity of phospholipases A2 and a phospholipase homologue isolated from the venom of the snake Bothrops asper. Toxins. 2012;4:1500–1516. doi: 10.3390/toxins4121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petricevich V.L., Mendonca R.Z. Inhibitory potential of Crotalus durissus terrificus venom on measles virus growth. Toxicon. 2003;42:143–153. doi: 10.1016/S0041-0101(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 18.Fenard D., Lambeau G., Valentin E., Lefebvre J.C., Lazdunski M., Doglio A. Secreted phospholipases A(2), a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Investig. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y.J., Wang J.H., Lee W.H., Wang Q., Liu H., Zheng Y.T., Zhang Y. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem. Biophys. Res. Commun. 2003;309:598–604. doi: 10.1016/j.bbrc.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi R., Bernardi M.M., Cury Y. Analgesic effect evoked by low molecular weight substances extracted from Crotalus durissus terrificus venom. Toxicon. 1993;31:1257–1265. doi: 10.1016/0041-0101(93)90399-4. [DOI] [PubMed] [Google Scholar]
- 21.Pu X.C., Wong P.T., Gopalakrishnakone P. A novel analgesic toxin (hannalgesin) from the venom of king cobra (Ophiophagus hannah) Toxicon. 1995;33:1425–1431. doi: 10.1016/0041-0101(95)00096-5. [DOI] [PubMed] [Google Scholar]
- 22.Mancin A.C., Soares A.M., Andriao-Escarso S.H., Faca V.M., Greene L.J., Zuccolotto S., Pela I.R., Giglio J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon. 1998;36:1927–1937. doi: 10.1016/S0041-0101(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.X., Zhang H.L., Gu Z.L., Chen B.W., Han R., Reid P.F., Raymond L.N., Qin Z.H. A long-form alpha-neurotoxin from cobra venom produces potent opioid-independent analgesia. Acta Pharmacol. Sin. 2006;27:402–408. doi: 10.1111/j.1745-7254.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W.J., Liang Y.X., Han L.P., Qiu P.X., Yuan J., Zhao S.J. Purification and characterization of a novel antinociceptive toxin from Cobra venom (Naja naja atra) Toxicon. 2008;52:638–646. doi: 10.1016/j.toxicon.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Dhanak A.C., Rishipathak D.D., Gide P.S. Multiple sclerosis & it´s treatment with alpha-cobratoxin: A review. Int. J. PharmTech Res. 2010;2:740–749. [Google Scholar]
- 26.Sunitha K., Hemshekhar M., Thushara R.M., Santhosh M.S., Sundaram M.S., Kemparaju K., Girish K.S. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon. 2015;98:89–97. doi: 10.1016/j.toxicon.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Rucavado A., Nicolau C.A., Escalante T., Kim J., Herrera C., Gutierrez J.M., Fox J.W. Viperid envenomation wound exudate contributes to increased vascular permeability via a DAMPs/TLR-4 mediated pathway. Toxins. 2016;8:349. doi: 10.3390/toxins8120349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher P.G., Bao Y., Serrano S.M., Kamiguti A.S., Theakston R.D., Fox J.W. Use of microarrays for investigating the subtoxic effects of snake venoms: Insights into venom-induced apoptosis in human umbilical vein endothelial cells. Toxicon. 2003;41:429–440. doi: 10.1016/S0041-0101(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher P., Bao Y., Serrano S.M., Laing G.D., Theakston R.D., Gutierrez J.M., Escalante T., Zigrino P., Moura-da-Silva A.M., Nischt R., et al. Role of the snake venom toxin jararhagin in proinflammatory pathogenesis: In vitro and in vivo gene expression analysis of the effects of the toxin. Arch. Biochem. Biophys. 2005;441:1–15. doi: 10.1016/j.abb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Fox J.W. Insights in to Venom and Toxin Activities and Pharmacological/Therapeutic Potential Using Gene Expression Profiling. In: Kini R.M., Clemetson K.J., Markland F.S., McLane M.A., Morita T., editors. Toxins and Hemostasis from the Bench to Bedside. Springer; Dordrecht, The Netherlands: 2010. pp. 73–81. [Google Scholar]
- 31.Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.P., Subramanian A., Ross K.N., et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 32.Lamb J. The Connectivity Map: A new tool for biomedical research. Nat. Rev. Cancer. 2007;7:54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- 33.Aramadhaka L.R., Prorock A., Dragulev B., Bao Y., Fox J.W. Connectivity maps for biosimilar drug discovery in venoms: The case of Gila monster venom and the anti-diabetes drug Byetta(R) Toxicon. 2013;69:160–167. doi: 10.1016/j.toxicon.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Nicolau C.A., Carvalho P.C., Junqueira-de-Azevedo I.L., Teixeira-Ferreira A., Junqueira M., Perales J., Neves-Ferreira A.G., Valente R.H. An in-depth snake venom proteopeptidome characterization: Benchmarking Bothrops jararaca. J. Proteom. 2017;151:214–231. doi: 10.1016/j.jprot.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Fox J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon. 2013;62:75–82. doi: 10.1016/j.toxicon.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 36.McCleary R.J., Kini R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon. 2013;62:56–74. doi: 10.1016/j.toxicon.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Boldrini-Franca J., Cologna C.T., Pucca M.B., Bordon K.C., Amorim F.G., Anjolette F.A., Cordeiro F.A., Wiezel G.A., Cerni F.A., Pinheiro-Junior E.L., et al. Minor snake venom proteins: Structure, function and potential applications. Biochim. Biophys. Acta. 2017;1861:824–838. doi: 10.1016/j.bbagen.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Bjarnason J.B., Fox J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 39.Jia Y., Perez J.C. Molecular cloning and characterization of cDNAs encoding metalloproteinases from snake venom glands. Toxicon. 2010;55:462–469. doi: 10.1016/j.toxicon.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox J.W., Serrano S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Kini R.M. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005;45:1147–1161. doi: 10.1016/j.toxicon.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Serrano S.M., Maroun R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon. 2005;45:1115–1132. doi: 10.1016/j.toxicon.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Kini R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006;397:377–387. doi: 10.1042/BJ20060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du X.-Y., Clemetson K.J. Reptile C-Type Lectins. In: Mackessy S.P., editor. Handbook of Venoms and Toxins of Reptiles. CRC Press; New York, NY, USA: 2010. pp. 359–375. [Google Scholar]
- 45.Yamazaki Y., Morita T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon. 2004;44:227–231. doi: 10.1016/j.toxicon.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Lodovicho M.E., Costa T.R., Bernardes C.P., Menaldo D.L., Zoccal K.F., Carone S.E., Rosa J.C., Pucca M.B., Cerni F.A., Arantes E.C., et al. Investigating possible biological targets of Bj-CRP, the first cysteine-rich secretory protein (CRISP) isolated from Bothrops jararaca snake venom. Toxicol. Lett. 2017;265:156–169. doi: 10.1016/j.toxlet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Kini R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Du X.Y., Clemetson K.J. Snake venom L-amino acid oxidases. Toxicon. 2002;40:659–665. doi: 10.1016/S0041-0101(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 49.Junqueira de Azevedo I.L., Farsky S.H., Oliveira M.L., Ho P.L. Molecular cloning and expression of a functional snake venom vascular endothelium growth factor (VEGF) from the Bothrops insularis pit viper. A new member of the VEGF family of proteins. J. Biol. Chem. 2001;276:39836–39842. doi: 10.1074/jbc.M106531200. [DOI] [PubMed] [Google Scholar]
- 50.Junqueira-de-Azevedo Ide L., da Silva M.B., Chudzinski-Tavassi A.M., Ho P.L. Identification and cloning of snake venom vascular endothelial growth factor (svVEGF) from Bothrops erythromelas pitviper. Toxicon. 2004;44:571–575. doi: 10.1016/j.toxicon.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Higuchi S., Murayama N., Saguchi K., Ohi H., Fujita Y., Camargo A.C., Ogawa T., Deshimaru M., Ohno M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology. 1999;44:129–135. doi: 10.1016/S0162-3109(99)00119-8. [DOI] [PubMed] [Google Scholar]
- 52.Aird S.D. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393. doi: 10.1016/S0041-0101(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 53.Santoro M.L., Vaquero T.S., Leme A.F., Serrano S.M. NPP-BJ, a nucleotide pyrophosphatase/phosphodiesterase from Bothrops jararaca snake venom, inhibits platelet aggregation. Toxicon. 2009;54:499–512. doi: 10.1016/j.toxicon.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Kemparaju K., Girish K.S. Snake venom hyaluronidase: A therapeutic target. Cell Biochem. Funct. 2006;24:7–12. doi: 10.1002/cbf.1261. [DOI] [PubMed] [Google Scholar]
- 55.Valente R.H., Dragulev B., Perales J., Fox J.W., Domont G.B. BJ46a, a snake venom metalloproteinase inhibitor. Isolation, characterization, cloning and insights into its mechanism of action. Eur. J. Biochem. 2001;268:3042–3052. doi: 10.1046/j.1432-1327.2001.02199.x. [DOI] [PubMed] [Google Scholar]
- 56.Calvete J.J. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Vogel C.W., Muller-Eberhard H.J. Cobra venom factor: Improved method for purification and biochemical characterization. J. Immunol. Methods. 1984;73:203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- 58.Kini R.M., Doley R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon. 2010;56:855–867. doi: 10.1016/j.toxicon.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Fry B.G., Wuster W., Kini R.M., Brusic V., Khan A., Venkataraman D., Rooney A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003;57:110–129. doi: 10.1007/s00239-003-2461-2. [DOI] [PubMed] [Google Scholar]
- 60.Hrycay E.G., Bandiera S.M. The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch. Biochem. Biophys. 2012;522:71–89. doi: 10.1016/j.abb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Galluzzi L., Kepp O., Kroemer G. Mitochondria: Master regulators of danger signalling. Nat. Rev. Mol. Cell. Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 62.Singh D., Kashyap A., Pandey R.V., Saini K.S. Novel advances in cytochrome P450 research. Drug Discov. Today. 2011;16:793–799. doi: 10.1016/j.drudis.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Zordoky B.N., El-Kadi A.O. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol. Ther. 2010;125:446–463. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Teixeira C.F., Landucci E.C., Antunes E., Chacur M., Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Allen A.C., Gammon C.M., Ousley A.H., McCarthy K.D., Morell P. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. J. Neurochem. 1992;58:1130–1139. doi: 10.1111/j.1471-4159.1992.tb09372.x. [DOI] [PubMed] [Google Scholar]
- 66.Albrecht E.A., Dhanasekaran S.M., Tomlins S. Immediate early inflammatory gene responses of human umbilical vein endothelial cells to hemorrhagic venom. Inflamm. Res. 2011;60:213–217. doi: 10.1007/s00011-010-0286-1. [DOI] [PubMed] [Google Scholar]
- 67.Seixas E., Gozzelino R., Chora A., Ferreira A., Silva G., Larsen R., Rebelo S., Penido C., Smith N.R., Coutinho A., et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl. Acad. Sci. USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Gozzelino R., Jeney V., Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev. Pharmacol. Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 70.Petricevich V.L., Teixeira C.F., Tambourgi D.V., Gutierrez J.M. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon. 2000;38:1253–1266. doi: 10.1016/S0041-0101(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 71.Chaves F., Teixeira C.F., Gutierrez J.M. Role of TNF-alpha, IL-1beta and IL-6 in the local tissue damage induced by Bothrops asper snake venom: An experimental assessment in mice. Toxicon. 2005;45:171–178. doi: 10.1016/j.toxicon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Gutierrez J.M., Rucavado A., Chaves F., Diaz C., Escalante T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon. 2009;54:958–975. doi: 10.1016/j.toxicon.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 73.Loibl S., von Minckwitz G., Weber S., Sinn H.P., Schini-Kerth V.B., Lobysheva I., Nepveu F., Wolf G., Strebhardt K., Kaufmann M. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer. 2002;95:1191–1198. doi: 10.1002/cncr.10817. [DOI] [PubMed] [Google Scholar]
- 74.Bulut A.S., Erden E., Sak S.D., Doruk H., Kursun N., Dincol D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: An immunohistochemical study of 151 cases. Virchows Arch. 2005;447:24–30. doi: 10.1007/s00428-005-1250-2. [DOI] [PubMed] [Google Scholar]
- 75.Choudhari S.K., Chaudhary M., Bagde S., Gadbail A.R., Joshi V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faria C.C., Agnihotri S., Mack S.C., Golbourn B.J., Diaz R.J., Olsen S., Bryant M., Bebenek M., Wang X., Bertrand K.C., et al. Identification of alsterpaullone as a novel small molecule inhibitor to target group 3 medulloblastoma. Oncotarget. 2015;6:21718–21729. doi: 10.18632/oncotarget.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Izidoro L.F., Ribeiro M.C., Souza G.R., Sant’Ana C.D., Hamaguchi A., Homsi-Brandeburgo M.I., Goulart L.R., Beleboni R.O., Nomizo A., Sampaio S.V., et al. Biochemical and functional characterization of an L-amino acid oxidase isolated from Bothrops pirajai snake venom. Bioorg. Med. Chem. 2006;14:7034–7043. doi: 10.1016/j.bmc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 78.De Menezes R.R., Torres A.F., da Silva T.S., de Sousa D.F., Lima D.B., Norjosa D.B., Nogueira N.A., Oliveira M.F., de Oliveira M.R., Monteiro H.S., et al. Antibacterial and antiparasitic effects of Bothropoides lutzi venom. Nat. Prod. Commun. 2012;7:71–74. [PubMed] [Google Scholar]
- 79.Goncalves A.R., Soares M.J., de Souza W., DaMatta R.A., Alves E.W. Ultrastructural alterations and growth inhibition of Trypanosoma cruzi and Leishmania major induced by Bothrops jararaca venom. Parasitol. Res. 2002;88:598–602. doi: 10.1007/s00436-002-0626-3. [DOI] [PubMed] [Google Scholar]
- 80.Passero L.F., Laurenti M.D., Tomokane T.Y., Corbett C.E., Toyama M.H. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol. Res. 2008;102:1025–1033. doi: 10.1007/s00436-007-0871-6. [DOI] [PubMed] [Google Scholar]
- 81.Grabner A.N., Alfonso J., Kayano A.M., Moreira-Dill L.S., Dos Santos A.P.A., Caldeira C.A.S., Sobrinho J.C., Gomez A., Grabner F.P., Cardoso F.F., et al. BmajPLA2-II, a basic Lys49-phospholipase A2 homologue from Bothrops marajoensis snake venom with parasiticidal potential. Int. J. Biol. Macromol. 2017;102:571–581. doi: 10.1016/j.ijbiomac.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 82.Carone S.E.I., Costa T.R., Burin S.M., Cintra A.C.O., Zoccal K.F., Bianchini F.J., Tucci L.F.F., Franco J.J., Torqueti M.R., Faccioli L.H., et al. A new l-amino acid oxidase from Bothrops jararacussu snake venom: Isolation, partial characterization, and assessment of pro-apoptotic and antiprotozoal activities. Int. J. Biol. Macromol. 2017;103:25–35. doi: 10.1016/j.ijbiomac.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 83.Mello C.P., Lima D.B., Menezes R.R., Bandeira I.C., Tessarolo L.D., Sampaio T.L., Falcao C.B., Radis-Baptista G., Martins A.M. Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon. 2017;130:56–62. doi: 10.1016/j.toxicon.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 84.Guillaume C., Deregnaucourt C., Clavey V., Schrevel J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004;43:311–318. doi: 10.1016/j.toxicon.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Quintana J.C., Chacon A.M., Vargas L., Segura C., Gutierrez J.M., Alarcon J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012;124:126–132. doi: 10.1016/j.actatropica.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Morais K.L., Hayashi M.A., Bruni F.M., Lopes-Ferreira M., Camargo A.C., Ulrich H., Lameu C. Bj-PRO-5a, a natural angiotensin-converting enzyme inhibitor, promotes vasodilatation mediated by both bradykinin B(2)and M1 muscarinic acetylcholine receptors. Biochem. Pharmacol. 2011;81:736–742. doi: 10.1016/j.bcp.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 87.Da Silva C.A., Kassel O., Mathieu E., Massard G., Gasser B., Frossard N. Inhibition by glucocorticoids of the interleukin-1beta-enhanced expression of the mast cell growth factor SCF. Br. J. Pharmacol. 2002;135:1634–1640. doi: 10.1038/sj.bjp.0704617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa T.R., Burin S.M., Menaldo D.L., de Castro F.A., Sampaio S.V. Snake venom L-amino acid oxidases: An overview on their antitumor effects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014;20:23. doi: 10.1186/1678-9199-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ande S.R., Kommoju P.R., Draxl S., Murkovic M., Macheroux P., Ghisla S., Ferrando-May E. Mechanisms of cell death induction by L-amino acid oxidase, a major component of ophidian venom. Apoptosis. 2006;11:1439–1451. doi: 10.1007/s10495-006-7959-9. [DOI] [PubMed] [Google Scholar]
- 90.Fung S.Y., Lee M.L., Tan N.H. Molecular mechanism of cell death induced by king cobra (Ophiophagus hannah) venom l-amino acid oxidase. Toxicon. 2015;96:38–45. doi: 10.1016/j.toxicon.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Mukherjee A.K., Saviola A.J., Burns P.D., Mackessy S.P. Apoptosis induction in human breast cancer (MCF-7) cells by a novel venom l-amino acid oxidase (Rusvinoxidase) is independent of its enzymatic activity and is accompanied by caspase-7 activation and reactive oxygen species production. Apoptosis. 2015;20:1358–1372. doi: 10.1007/s10495-015-1157-6. [DOI] [PubMed] [Google Scholar]
- 92.Martins N.M., Santos N.A., Sartim M.A., Cintra A.C., Sampaio S.V., Santos A.C. A tripeptide isolated from Bothrops atrox venom has neuroprotective and neurotrophic effects on a cellular model of Parkinson’s disease. Chem. Biol. Interact. 2015;235:10–16. doi: 10.1016/j.cbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Gomes A., Datta P., Das T., Biswas A.K. Anti arthritic and anti inflammatory activity of a cytotoxic protein NN-32 from Indian spectacle cobra (Naja naja) venom in male albino rats. Toxicon. 2014;90:106–110. doi: 10.1016/j.toxicon.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Ruan Y., Yao L., Zhang B., Zhang S., Guo J. Anti-inflammatory effects of Neurotoxin-Nna, a peptide separated from the venom of Naja naja atra. BMC Complement. Altern. Med. 2013;13:86. doi: 10.1186/1472-6882-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen T.T., Folch B., Letourneau M., Vaudry D., Truong N.H., Doucet N., Chatenet D., Fournier A. Cardiotoxin-I: An unexpectedly potent insulinotropic agent. Chembiochem. 2012;13:1805–1812. doi: 10.1002/cbic.201200081. [DOI] [PubMed] [Google Scholar]
- 96.Moore S.W., Bhat V.K., Flatt P.R., Gault V.A., McClean S. Isolation and characterisation of insulin-releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis venom. Toxicon. 2015;101:48–54. doi: 10.1016/j.toxicon.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Toyama O.D., Boschero C.A., Martins A.M., Fonteles C.M., Monteiro S.H., Toyama H.M. Structure-function relationship of new crotamine isoform from the Crotalus durissus cascavella. Protein J. 2005;24:9–19. doi: 10.1007/s10930-004-0601-1. [DOI] [PubMed] [Google Scholar]
- 98.Lajus S., Vacher P., Huber D., Dubois M., Benassy M.N., Ushkaryov Y., Lang J. Alpha-latrotoxin induces exocytosis by inhibition of voltage-dependent K+ channels and by stimulation of L-type Ca2+ channels via latrophilin in beta-cells. J. Biol. Chem. 2006;281:5522–5531. doi: 10.1074/jbc.M510528200. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto S., Nakaki T., Nakadate T., Kato R. Insulinotropic effects of exogenous phospholipase A2 and C in isolated pancreatic islets. Eur. J. Pharmacol. 1982;86:121–124. doi: 10.1016/0014-2999(82)90409-5. [DOI] [PubMed] [Google Scholar]
- 100.Nogueira T.C., Ferreira F., Toyama M.H., Stoppiglia L.F., Marangoni S., Boschero A.C., Carneiro E.M. Characterization of the insulinotropic action of a phospholipase A2 isolated from Crotalus durissus collilineatus rattlesnake venom on rat pancreatic islets. Toxicon. 2005;45:243–248. doi: 10.1016/j.toxicon.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 101.Nicastro G., Franzoni L., de Chiara C., Mancin A.C., Giglio J.R., Spisni A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur. J. Biochem. 2003;270:1969–1979. doi: 10.1046/j.1432-1033.2003.03563.x. [DOI] [PubMed] [Google Scholar]
- 102.WHO . Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 103.WHO . Guidelines for Malaria Treatment. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 104.Baird J.K., Rieckmann K.H. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003;19:115–120. doi: 10.1016/S1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 105.Price R.N., Douglas N.M., Anstey N.M., von Seidlein L. Plasmodium vivax treatments: What are we looking for? Curr. Opin. Infect. Dis. 2011;24:578–585. doi: 10.1097/QCO.0b013e32834c61e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howes R.E., Battle K.E., Satyagraha A.W., Baird J.K., Hay S.I. G6PD deficiency: Global distribution, genetic variants and primaquine therapy. Adv. Parasitol. 2013;81:133–201. doi: 10.1016/B978-0-12-407826-0.00004-7. [DOI] [PubMed] [Google Scholar]
- 107.Thomas D., Tazerouni H., Sundararaj K.G., Cooper J.C. Therapeutic failure of primaquine and need for new medicines in radical cure of Plasmodium vivax. Acta Trop. 2016;160:35–38. doi: 10.1016/j.actatropica.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Fitch C.D., Chevli R., Banyal H.S., Phillips G., Pfaller M.A., Krogstad D.J. Lysis of Plasmodium falciparum by ferriprotoporphyrin IX and a chloroquine-ferriprotoporphyrin IX complex. Antimicrob. Agents Chemother. 1982;21:819–822. doi: 10.1128/AAC.21.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Villiers K.A., Marques H.M., Egan T.J. The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J. Inorg. Biochem. 2008;102:1660–1667. doi: 10.1016/j.jinorgbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Dorn A., Vippagunta S.R., Matile H., Jaquet C., Vennerstrom J.L., Ridley R.G. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 1998;55:727–736. doi: 10.1016/S0006-2952(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 111.Werlinder V., Backlund M., Zhukov A., Ingelman-Sundberg M. Transcriptional and post-translational regulation of CYP1A1 by primaquine. J. Pharmacol. Exp. Ther. 2001;297:206–214. [PubMed] [Google Scholar]
- 112.Nagaraj V.A., Sundaram B., Varadarajan N.M., Subramani P.A., Kalappa D.M., Ghosh S.K., Padmanaban G. Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 2013;9:e1003522. doi: 10.1371/journal.ppat.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ke H., Sigala P.A., Miura K., Morrisey J.M., Mather M.W., Crowley J.R., Henderson J.P., Goldberg D.E., Long C.A., Vaidya A.B. The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J. Biol. Chem. 2014;289:34827–34837. doi: 10.1074/jbc.M114.615831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pamplona A., Ferreira A., Balla J., Jeney V., Balla G., Epiphanio S., Chora A., Rodrigues C.D., Gregoire I.P., Cunha-Rodrigues M., et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 115.Ferreira I.D., Martinelli A., Rodrigues L.A., do Carmo E.L., do Rosario V.E., Povoa M.M., Cravo P. Plasmodium falciparum from Pará state (Brazil) shows satisfactory in vitro response to artemisinin derivatives and absence of the S769N mutation in the SERCA-type PfATPase6. Trop. Med. Int. Health. 2008;13:199–207. doi: 10.1111/j.1365-3156.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 116.Sivaraman P., Rattehalli R.D., Jayaram M.B. Levomepromazine for schizophrenia. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.CD007779.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bymaster F.P., Felder C.C., Tzavara E., Nomikos G.G., Calligaro D.O., McKinzie D.L. Muscarinic mechanisms of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 118.Thanacoody R.H. Thioridazine: The good and the bad. Recent Pat. Anti-Infect. Drug Discov. 2011;6:92–98. doi: 10.2174/157489111796064588. [DOI] [PubMed] [Google Scholar]
- 119.Jayakody K., Gibson R.C., Kumar A., Gunadasa S. Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses. Cochrane Database Syst. Rev. 2012;4:CD000525. doi: 10.1002/14651858.CD000525.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tardy M., Huhn M., Engel R.R., Leucht S. Fluphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014;8:CD009230. doi: 10.1002/14651858.CD009230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Selbie L.A., Hill S.J. G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 1998;19:87–93. doi: 10.1016/S0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 122.Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cryan J.F., Kaupmann K. Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Bettler B., Kaupmann K., Mosbacher J., Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 126.Kumar K., Sharma S., Kumar P., Deshmukh R. Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol. Biochem. Behav. 2013;110:174–184. doi: 10.1016/j.pbb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 127.Da Silva D.C., de Medeiros W.A., Batista Ide F., Pimenta D.C., Lebrun I., Abdalla F.M., Sandoval M.R. Characterization of a new muscarinic toxin from the venom of the Brazilian coral snake Micrurus lemniscatus in rat hippocampus. Life Sci. 2011;89:931–938. doi: 10.1016/j.lfs.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 128.Liang J.S., Carsi-Gabrenas J., Krajewski J.L., McCafferty J.M., Purkerson S.L., Santiago M.P., Strauss W.L., Valentine H.H., Potter L.T. Anti-muscarinic toxins from Dendroaspis angusticeps. Toxicon. 1996;34:1257–1267. doi: 10.1016/S0041-0101(96)00109-2. [DOI] [PubMed] [Google Scholar]
- 129.Bradley K.N. Muscarinic toxins from the green mamba. Pharmacol. Ther. 2000;85:87–109. doi: 10.1016/S0163-7258(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 130.Karlsson E., Jolkkonen M., Mulugeta E., Onali P., Adem A. Snake toxins with high selectivity for subtypes of muscarinic acetylcholine receptors. Biochimie. 2000;82:793–806. doi: 10.1016/S0300-9084(00)01176-7. [DOI] [PubMed] [Google Scholar]
- 131.Servent D., Fruchart-Gaillard C. Muscarinic toxins: Tools for the study of the pharmacological and functional properties of muscarinic receptors. J. Neurochem. 2009;109:1193–1202. doi: 10.1111/j.1471-4159.2009.06092.x. [DOI] [PubMed] [Google Scholar]
- 132.Maiga A., Merlin J., Marcon E., Rouget C., Larregola M., Gilquin B., Fruchart-Gaillard C., Lajeunesse E., Marchetti C., Lorphelin A., et al. Orthosteric binding of rho-Da1a, a natural peptide of snake venom interacting selectively with the alpha1A-adrenoceptor. PLoS ONE. 2013;8:e68841. doi: 10.1371/journal.pone.0068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maiga A., Mourier G., Quinton L., Rouget C., Gales C., Denis C., Lluel P., Senard J.M., Palea S., Servent D., et al. G protein-coupled receptors, an unexploited animal toxin targets: Exploration of green mamba venom for novel drug candidates active against adrenoceptors. Toxicon. 2012;59:487–496. doi: 10.1016/j.toxicon.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 134.Nareoja K., Nasman J. Selective targeting of G-protein-coupled receptor subtypes with venom peptides. Acta Physiol. 2011;204:186–201. doi: 10.1111/j.1748-1716.2011.02305.x. [DOI] [PubMed] [Google Scholar]
- 135.Blanchet G., Upert G., Mourier G., Gilquin B., Gilles N., Servent D. New alpha-adrenergic property for synthetic MTbeta and CM-3 three-finger fold toxins from black mamba. Toxicon. 2013;75:160–167. doi: 10.1016/j.toxicon.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 136.Gorbacheva E.V., Starkov V.G., Tsetlin V.I., Utkin Y.N., Vulfius C.A. Viperidae Snake Venoms Block Nicotinic Acetylcholine Receptors and Voltage-Gated Ca2+ Channels in Identified Neurons of Fresh-Water Snail Lymnaea stagnalis. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2008;2:14–18. [Google Scholar]
- 137.Lally J., MacCabe J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015;114:169–179. doi: 10.1093/bmb/ldv017. [DOI] [PubMed] [Google Scholar]
- 138.Milelli A., Turrini E., Catanzaro E., Maffei F., Fimognari C. Perspectives in Designing Multifunctional Molecules in Antipsychotic Drug Discovery. Drug Dev. Res. 2016;77:437–443. doi: 10.1002/ddr.21334. [DOI] [PubMed] [Google Scholar]
- 139.Horacek J., Bubenikova-Valesova V., Kopecek M., Palenicek T., Dockery C., Mohr P., Hoschl C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 140.Divac N., Prostran M., Jakovcevski I., Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed. Res. Int. 2014;2014:656370. doi: 10.1155/2014/656370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meltzer H.Y. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 142.Meltzer H.Y. Clinical studies on the mechanism of action of clozapine: The dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99:S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 143.Kane J.M., Honigfeld G., Singer J., Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol. Bull. 1988;24:62–67. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 144.Kane J.M., Correll C.U. The Role of Clozapine in Treatment-Resistant Schizophrenia. JAMA Psychiatry. 2016;73:187–188. doi: 10.1001/jamapsychiatry.2015.2966. [DOI] [PubMed] [Google Scholar]
- 145.Ayati A., Emami S., Foroumadi A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016;109:380–392. doi: 10.1016/j.ejmech.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 146.Masini E., Carta F., Scozzafava A., Supuran C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013;23:705–716. doi: 10.1517/13543776.2013.794788. [DOI] [PubMed] [Google Scholar]
- 147.Aggarwal M., Kondeti B., McKenna R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013;23:717–724. doi: 10.1517/13543776.2013.782394. [DOI] [PubMed] [Google Scholar]
- 148.Cosci F., Fava G.A., Sonino N. Mood and anxiety disorders as early manifestations of medical illness: A systematic review. Psychother. Psychosom. 2014;84:22–29. doi: 10.1159/000367913. [DOI] [PubMed] [Google Scholar]
- 149.Sliz D., Hayley S. Major depressive disorder and alterations in insular cortical activity: A review of current functional magnetic imaging research. Front. Hum. Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morilak D.A., Frazer A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 151.Dickson D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012;2:a009258. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexander G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004;6:259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ye Z., Altena E., Nombela C., Housden C.R., Maxwell H., Rittman T., Huddleston C., Rae C.L., Regenthal R., Sahakian B.J., et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson’s disease. Brain. 2014;137:1145–1155. doi: 10.1093/brain/awu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Syvalahti E.K., Kunelius R., Lauren L. Effects of antiparkinsonian drugs on muscarinic receptor binding in rat brain, heart and lung. Pharmacol. Toxicol. 1988;62:90–94. doi: 10.1111/j.1600-0773.1988.tb01852.x. [DOI] [PubMed] [Google Scholar]
- 155.Sousa L.F., Nicolau C.A., Peixoto P.S., Bernardoni J.L., Oliveira S.S., Portes-Junior J.A., Mourao R.H., Lima-Dos-Santos I., Sano-Martins I.S., Chalkidis H.M., et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl. Trop. Dis. 2013;7:e2442. doi: 10.1371/journal.pntd.0002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gasanov S.E., Dagda R.K., Rael E.D. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J. Clin. Toxicol. 2014;4:1000181. doi: 10.4172/2161-0495.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Villard E., Soubrier F. Molecular biology and genetics of the angiotensin-I-converting enzyme: Potential implications in cardiovascular diseases. Cardiovasc. Res. 1996;32:999–1007. doi: 10.1016/S0008-6363(96)00170-8. [DOI] [PubMed] [Google Scholar]
- 158.Cotton J., Hayashi M.A., Cuniasse P., Vazeux G., Ianzer D., De Camargo A.C., Dive V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry. 2002;41:6065–6071. doi: 10.1021/bi012121x. [DOI] [PubMed] [Google Scholar]
- 159.Fiszman G.L., Middonno M.C., de la Torre E., Farina M., Espanol A.J., Sales M.E. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol. Ther. 2007;6:1106–1113. doi: 10.4161/cbt.6.7.4330. [DOI] [PubMed] [Google Scholar]
- 160.Da Costa P.L., Sirois P., Tannock I.F., Chammas R. The role of kinin receptors in cancer and therapeutic opportunities. Cancer Lett. 2014;345:27–38. doi: 10.1016/j.canlet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 161.Michel T., Hoffman B.B. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. Brunton L.L., Chabner B.A., Knollman B.C., editors. McGraw-Hill; New York, NY, USA: 2011. pp. 745–788. [Google Scholar]
- 162.Rajagopal V., Kreitman R.J. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the a2-macroglobulin receptor. J. Biol. Chem. 2000;275:7566–7573. doi: 10.1074/jbc.275.11.7566. [DOI] [PubMed] [Google Scholar]
- 163.Lewis R.J., Garcia M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003;2:790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- 164.Joseph R., Pahari S., Hodgson W.C., Kini R.M. Hypotensive agents from snake venoms. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4:437–459. doi: 10.2174/1568006043335808. [DOI] [PubMed] [Google Scholar]
- 165.Koh C.Y., Kini R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon. 2011;59:497–506. doi: 10.1016/j.toxicon.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 166.Horta C.C.R., Chatzaki M., Rezende B.A., Magalhães B.F., Duarte C.G., Felicori L.F., Oliveira-Mendes B.B.R., do Carmo A.O., hávez-Olórtegui C., Kalapothakis E. Cardiovascular-Active Venom Toxins: An Overview. Curr. Med. Chem. 2016;23:603–622. doi: 10.2174/0929867323666160126142837. [DOI] [PubMed] [Google Scholar]
- 167.Accary C., Hraoui-Bloquet S., Sadek R., Alameddine A., Fajloun Z., Desfontis J.C., Mallem Y. The relaxant effect of the Montivipera bornmuelleri snake venom on vascular contractility. J. Venom. Res. 2016;7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 168.Santos S.S., Jesus R.L.C., Simoes L.O., Vasconcelos W.P., Medeiros I.A., Veras R.C., Casais E.S.L.L., Silva D.F. NO production and potassium channels activation induced by Crotalus durissus cascavella underlie mesenteric artery relaxation. Toxicon. 2017;133:10–17. doi: 10.1016/j.toxicon.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 169.Tambourgi D.V., dos Santos M.C., Furtado Mde F., de Freitas M.C., da Silva W.D., Kipnis T.L. Pro-inflammatory activities in elapid snake venoms. Br. J. Pharmacol. 1994;112:723–727. doi: 10.1111/j.1476-5381.1994.tb13137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teixeira C.F.P., Fernandes C.M., Zuliani J.P., Zamuner S.F. Inflammatory effects of snake venom metalloproteinases. Mem. Inst. Butantan. 2005;100:181–184. doi: 10.1590/S0074-02762005000900031. [DOI] [PubMed] [Google Scholar]
- 171.Moura-da-Silva A.M., Butera D., Tanjoni I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007;13:2893–2905. doi: 10.2174/138161207782023711. [DOI] [PubMed] [Google Scholar]
- 172.Nunes D.C., Rodrigues R.S., Lucena M.N., Cologna C.T., Oliveira A.C., Hamaguchi A., Homsi-Brandeburgo M.I., Arantes E.C., Teixeira D.N., Ueira-Vieira C., et al. Isolation and functional characterization of proinflammatory acidic phospholipase A2 from Bothrops leucurus snake venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;154:226–233. doi: 10.1016/j.cbpc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 173.Gutierrez J.M., Escalante T., Rucavado A., Herrera C., Fox J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins. 2016;8:304. doi: 10.3390/toxins8100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alcaraz M.J., Fernandez P., Guillen M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003;9:2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 175.Otterbein L.E., Choi A.M. Heme oxygenase: Colors of defense against cellular stress. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 176.Gullotta F., di Masi A., Coletta M., Ascenzi P. CO metabolism, sensing, and signaling. Biofactors. 2012;38:1–13. doi: 10.1002/biof.192. [DOI] [PubMed] [Google Scholar]
- 177.Motterlini R., Green C.J., Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid. Redox Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- 178.Zhu X., Fan W.G., Li D.P., Kung H., Lin M.C. Heme oxygenase-1 system and gastrointestinal inflammation: A short review. World J. Gastroenterol. 2011;17:4283–4288. doi: 10.3748/wjg.v17.i38.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wegiel B., Otterbein L.E. Go green: The anti-inflammatory effects of biliverdin reductase. Front. Pharmacol. 2012;3:47. doi: 10.3389/fphar.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jain D., Kumar S. Snake venom: A potent anticancer agent. Asian Pac. J. Cancer Prev. 2012;13:4855–4860. doi: 10.7314/APJCP.2012.13.10.4855. [DOI] [PubMed] [Google Scholar]
- 181.Calderon L.A., Sobrinho J.C., Zaqueo K.D., de Moura A.A., Grabner A.N., Mazzi M.V., Marcussi S., Nomizo A., Fernandes C.F., Zuliani J.P., et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014;2014:203639. doi: 10.1155/2014/203639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lucena S., Castro R., Lundin C., Hofstetter A., Alaniz A., Suntravat M., Sanchez E.E. Inhibition of pancreatic tumoral cells by snake venom disintegrins. Toxicon. 2015;93:136–143. doi: 10.1016/j.toxicon.2014.11.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dhananjaya B.L., Sivashankari P.R. Snake venom derived molecules in tumor angiogenesis and its application in cancer therapy; an overview. Curr. Top. Med. Chem. 2015;15:649–657. doi: 10.2174/1568026615666150225113402. [DOI] [PubMed] [Google Scholar]
- 184.Mizuno C.S., Chittiboyina A.G., Kurtz T.W., Pershadsingh H.A., Avery M.A. Type 2 diabetes and oral antihyperglycemic drugs. Curr. Med. Chem. 2008;15:61–74. doi: 10.2174/092986708783330656. [DOI] [PubMed] [Google Scholar]
- 185.Staels B., Fruchart J. Therapeutic Roles of Peroxisome Proliferator—Activated Receptor Agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 186.American Association Diabetes Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl. 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chandra R., Liddle R.A. Neural and hormonal regulation of pancreatic secretion. Curr. Opin. Gastroenterol. 2009;25:441–446. doi: 10.1097/MOG.0b013e32832e9c41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fu Z., Gilbert E.R., Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013;9:25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Madiraju S.R., Poitout V. G protein-coupled receptors and insulin secretion: 119 and counting. Endocrinology. 2007;148:2598–2600. doi: 10.1210/en.2007-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boyd A.E., III The Role of Ion Channels in Insulin Secretion. J. Cell. Biochem. 1992;48:234–241. doi: 10.1002/jcb.240480303. [DOI] [PubMed] [Google Scholar]
- 191.Fridlyand L.E., Jacobson D.A., Philipson L.H. Ion channels and regulation of insulin secretion in human β-cells: A computational systems analysis. Islets. 2013;5:1–15. doi: 10.4161/isl.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Diaz-Garcia C.M., Sanchez-Soto C., Hiriart M. Toxins that modulate ionic channels as tools for exploring insulin secretion. Cell. Mol. Neurobiol. 2010;30:1275–1281. doi: 10.1007/s10571-010-9586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toyama M.H., Carneiro E.M., Marangoni S., Barbosa R.L., Corso G., Boschero A.C. Biochemical characterization of two crotamine isoforms isolated by a single step RP-HPLC from Crotalus durissus terrificus (South American rattlesnake) venom and their action on insulin secretion by pancreatic islets. Biochim. Biophys. Acta. 2000;1474:56–60. doi: 10.1016/S0304-4165(99)00211-1. [DOI] [PubMed] [Google Scholar]
- 194.Eng J., Kleinman W.A., Singh L., Singh G., Raufman J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 195.Furman B.L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59:464–471. doi: 10.1016/j.toxicon.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 196.Amin R.H., Chen H.Q., Veluthakal R., Silver R.B., Li J., Li G., Kowluru A. Mastoparan-induced insulin secretion from insulin-secreting betaTC3 and INS-1 cells: Evidence for its regulation by Rho subfamily of G proteins. Endocrinology. 2003;144:4508–4518. doi: 10.1210/en.2003-0106. [DOI] [PubMed] [Google Scholar]
- 197.Baptista-Saidemberg N.B., Saidemberg D.M., Ribeiro R.A., Arcuri H.A., Palma M.S., Carneiro E.M. Agelaia MP-I: A peptide isolated from the venom of the social wasp, Agelaia pallipes pallipes, enhances insulin secretion in mice pancreatic islets. Toxicon. 2012;60:596–602. doi: 10.1016/j.toxicon.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 198.Sankaran H., Deveney C.W., Bartholomew C., Raghupathy E. Action of the venom of the scorpion Tityus trinitatis on pancreatic insulin secretion. Biochem. Pharmacol. 1983;32:1101–1104. doi: 10.1016/0006-2952(83)90632-9. [DOI] [PubMed] [Google Scholar]
- 199.Goncalves A.A., Toyama M.H., Carneiro E.M., Marangoni S., Arantes E.C., Giglio J.R., Boschero A.C. Participation of Na+ channels in the potentiation by Tityus serrulatus alpha-toxin TsTx-V of glucose-induced electrical activity and insulin secretion in rodent islet beta-cells. Toxicon. 2003;41:1039–1045. doi: 10.1016/S0041-0101(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 200.Holz G.G., Habener J.F. Black widow spider alpha-latrotoxin: A presynaptic neurotoxin that shares structural homology with the glucagon-like peptide-1 family of insulin secretagogic hormones. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;121:177–184. doi: 10.1016/S0305-0491(98)10088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mousavi S.M., Imani S., Haghighi S., Mousavi S.E., Karimi A. Effect of Iranian Honey bee (Apis mellifera) Venom on Blood Glucose and Insulin in Diabetic Rats. J. Arthropod-Borne Dis. 2013;6:136–143. [PMC free article] [PubMed] [Google Scholar]
- 202.Vela M.F. Medical treatments of GERD: The old and new. Gastroenterol. Clin. N. Am. 2014;43:121–133. doi: 10.1016/j.gtc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 203.Iwakiri K., Kinoshita Y., Habu Y., Oshima T., Manabe N., Fujiwara Y., Nagahara A., Kawamura O., Iwakiri R., Ozawa S., et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J. Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8. [DOI] [PubMed] [Google Scholar]
- 204.Patrick L. Gastroesophageal reflux disease (GERD): A review of conventional and alternative treatments. Altern. Med. Rev. 2011;16:116–133. [PubMed] [Google Scholar]
- 205.Gremse D.A. Lansoprazole: Pharmacokinetics, pharmacodynamics and clinical uses. Expert Opin. Pharmacother. 2001;2:1663–1670. doi: 10.1517/14656566.2.10.1663. [DOI] [PubMed] [Google Scholar]
- 206.Krusekopf S., Kleeberg U., Hildebrandt A.G., Ruckpaul K. Effects of benzimidazole derivatives on cytochrome P450 1A1 expression in a human hepatoma cell line. Xenobiotica. 1997;27:1–9. doi: 10.1080/004982597240721. [DOI] [PubMed] [Google Scholar]
- 207.Curi-Pedrosa R., Daujat M., Pichard L., Ourlin J.C., Clair P., Gervot L., Lesca P., Domergue J., Joyeux H., Fourtanier G., et al. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J. Pharmacol. Exp. Ther. 1994;269:384–392. [PubMed] [Google Scholar]
- 208.Van der Pol R., Langendam M., Benninga M., van Wijk M., Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014;168:947–954. doi: 10.1001/jamapediatrics.2014.1273. [DOI] [PubMed] [Google Scholar]
- 209.Galvao Nascimento N., Sampaio M.C., Amaral Olivo R., Teixeira C. Contribution of mast cells to the oedema induced by Bothrops moojeni snake venom and a pharmacological assessment of the inflammatory mediators involved. Toxicon. 2010;55:343–352. doi: 10.1016/j.toxicon.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 210.Simons F.E. The antiallergic effects of antihistamines (H1-receptor antagonists) J. Allergy Clin. Immunol. 1992;90:705–715. doi: 10.1016/0091-6749(92)90156-V. [DOI] [PubMed] [Google Scholar]
- 211.Stojković N., Cekić S., Ristov M., Ristić M., Đukić D., Binić M., Virijević D. Histamine and Antihistamines. Sci. J. Fac. Med. 2015;32:7–22. [Google Scholar]
- 212.Xie H., He S.H. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J. Gastroenterol. 2005;11:2851–2857. doi: 10.3748/wjg.v11.i19.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Damerau B., Lege L., Oldigs H.D., Vogt W. Histamine release, formation of prostaglandin-like activity (SRS-C) and mast cell degranulation by the direct lytic factor (DLF) and phospholipase A of cobra venom. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1975;287:141–156. doi: 10.1007/BF00510446. [DOI] [PubMed] [Google Scholar]
- 214.Chen I.J., Chiu H.F., Huang H.T., Teng C.M. Edema formation and degranulation of mast cells by Trimeresurus mucrosquamatus snake venom. Toxicon. 1984;22:17–28. doi: 10.1016/0041-0101(84)90134-X. [DOI] [PubMed] [Google Scholar]
- 215.Wei J.F., Mo Y.Z., Qiao L.Y., Wei X.L., Chen H.Q., Xie H., Fu Y.L., Wang W.Y., Xiong Y.L., He S.H. Potent histamine-releasing activity of atrahagin, a novel snake venom metalloproteinase. Int. J. Biochem. Cell Biol. 2006;38:510–520. doi: 10.1016/j.biocel.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 216.Bonavita A.G., da Costa A.S., Pires A.L., Neves-Ferreira A.G., Perales J., Cordeiro R.S., Martins M.A., e Silva P.M. Contribution of mast cells and snake venom metalloproteinases to the hyperalgesia induced by Bothrops jararaca venom in rats. Toxicon. 2006;47:885–893. doi: 10.1016/j.toxicon.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 217.Ogawa T., Chijiwa T., Oda-Ueda N., Ohno M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon. 2005;45:1–14. doi: 10.1016/j.toxicon.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 218.Medina V.A., Rivera E.S. Histamine receptors and cancer pharmacology. Br. J. Pharmacol. 2010;161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Corboz M.R., Mutter J.C., Rivelli M.A., Mingo G.G., McLeod R.L., Varty L., Jia Y., Cartwright M., Hey J.A. α2-adrenoceptor agonists as nasal decongestants. Pulm. Pharmacol. Ther. 2007;20:149–156. doi: 10.1016/j.pupt.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 220.Chung C.H., Au L.C., Huang T.F. Molecular cloning and sequence analysis of aggretin, a collagen-like platelet aggregation inducer. Biochem. Biophys. Res. Commun. 1999;263:723–727. doi: 10.1006/bbrc.1999.1457. [DOI] [PubMed] [Google Scholar]
- 221.Chung C.H., Lin K.T., Chang C.H., Peng H.C., Huang T.F. The integrin alpha2beta1 agonist, aggretin, promotes proliferation and migration of VSMC through NF-kB translocation and PDGF production. Br. J. Pharmacol. 2009;156:846–856. doi: 10.1111/j.1476-5381.2008.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.