Summary
Cordyceps militaris, a medicinal and edible mushroom, was used to ferment buckwheat and embryo rice by solid-state fermentation (SSF). Our aim was to investigate the effect of ultraviolet B (UVB) light irradiation on the content of vitamin D2 and biologically effective components, and antioxidant properties of buckwheat and embryo rice in SSF with C. militaris. Irradiated samples of buckwheat and embryo rice fermented by C. militaris had significantly increased vitamin D2 content, from 0-0.3 to 1.18-16.79 μg/g, while the increase in fresh embryo rice fermented by C. militaris was up to 16.79 μg/g. The content of adenosine, cordycepin and polysaccharide in irradiated dry samples fermented by C. militaris was 0.08 to 11.15 mg/g, higher than that of the irradiated fresh samples fermented by C. militaris (0.07–8.40 mg/g). Samples fermented by C. militaris had lower EC50 values and higher content of antioxidants than did unfermented samples. When the solid-state fermented sample was irradiated with UVB light, the content of biologically effective and antioxidant components and antioxidant property of sample decreased. However, it still contained enough of these biologically effective and antioxidant components.
Key words: Cordyceps militaris, UVB irradiation, solid-state fermentation, vitamin D2, antioxidant activity
Introduction
The medicinal caterpillar fungus Cordyceps militaris (L.) Link (Clavicipitaceae, Ascomycetes) is the only cultivated caterpillar fungus whose fruiting bodies can be formed without the process of caterpillar infection (1). It contains many bioactive components, such as adenosine, cordycepin and polysaccharides. Because of its various physiological activities, including anticancer, antiageing, antiviral, anti- -inflammatory and hypoglycaemic activities, it is used for multiple medicinal purposes (2-8). Currently, cultivation methods of C. militaris mainly include solid-state fermentation, submerged fermentation and membrane-surface liquid cultivation (9, 10). Furthermore, the solid-state fermentation (SSF) of grains by C. militaris results in biotransformed grains with high antioxidant activity, DNA damage protection, and angiotensin I-converting enzyme (ACE) inhibitory activity, thereby providing a method to obtain oats and chickpeas with enhanced functional value (11, 12). Therefore, in the present study, SSF of grains by C. militaris was studied to find a technological method to potentially produce functional foods and nutraceutical formulations.
The SSF technique involves the growth and metabolism of microorganisms on solid materials, which generally have a low water content to reduce the risk of contamination (13). It is particularly suitable for propagating fungi that are used as food or medicine. There are several recent publications describing the development of SSF for utilizing such raw materials for the production of food matrices and value- -added fine products such as enzymes, flavonoids, phenols, amino acids, organic acids, and biologically active secondary metabolites, among others (14-17).
Vitamin D is important for calcium absorption and bone health. In addition, vitamin D deficiency causes many health problems, including immune-mediated diseases, cardiovascular diseases, diabetes, cancer and infections (18). Therefore, vitamin D intake has attracted increasing interest in recent years, and consuming foods rich in vitamin D has become a general preference (19). Edible and medicinal mushrooms contain many physiologically active substances, including ergothioneine, ergosterol, vitamin D2, polysaccharides and triterpenoids (20). Mushrooms are a natural and non-animal food source of vitamin D2. Fortunately, when mushrooms are exposed to UV light, ergosterol undergoes photolysis and results in an increase in the amount of vitamin D2 (21-26).
Most studies have worked on fruiting bodies to enhance their vitamin D2 content. However, information is lacking about the application of UV light on solid-state fermented products. Since they are easily produced compared to fruiting bodies, there is a great potential for the development of healthy food with high content of vitamin D2 and enhanced functional value. In this regard, we cultivated C. militaris on buckwheat and embryo rice with ultraviolet B (UVB) irradiation, which might provide abundant nutraceutical compounds derived from the biologically active components. Therefore, the objective of this work is to assay the influence of C. militaris fermentation with UVB irradiation on the biologically active components, antioxidant properties and antioxidant components of buckwheat and embryo rice. Furthermore, the analysis of biologically active components was conducted to determine the vitamin D2, ergosterol, adenosine, cordycepin and polysaccharide contents of irradiated and unirradiated samples. The antioxidant properties of the ethanolic and hot water extracts of irradiated samples, including their reducing power, radical scavenging ability and ferrous ion chelating ability, were determined and compared with those of the unirradiated samples. The content of potential antioxidant components (flavonoids and total phenols) was also determined.
Materials and Methods
Materials and microorganism
Buckwheat and embryo rice were purchased from a local market in Taichung City, Taiwan, and the mycelium of Cordyceps militaris was obtained from the Star-Ocean International Co., Taichung City, Taiwan. Chemicals and reagents were of analytical grade. C. militaris was cultivated and maintained on potato dextrose agar (PDA; Merck, Darmstadt, Germany) slants and subcultured every month. Slants were incubated at 25 °C for seven days, and then stored at 4 °C.
Inoculum preparation and SSF of buckwheat and embryo rice
Preparation of fermented buckwheat and embryo rice started with the propagation of C. militaris on a PDA slant at 25 °C for seven days, and then the 0.5-cm2 mycelial block was inoculated into a 250-mL flask containing 100 mL of liquid culture medium. The liquid culture medium was composed of the following components (in %): glucose 2, yeast extract 0.5, MgSO4·7H2O 0.05, KH2PO4 0.05 and KHP2O4 0.05. The culture was incubated at 25 °C for five days on a rotary shaker (100 rpm; Hsin Chien Xiang Precision Industry Co., Kaohsiung, Taiwan). This C. militaris seed culture containing mycelia was ready to serve as inoculum for buckwheat and embryo rice fermentation. The buckwheat or embryo rice (15 g) was soaked with 45 mL of water for 2 h and then sterilized at 121 °C for 15 min. The culture was homogenized for 10 s in a Waring blender (7011G; Torrington, CT, USA). Then, 5 mL of the homogenized seed culture (7.4 g of culture per L of dry biomass) were inoculated into the autoclaved buckwheat and embryo rice, mixed thoroughly, placed in a 90 mm×15 mm Petri dish and incubated at 25 °C for 12 days.
UVB irradiation of SSF substrates
An open Petri dish of SSF substrate was placed 19 cm from the source of irradiation, a UVB lamp (λ=280-360 nm, G15T8E; Sankyo Denki, Tokyo, Japan) for 2 h at room temperature (25 °C). The UVB irradiation intensity was measured by a UVX 31 radiometer (UVP, Upland, CA, USA) to be 0.36 mW/cm2, and the irradiation doses for 2 h were 25.9 kJ/m2 (27). The sample was hot-air-dried at 50 °C, then ground in an RT-08 pulverizing machine (Rong Tsong Precision Technology, Taichung, Taiwan). The samples were sequentially ground and sieved until all particles were <0.4 mm and were then stored in darkness at 4 °C before use.
Samples obtained using three processing methods before UVB irradiation were examined in the following experiments. The first treatment included solid-state fermentation and then drying of buckwheat or embryo rice, followed by UVB irradiation. In the second treatment, after solid-state fermentation, followed by UVB irradiation, the samples were dried. The third approach started with UVB irradiation, followed by solid-state fermentation and finally drying of the samples of buckwheat and embryo rice. Other unfermented samples were used as control groups for comparison, and they included: buckwheat, UVB irradiated buckwheat, buckwheat mycelia, embryo rice, UVB irradiated embryo rice and embryo rice mycelia.
Determination of biologically active components
Adenosine and cordycepin were extracted and analysed according to the method of Masuda et al. (28) with some modifications. A sample (5 g) was mixed with 100 mL of distilled water, sonicated at 40 °C for 30 min and centrifuged (CN5101; Hsiangtai Machinery Industry Co., Taipei, Taiwan) at 3000×g for 10 min. The residue was then extracted twice and centrifuged as described above. The combined filtrate was dried in a rotary evaporator (N-1000; EYELA, Tokyo Rikakikai Co., Tokyo, Japan) at 40 °C until the solvent was evaporated, redissolved in distilled water, and filtered prior to injection into high-performance liquid chromatograph (HPLC) which consisted of L-2130 pump and L-2400 UV detector (Hitachi, Tokyo, Japan), and a LiChrospher 100 RP-18e column (4.6 mm×250 mm, 5 μm i.d.; Merck). The mobile phase was methanol/0.02 M potassium dihydrogen phosphate (18:85 by volume) at a flow rate of 1.0 mL/min, and UV detection was done at λ=254 nm. The content of adenosine and cordycepin was calculated on the basis of the calibration curves of authentic adenosine and cordycepin (Sigma-Aldrich; St. Louis, MO, USA).
Vitamin D2 and ergosterol were extracted and analysed according to the method of Huang et al. (23) with some modifications. A sample (5 g) was mixed with 10 mL of dimethyl sulfoxide (Merck) and sonicated at 45 °C for 30 min. Then, 10 mL of methanol and water (1:1 by volume) and 20 mL of hexane were added, the mixture was sonicated at 45 °C for another 30 min and centrifuged (CN-5101; Jusun Instruments Co. Ltd, Taiwan, ROC) at 3000×g for 10 min. The residue was extracted twice with 20 mL of hexane and centrifuged. The combined filtrate was dried in a rotary evaporator (N-1000; EYELA) at 40 °C until the solvent was evaporated, redissolved in 1 mL of methanol (LC grade, Merck) and filtered using a 0.45-µm polyvinylidene difluoride filter (Millipore, Billerica, MA, USA) prior to HPLC injection in the same manner as in the adenosine and cordycepin assay. The used HPLC system and column were the same as for the adenosine and cordycepin assay. The mobile phase was methanol/H2O (95:5 by volume) at a flow rate of 1.0 mL/min, and UV detection was done at λ=254 nm. The content of vitamin D2 and ergosterol was calculated on the basis of the calibration curve of authentic vitamin D2 and ergosterol (Sigma-Aldrich).
Polysaccharides were extracted and analysed according to the method of Huang et al. (23). A sample (1 g) was refluxed with 50 mL of deionized water for 30 min. The mixture was cooled to room temperature and filtered through Whatman No. 4 filter paper (Sigma-Aldrich). The residue was then refluxed with two additional 10-mL portions of deionized water for 30 min, cooled and filtered. The combined filtrate was dialyzed using a Cellu Sep T2 tubular membrane (MMCO=6000-8000; Membrane Filtration Products, Inc., Seguin, TX, USA) for 24 h. The water soluble polysaccharide content was determined by the phenol- -sulfuric acid assay (29).
Preparation of extracts
For ethanolic extraction, a subsample (10 g, dry basis) was extracted by stirring with 100 mL of 95% (by volume) ethanol at 25 °C and 150 rpm for 24 h and filtered through Whatman No. 1 filter paper (Sigma-Aldrich). The residue was then extracted with two additional 100-mL portions of ethanol and filtered. The combined ethanolic extracts were then dried in a rotary evaporator (N-1000; EYELA) at 40 °C until the solvent was evaporated. For hot water extraction, a subsample (10 g) was extracted by stirring with 100 mL of boiling water at 100 °C and 150 rpm for 10 min and filtered. The residue was extracted with two additional 100-mL portions of boiling water as described above. The combined hot water extracts were then freeze dried. The dried ethanolic and hot water extracts were redissolved in ethanol and water respectively to concentrations of 50 mg/mL and stored at 4 °C for further use.
Determination of antioxidant properties
The reducing power was determined according to the method of Oyaizu (30). The reducing power assayed the ability of the extracts to form a coloured complex with ferricyanide, which is an electron acceptor. The ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich) radicals was determined according to Shimada et al. (31). The sample chelating ability was determined according to the method of Dinis et al. (32). EC50 values were obtained by interpolation from linear regression analysis.
Determination of antioxidant components
Flavonoids were determined according to the method of Zhishen et al. (33). An aliquot (0.5 mL) of an appropriately diluted sample or a standard solution of rutin (Sigma--Aldrich) was added to the flask that contained 0.1 mL of 5% aqueous NaNO2 solution. After 6 min, 0.1 mL of 10% aqueous AlCl3 solution was added, and after another 6 min, 1 mL of 5% aqueous NaOH solution was added to the mixture. Next, the mixture was diluted with 0.8 mL of deionized water and thoroughly mixed. The absorbance of the mixture was determined at 510 nm against a blank using spectrophotometer (U-2900; Hitachi, Tokyo, Japan). The flavonoid content in the sample was expressed in mg of rutin equivalents (RE) per g of sample.
Total phenols were determined according to the method of Taga et al. (34). Each sample (20 mg) was dissolved in a solution of 5 mL of 1.3% HCl in methanol/deionized water (60:40 by volume), and the resulting mixture (100 μL) was added to 2 mL of 2% aqueous Na2CO3 solution. After 3 min, 100 μL of 50% Folin-Ciocalteu’s phenol reagent (Sigma-Aldrich) were added to the mixture. After resting for 30 min, the absorbance was measured at 750 nm against a blank using spectrophotometer model U-2900 (Hitachi). The content of total phenols in the sample was calculated on the basis of the calibration curve of gallic acid (Sigma--Aldrich) and was expressed in mg of gallic acid equivalents (GAE) per g of sample.
Statistical analysis
A one-way analysis of variance (ANOVA) for a completely random design was used, with Statistical Analysis System v. 9.4 (SAS Institute, Inc., Cary, NC, USA). The results presented in this paper were the average of three independent assays and expressed as the mean value±standard deviation (S.D.). The difference among the mean values was determined using Duncan’s multiple range tests at the level of α=0.05. The EC50 values were obtained from linear regression analysis. Principal component analysis (PCA) provided an overview of the relationships between irradiation and the biologically active components, antioxidant properties and antioxidant components of buckwheat and embryo rice. PCA analysis was performed using XLSTAT-Pro v. 2016 software (Addinsoft, Inc., Brooklyn, NY, USA), which provides the internal structure of the data and gives good dispersion of data (35).
Results and Discussion
Biologically active components
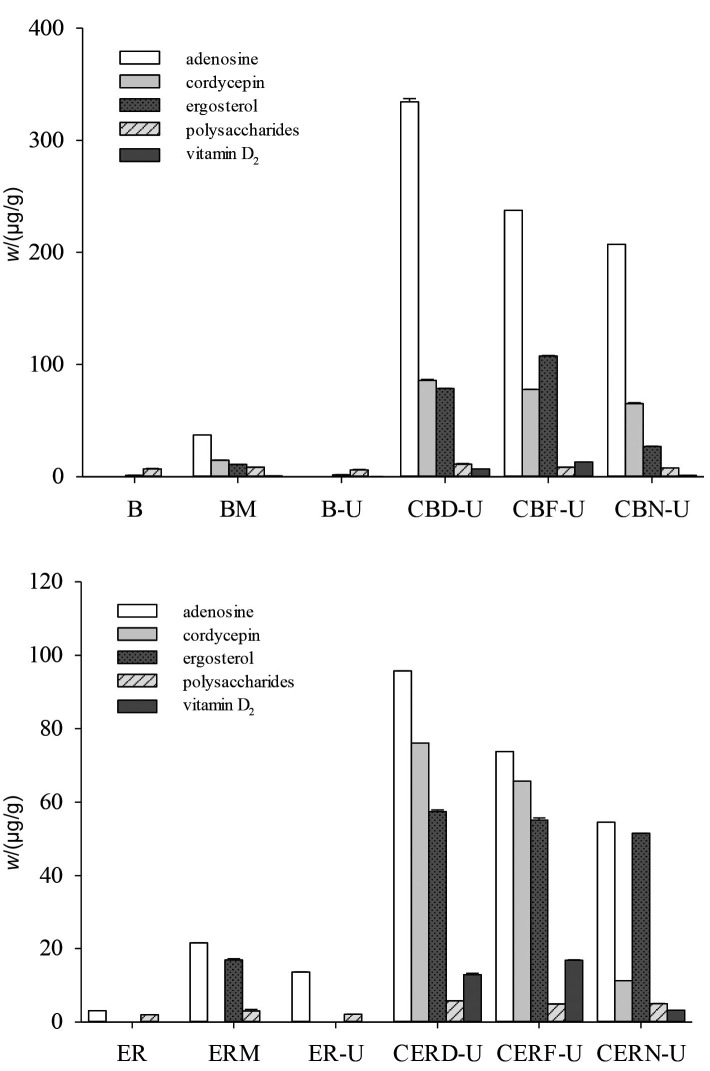
Three types of unfermented controls (substrates, substrates containing 5% mycelia, and UVB irradiated substrates) contained trace amounts of vitamin D2 (<0.71 µg/g) (Fig. 1). With UVB irradiation of the three differently treated fermented products, the vitamin D2 content was in the descending order: fresh fermented products>dried fermented products>untreated fresh products. The fermented and UVB irradiated buckwheat and embryo rice contained the highest mass fractions of vitamin D2 (13.03 and 16.79 µg/g, respectively). According to the results, these samples with higher moisture content absorb more irradiation energy for vitamin D2 conversion. Drying of buckwheat and embryo rice resulted in less efficient absorption of UVB irradiation. However, pre-fermentation with UVB irradiation could denature proteins and damage the DNA in living organisms, and hence suppress the growth of microorganisms, resulting in lower conversion of vitamin D2.
Fig. 1.
Effect of UVB irradiation on the functional components of samples fermented by Cordyceps militaris and grain substrates: a) buckwheat and b) embryo rice. B=buckwheat, BM=buckwheat mycelia, B-U=UVB irradiated buckwheat, CBD-U=fermented then dried and UVB irradiated buckwheat, CBF-U=fermented and UVB irradiated buckwheat, CBN-U=fermented unirradiated buckwheat, ER=embryo rice, ERM=embryo rice mycelia, ER-U=UVB irradiated embryo rice, CERD-U=fermented then dried and UVB irradiated embryo rice, CERF-U=fermented and UVB irradiated embryo rice, CERN-U=fermented unirradiated embryo rice
The three variously treated fermented products contained higher mass fractions of ergosterol (26.91-107.46 µg/g) than the unfermented controls (0-16.89 µg/g) (Fig. 1). Generally, ergosterol content has been positively correlated with mycelial biomass in products obtained by solid-state fermentation (36). Therefore, C. militaris-fermented buckwheat samples contained higher mass fractions of ergosterol than C. militaris-fermented embryo rice samples, except for the non-irradiated buckwheat sample. Simon et al. (37) reported that the vitamin D2 content in mushrooms was due to photosynthetic/thermal processes occurring from the exposure of ergosterol to UV light. UVB irradiation of SSF products resulted in nonsignificant decreases in ergosterol content, whereas vitamin D2 content significantly increased. Guan et al. (22) reported that ergosterol may not be the limiting factor of vitamin D2 conversion since previtamin D2 can undergo several reversible photoreactions.
Process operating conditions, mushroom morphology and fermentation types have been shown to play important roles in vitamin D2 accumulation in mushrooms. Jasinghe and Perera (38) reported that the optimum moisture content of shiitake for the conversion of ergosterol to vitamin D2 was approx. 70-80% on a wet mass basis. Therefore, we used the fresh fermented and UVB irradiated buckwheat and embryo rice that contained 70-80% moisture, which could enhance such vitamin D2 conversion. Furthermore, both the fresh and dried samples have the ability to produce vitamin D2 when they are subjected to the UVB irradiation.
Adenosine and cordycepin are metabolic products resulting from mycelial growth on grains. Polysaccharides are the most abundant and well-known bioactive constituents of edible and medicinal mushrooms. Dried fermented and UVB irradiated buckwheat and embryo rice contained higher mass fractions of adenosine (95.75 and 334.34 µg/g), cordycepin (76.11 and 85.78 µg/g) and polysaccharides (5.73 and 11.15 mg/g), respectively, than the fresh and untreated fermented products (Fig. 1). After UVB irradiation, the adenosine, cordycepin and polysaccharide contents of dried fermented products demonstrated a 1.10 to 1.41-fold greater increase than the fresh products. The UVB irradiation in the presence of 70-80% moisture might cause the decomposition of adenosine, cordycepin and polysaccharide in fresh products. In other words, refermentation with UVB irradiation could denature proteins and damage DNA in living organisms and hence suppress the growth of microorganisms, resulting in lower conversion of bioactive compounds.
Huang et al. (20) reported that UVB irradiation could increase the vitamin D2 content of mushroom fruiting bodies and mycelia. Based on the obtained results, the following experiment on UVB irradiation can be applied in solid--state fermentation of mushroom.
Antioxidant properties
The antioxidant properties assayed herein are summarized in Table 1, and the EC50 values (mg of ethanolic and hot water extracts per mL) were calculated for comparison. The effectiveness of antioxidant properties correlates inversely with their EC50 values. The EC50 values of the ethanolic and hot water extracts of C. militaris-fermented buckwheat (0.85-6.8 and 0.58-17.4 mg/mL, respectively) were lower than those of both extracts of unfermented buckwheat (1.58-24.0 and 0.68-35.4 mg/mL, respectively). Similarly, the EC50 values of the ethanolic and hot water extracts of C. militaris-fermented embryo rice (1.3-10.9 and 6.37-26.2 mg/mL, respectively) were lower than those of both extracts of unfermented embryo rice (2.7-31.1 and 8.0-46.8 mg/mL, respectively).
Table 1. Effects of UVB irradiation on the EC50 values of ethanolic and hot water extracts of samples fermented by Cordyceps militaris and grain substrates.
| Extract | EC50/(mg extract per mL) | ||||||
|---|---|---|---|---|---|---|---|
| B | BM | B-U | CBD-U | CBF-U | CBN-U | ||
| Ethanolic | Reducing power | (16.0±0.9)b | (1.94±0.03)c | (24.0±0.9)a | (0.85±0.01)d | (0.96±0.01)d | (0.99±0.02)d |
| Scavenging ability | (1.65±0.06)b | (1.94±0.01)a | (1.58±0.02)b | (1.38±0.04)c | (0.95±0.01)e | (1.28±0.05)d | |
| Chelating ability | (8.2±0.1)a | (6.9±0.4)b | (8.7±0.4)a | (5.4±0.2)c | (3.7±0.1)d | (6.8±0.4)b | |
| Hot water | Reducing power | (21.0±0.7)b | (20.8±0.8)b | (26.5±2.8)a | (10.6±0.5)d | (12.6±0.1)d | (17.4±0.5)c |
| Scavenging ability | (14.0±0.7)b | (20.8±0.4)b | (35.4±2.2)a | (7.5±0.4)e | (8.4±0.1)d | (9.7±0.7)d | |
| Chelating ability | (0.69±0.01)b | (0.68±0.01)b | (0.87±0.01)a | (0.58±0.02)d | (0.65±0.01)c | (0.68±0.02)b | |
| ER | ERM | ER-U | CERD-U | CERF-U | CERN-U | ||
| Ethanolic | Reducing power | (28.5±0.3)b | (14.73±0.71)c | (31.11±0.62)a | (1.3±0.2)d | (1.6±0.9)d | (1.4±0.3)d |
| Scavenging ability | (2.72±0.06)d | (2.92±0.04)b | (3.16±0.02)a | (2.84±0.06)bc | (2.79±0.02)cd | (2.79±0.07)cd | |
| Chelating ability | (23.9±0.6)b | (15.1±0.5)c | (26.0±0.4)a | (10.9±0.8)d | (7.8±0.4)e | (7.6±0.1)e | |
| Hot water | Reducing power | (36.1±1.3)b | (26.5±0.5)c | (46.8±1.1)a | (25.0±0.6)d | (22.9±0.2)e | (26.2±0.2)cd |
| Scavenging ability | (26.8±0.7)b | (17.78±0.04)c | (43.7±0.6)a | (12.2±0.7)f | (15.3±0.2)e | (16.2±0.1)d | |
| Chelating ability | (12.2±0.6)a | (8.3±0.7)b | (8.0±0.2)bc | (7.6±0.1)c | (6.37±0.03)d | (6.8±0.2)d | |
B=buckwheat, BM=buckwheat mycelia, B-U=UVB irradiated buckwheat, CBD-U=fermented then dried and UVB irradiated buckwheat, CBF-U=fermented and UVB irradiated buckwheat, CBN-U=fermented unirradiated buckwheat, ER=embryo rice, ERM=embryo rice mycelia, ER-U=UVB irradiated embryo rice, CERD-U=fermented then dried and UVB irradiated embryo rice, CERF-U=fermented and UVB irradiated embryo rice, CERN-U=fermented embryo rice without UVB irradiation. EC50 values were obtained by interpolation from linear regression analysis A750 nm=0.5 in reducing power assay, 1,1-diphenyl-2-picrylhydrazyl radicals were scavenged by 50% and ferrous ions were chelated by 50%. Each value is expressed as the mean±standard error (N=3). Mean values with different superscript letters within a row differ significantly (p<0.05)
For the reducing power, scavenging ability and chelating ability assays, the EC50 values of the ethanolic extracts of fresh fermented and UVB irradiated buckwheat and embryo rice were more effective than those of the dried fermented and irradiated both samples and unirradiated buckwheat, while fermented unirradiated embryo rice was more effective than buckwheat. In addition, the EC50 values of the hot water extracts from fermented then dried and UVB irradiated dried buckwheat, and fermented and UVB irradiated embryo rice demonstrated greater effectiveness than those of the fresh fermented and UVB irradiated buckwheat, fermented and unirradiated buckwheat, fermented then dried and UVB irradiated embryo rice and fermented unirradiated embryo rice. Overall, the ethanolic extracts of UVB irradiated fresh buckwheat and embryo rice fermented by C. militaris demonstrated greater antioxidant properties than irradiated dried and unfermented substrates.
Zhang et al. (39) found that the EC50 values of the 70% ethanolic extracts of unfermented wheat and wheat fermented by C. militaris were 0.52 and 0.58 mg/mL for reducing power, 0.08 and 0.06 mg/mL for scavenging ability, and 0.61 and 0.26 mg/mL for chelating ability, respectively. Furthermore, regarding the three antioxidant properties assayed, it is clear that both extracts of the C. militaris--fermented products were more effective than those of the unfermented substrates. In addition, the hot water extracts were less effective than the ethanolic extracts, as evidenced by their higher EC50 values, except that the reducing power of the ethanolic extracts was less effective than that of the hot water extracts. Both phenomena were consistent with previous findings (40).
Antioxidant components
Flavonoids act as antioxidants, antimicrobials, photoreceptors, visual attractors, feeding repellents, and light screening substances in plants. In both extracts, the samples of buckwheat and embryo rice dried after incubation and UVB irradiation had the highest flavonoid content, except for irradiated dried buckwheat (Fig. 2). In addition, in both extracts, the flavonoid content of C. militaris-fermented substances (0.57-6.29 mg/mL) was higher than those of both extracts of unfermented substrates (0.48-5.15 mg/mL). Zhang et al. (39) found that in ethanolic and water extracts, the total phenolic content of C. militaris-fermented wheat (32.79-41.63 mg/g) was higher than of unfermented wheat (29.37-36.94 mg/g). Liu et al. (41) found that postharvest irradiation with UVB enhanced the levels of flavonoids, phenols and total phenols in fruiting bodies on tomato. It appears that UVB irradiation of fresh products could increase their flavonoid content. UVB irradiation of dried products seems to have had only a slight influence on the flavonoid content.
Fig. 2.
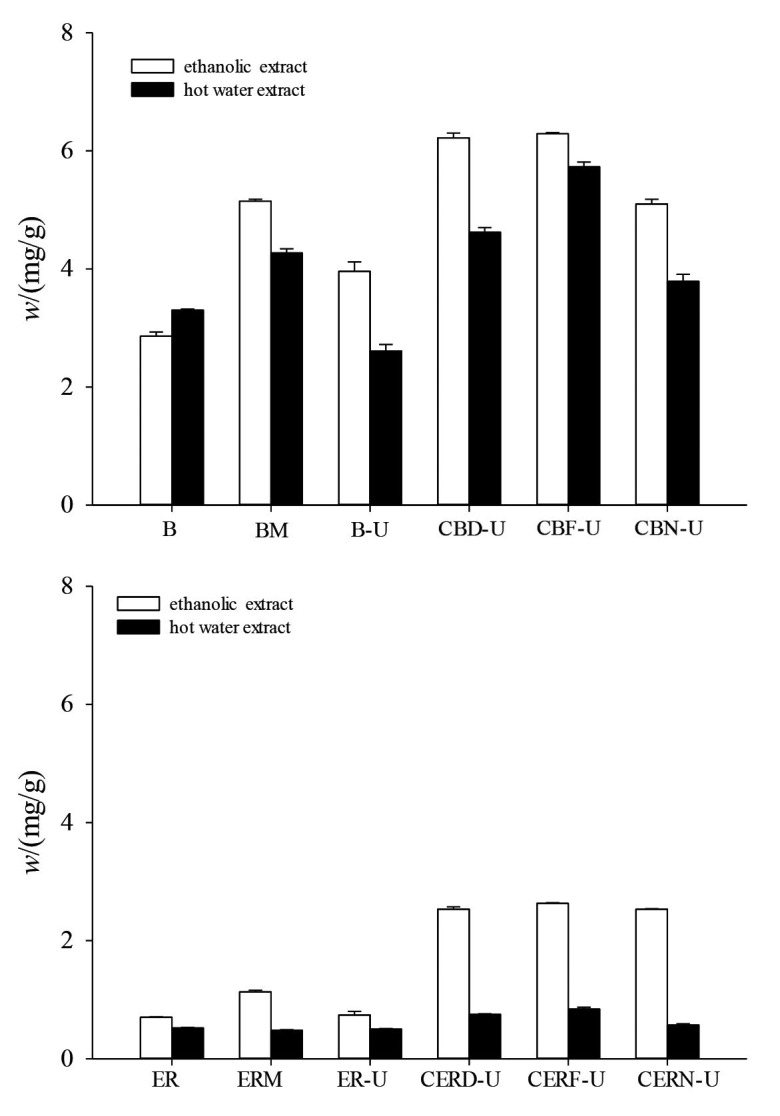
Effect of UVB irradiation on the flavonoid content of samples fermented by Cordyceps militaris and grain substrates: a) buckwheat and b) embryo rice. B=buckwheat, BM=buckwheat mycelia, B-U=UVB irradiated buckwheat, CBD-U=fermented then dried and UVB irradiated buckwheat, CBF-U=fermented and UVB irradiated buckwheat, CBN-U=fermented unirradiated buckwheat, ER=embryo rice, ERM=embryo rice mycelia, ER-U=UVB irradiated embryo rice, CERD-U=fermented then dried and UVB irradiated embryo rice, CERF-U=fermented and UVB irradiated embryo rice, CERN-U=fermented unirradiated embryo rice
In both ethanolic and hot water extracts, the total phenolic content (3.28-20.37 mg/g) (Fig. 3) was higher than the flavonoid content (0.48-6.29 mg/g) (Fig. 2). Phenolic compounds are widely distributed in mushrooms. As shown in Fig. 3, in both extracts, UVB irradiated samples of dried buckwheat and embryo rice contained the highest total phenolic contents, except for the embryo rice dried after incubation and UVB irradiation (Fig. 3). The high mass fractions of total phenols and flavonoids in the ethanolic and hot water extracts might explain their higher reducing power and scavenging ability of DPPH radicals. These results are consistent with their assayed effectiveness in antioxidant properties. Phenols such as BHT (butylated hydroxytoluene) and gallate are known to be effective antioxidants (42). Due to their free radical scavenging and ferrous ion chelating abilities, phenols may possess good antioxidant, antimutagenic and anticancer properties (43). Overall, C. militaris can enhance flavonoid and total phenolic contents by solid-state fermentation. Furthermore, UVB irradiation has no influence on the flavonoid and total phenolic content during the solid-state fermentation, as these irradiated samples still contained sufficient quantities of these antioxidant components.
Fig. 3.
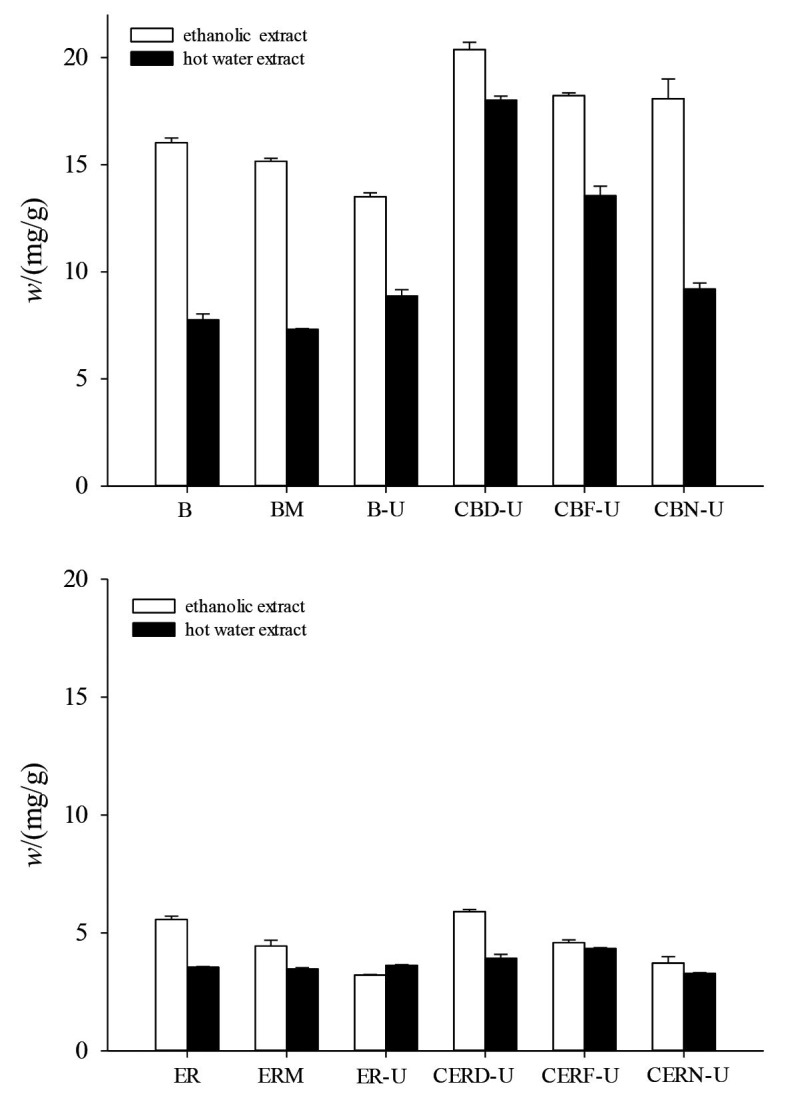
Effect of UVB irradiation on total phenolic content of samples fermented by Cordyceps militaris and grain substrates: a) buckwheat and b) embryo rice. B=buckwheat, BM=buckwheat mycelia, B-U=UVB irradiated buckwheat, CBD-U=fermented then dried and UVB irradiated buckwheat, CBF-U=fermented and UVB irradiated buckwheat, CBN-U=fermented unirradiated buckwheat, ER=embryo rice, ERM=embryo rice mycelia, ER-U=UVB irradiated embryo rice, CERD-U=fermented then dried and UVB irradiated embryo rice, CERF-U=fermented and UVB irradiated embryo rice, CERN-U=fermented unirradiated embryo rice
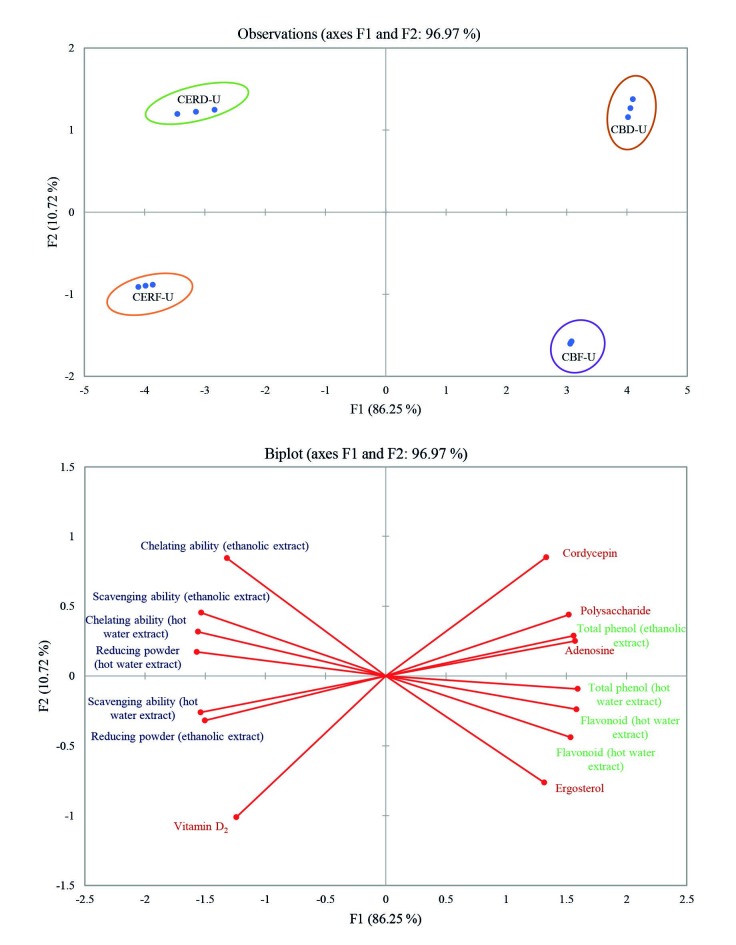
Principal components analysis
To gain an overview of the relationships among the biologically effective components, antioxidant properties and antioxidant components in the irradiated samples, principal component analysis (PCA) was performed, and the results are shown in Fig. 4. The multivariate treatment of the data obtained for the samples permitted the reduction of the variables to two principal components, which together explained 96.97% of the total variability. The first axis accounted for 86.25% and the second axis for 10.72%. A PCA score plot shows that irradiated C. militaris-fermented products were quantitatively distinguished as four sets of three replicates, indicating that irradiated samples of both dried and fresh buckwheat and embryo rice had entirely different profiles of biologically active components, antioxidant properties and antioxidant components as well as a dispersed score plot. In addition, in plots for components and properties (Fig. 4a), irradiated both fresh and dried buckwheat had positive values in F1, while both irradiated dried samples had positive values in F2. The antioxidant properties and biological and antioxidant components in F1 and F2 were dispersed actinomorphically from the origin (Fig. 4b). Vitamin D2 and ergosterol seemed to correlate negatively. From the score and loading plots, irradiated dried buckwheat positively correlated with cordycepin, irradiated fresh embryo rice positively correlated with vitamin D2, irradiated fresh buckwheat positively correlated with ergosterol, and irradiated dried embryo rice positively correlated with chelating ability and scavenging ability with ethanolic extracts. In summary, irradiated products fermented by C. militaris were well separated in score plots, and their biologically active components, antioxidant properties and antioxidant components were individually dispersed using PCA. Therefore, PCA could be helpful to provide valuable information on relationships among the biologically active components, antioxidant properties and antioxidant components of irradiated products fermented by C. militaris.
Fig. 4.
Principal component analysis of the biologically active components, antioxidant properties and antioxidant components of samples fermented by Cordyceps militaris: a) score and b) loading plots. CBD-U=fermented then dried and UVB irradiated buckwheat, CBF-U=fermented and UVB irradiated buckwheat, CERD-U=fermented then dried and UVB irradiated embryo rice, CERF-U=fermented and UVB irradiated embryo rice
Conclusions
After UVB irradiation, fresh buckwheat and embryo rice fermented by C. militaris had higher vitamin D2 and flavonoid content, antioxidant properties of ethanolic extracts and antioxidant activity than dried fermented products. However, after UVB irradiation, adenosine, cordycepin, polysaccharide, and total phenolic contents were higher in dried products fermented by C. militaris than those of fresh fermented products. Therefore, to develop products rich in vitamin D2, fresh products fermented by C. militaris are the best choice; however, when considering cordycepin, polysaccharides or other biologically active components, fermentation of dried products by C. militaris may be better choice, because the dried samples are easy to store and process, while fresh samples require additional attention such as cold storage. This study presents an innovative approach to the improvement of biologically active ingredients of buckwheat and embryo rice fermented by C. militaris in SSF. Future research will be oriented towards the solid-state fermentation after grinding of buckwheat and embryo rice into a dry powder and then UVB irradiation, and development of functional ingredients for food, cosmetics or animal feed production.
Acknowledgements
The authors are indebted to the Ministry of Science and Technology (MOST), Taiwan, ROC (contract no. MOST 105-2221-E-468-022) and the Department of Medical Research, China Medical University Hospital, China Medical University, Taiwan, ROC (contract no. ASIA-105-CMUH-13) for financial support.
References
- 1.Chan JSL, Barseghyan GS, Asatiani MD, Wasser SP. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of cateripillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int J Med Mushrooms. 2015;17:649–59. 10.1615/IntJMedMushrooms.v17.i7.50 [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia. 2010;81:961–8. 10.1016/j.fitote.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Kim SB, Ahn B, Kim M, Ji HJ, Shin SK, Hong IP, et al. Effect of Cordyceps militaris extract and active constituents on metabolic parameters of obesity induced by high-fat diet in C58BL/6J mice. J Ethnopharmacol. 2014;151:478–84. 10.1016/j.jep.2013.10.064 [DOI] [PubMed] [Google Scholar]
- 4.Wang BS, Lee CP, Chen ZT, Yu HM, Duh PD. Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. J Funct Foods. 2012;4:489–95. 10.1016/j.jff.2012.02.009 [DOI] [Google Scholar]
- 5.Zhu SJ, Pan J, Zhao B, Liang J, Wu ZY, Yang JJ. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J Ethnopharmacol. 2013;149:713–9. 10.1016/j.jep.2013.07.037 [DOI] [PubMed] [Google Scholar]
- 6.Lin R, Liu H, Wu S, Pang L, Jia M, Fan K, et al. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int J Biol Macromol. 2012;51:153–7. 10.1016/j.ijbiomac.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Cui JD, Zhang YN. Evaluation of metal ions and surfactants effect on cell growth and exopolysaccharide production in two- -stage submerged culture of Cordyceps militaris. Appl Biochem Biotechnol. 2012;168:1394–404. 10.1007/s12010-012-9865-7 [DOI] [PubMed] [Google Scholar]
- 8.Cui JD, Zhu BZ. Comparison of culture methods on exopolysaccharide production in the submerged culture of Cordyceps militaris and process optimization. Lett Appl Microbiol. 2011;52:123–8. 10.1111/j.1472-765X.2010.02987.x [DOI] [PubMed] [Google Scholar]
- 9.Cui JD. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit Rev Biotechnol. 2015;35:475–84. 10.3109/07388551.2014.900604 [DOI] [PubMed] [Google Scholar]
- 10.Cui JD. The traditional Chinese medicine fungus Cordyceps and its biotechnological production. Res J Biotechnol. 2013;8:1–2. [Google Scholar]
- 11.Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, et al. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. Lebensm Wiss Technol. 2015;63:1317–24. 10.1016/j.lwt.2015.04.046 [DOI] [Google Scholar]
- 12.Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, et al. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J Funct Foods. 2014;10:210–22. 10.1016/j.jff.2014.06.008 [DOI] [Google Scholar]
- 13.Pandey A. Solid-state fermentation. Biochem Eng J. 2003;13:81–4. 10.1016/S1369-703X(02)00121-3 [DOI] [PubMed] [Google Scholar]
- 14.Zhang GP, Zhang F, Ru WM, Han JR. Solid-state fermentation of cornmeal with the ascomycete Morchella esculenta for degrading starch and upgrading nutritional value. World J Microbiol Biotechnol. 2010;26:5–20. 10.1007/s11274-009-0135-y [DOI] [Google Scholar]
- 15.Xiao Y, Rui X, Xing G, Wu H, Li W, Chen X, et al. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct Food. 2015;16:58–73. 10.1016/j.jff.2015.04.032 [DOI] [Google Scholar]
- 16.Razak DLA, Rashid NYA, Jamaluddin A, Sharifudin SA, Long K. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal Agric Biotechnol. 2015;4:33–8. 10.1016/j.bcab.2014.11.003 [DOI] [Google Scholar]
- 17.Chien RC, Ulziijargal E, Mau JL. Quality of bread supplemented with Antrodia salmonea-fermented grains. Food Technol Biotechnol. 2016;54:180–8. 10.17113/ftb.54.02.16.4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorde R, Grimnes G. Vitamin D and health: The need for more randomized controlled trials. J Steroid Biochem Mol Biol. 2015;148:269–74. 10.1016/j.jsbmb.2015.01.021 [DOI] [PubMed] [Google Scholar]
- 19.Cashman KD, Vitamin D. Dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol. 2015;148:19–26. 10.1016/j.jsbmb.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 20.Huang SJ, Lin CP, Tsai SY. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. J Food Compos Anal. 2015;42:38–45. 10.1016/j.jfca.2015.02.005 [DOI] [Google Scholar]
- 21.Sławińska A, Fornal E, Radzki W, Skrzypczak K, Zalewska-Korona M, Michalak-Majewska M, et al. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016;199:203–9. 10.1016/j.foodchem.2015.11.131 [DOI] [PubMed] [Google Scholar]
- 22.Guan W, Zhang J, Yan R, Shao S, Zhou T, Lei J, et al. Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus). Food Chem. 2016;210:129–34. 10.1016/j.foodchem.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 23.Huang SJ, Lin CP, Mau JL, Li YS, Tsai SY. Effect of UV-B irradiation on physiologically active substance content and antioxidant properties of the medicinal caterpillar fungus Cordyceps militaris (Ascomycetes). Int J Med Mushrooms. 2015;17:241–53. 10.1615/IntJMedMushrooms.v17.i3.40 [DOI] [PubMed] [Google Scholar]
- 24.Witting M, Krings U, Berger RG. Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J Food Compos Anal. 2013;31:266–74. 10.1016/j.jfca.2013.05.017 [DOI] [Google Scholar]
- 25.Wu WJ, Ahn BY. Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PLoS One. 2014;9:e95359. 10.1371/journal.pone.0095359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbain P, Valverde J, Jakobsen J. Impact on vitamin D2, vitamin D4 and agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods Hum Nutr. 2016;71:314–21. 10.1007/s11130-016-0562-5 [DOI] [PubMed] [Google Scholar]
- 27.Mau JL, Chen PR, Yang JH. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J Agric Food Chem. 1998;46:5269–72. 10.1021/jf980602q [DOI] [Google Scholar]
- 28.Masuda M, Urabe E, Sakurai A, Sakakibara M. Production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2006;39:641–6. 10.1016/j.enzmictec.2005.11.010 [DOI] [Google Scholar]
- 29.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. 10.1021/ac60111a017 [DOI] [Google Scholar]
- 30.Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. 10.5264/eiyogakuzashi.44.307 [DOI] [Google Scholar]
- 31.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. 10.1021/jf00018a005 [DOI] [Google Scholar]
- 32.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. 10.1006/abbi.1994.1485 [DOI] [PubMed] [Google Scholar]
- 33.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. 10.1016/S0308-8146(98)00102-2 [DOI] [Google Scholar]
- 34.Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc. 1984;61:928–31. 10.1007/BF02542169 [DOI] [Google Scholar]
- 35.Šamec D, Maretić M, Lugarić I, Mešić A, Salopek-Sondi B, Duralija B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016;194:828–34. 10.1016/j.foodchem.2015.08.095 [DOI] [PubMed] [Google Scholar]
- 36.Chen SY, Ho KJ, Liang CH, Tsai CH, Huang LY, Mau JL. Preparation of culinary-medicinal king oyster mushroom Pleurotus eryngii-fermented products with high ergothioneine content and their taste quality. Int J Med Mushrooms. 2012;14:85–93. 10.1615/IntJMedMushr.v14.i1.90 [DOI] [PubMed] [Google Scholar]
- 37.Simon RR, Borzelleca JF, DeLuca HF, Weaver CM. Safety assessment of the post-harvest treatment of button mushrooms (Agaricus bisporus) using ultraviolet light. Food Chem Toxicol. 2013;56:278–89. 10.1016/j.fct.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 38.Jasinghe VJ, Perera CO. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005;92:541–6. 10.1016/j.foodchem.2004.08.022 [DOI] [Google Scholar]
- 39.Zhang Z, Lv G, Pan H, Fan L, Soccol CR, Pandey A. Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol Biotechnol. 2012;50:32–9. [Google Scholar]
- 40.Chiang SS, Ulziijargal E, Chien RC, Mau JL. Antioxidant and anti-inflammatory properties of solid-state fermented products from a medicinal mushroom, Taiwanofungus salmoneus (higher Basidiomycetes) from Taiwan. Int J Med Mushrooms. 2015;17:21–32. 10.1615/IntJMedMushrooms.v17.i1.30 [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Han X, Cai L, Lu X, Ying T, Jiang Z. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant capacity in tomato fruiting during storage. Postharvest Biol Technol. 2011;59:232–7. 10.1016/j.postharvbio.2010.09.003 [DOI] [Google Scholar]
- 42.Madhavi DL, Singhal RS, Kulkarni PR. Technological aspects of food antioxidants. In: Madhavi DL, Deshpande SS, Salunkhe DK. Food antioxidants: technological, toxicological, and health perspectives. New York NY, USA: Marcel Dekker; 1996. pp. 159-265. [Google Scholar]
- 43.Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999;57:78–83. 10.1111/j.1753-4887.1999.tb06927.x [DOI] [PubMed] [Google Scholar]