Abstract
Objectives:
Management of eosinophilic esophagitis (EoE) requires repeated endoscopic mucosal sampling to assess disease activity. A less invasive and expensive means of monitoring of EoE is required. The objective of this study was to assess the accuracy, safety, and tolerability of the cytosponge compared to endoscopy and biopsy for histologic assessment of EoE.
Methods:
In this prospective two-center cross-sectional study, patients with known EoE underwent cytosponge sampling followed by endoscopy and biopsy. Sample adequacy and eosinophil counts (eos/HPF) were determined for both cytosponge and endoscopic samples. The cytosponge was assessed for diagnostic accuracy, safety, and patient preference as compared to endoscopy.
Results:
Six patients (7%) failed to swallow the sponge. One hundred and five procedures were successfully performed in 80 patients (66% male, 100% white, 19% stricture). The cytosponge sample was adequate in 102 and the biopsy in 104; 101 procedures had adequate samples by both techniques. Fifty-seven biopsies were graded as active EoE with ≥15 eos/HPF as the gold standard. Eosinophil counts highly correlated between the biopsy and cytosponge (r=0.78, P<0.0001). Using a cutoff of ≤15 eos/HPF for inactive disease, the sensitivity and specificity of the cytosponge was 75% and 86%, respectively. Six patients had active EoE on cytosponge not found on biopsy. For biopsies with inactive EoE, the cytosponge identified 38/44. No complications occurred, and cytosponge endoscopic abrasion scores were low (0.34/4). Patients preferred cytosponge to endoscopy with higher rating scores (7.27 vs. 6.11, P=0.002).
Conclusions:
Compared to endoscopy with biopsy, cytosponge provided a minimally invasive, safe, well tolerated, and accurate method to assess EoE histologic activity. (ClinicalTrial.gov number NCT01585103).
Introduction
Eosinophilic esophagitis is a relatively new disease in which esophageal eosinophilia leading to inflammation and stricture formation is thought to result from exposure to food antigens (1). As symptoms do not reliably reflect disease activity (1, 2, 3, 4) and ongoing inflammation commonly leads to fibrosis and stricture formation (3, 4, 5), consensus guidelines recommend assessing response to diet or steroid therapy by endoscopy and biopsy (6, 7). With the invasiveness and high costs of endoscopy (8, 9, 10), an alternative and less invasive form of monitoring therapeutic response in EoE is be highly desired (11, 12, 13, 14).
The cytosponge consists of an ingestible gelatin capsule containing a compressed mesh sponge attached to a string developed for esophageal cancer screening (15). The capsule is swallowed, and once in the stomach the gelatin dissolves and a spherical mesh of 3 cm diameter is released. The mesh is withdrawn through the mouth by traction on the attached string, and as the sponge passes through the esophagus a tissue specimen is collected (16, 17). Studies in patients with Barrett’s esophagus have demonstrated high diagnostic sensitivity and patient acceptance, lower cost, and an excellent safety profile (18, 19). In a pilot study of 20 patients using the cytosponge for assessing disease activity in eosinophilic esophagitis (20), the cytosponge demonstrated 83% sensitivity, was performed without adverse effects, and was well tolerated by patients.
The aim of this study was to expand these initial data to a larger two-center study to further determine the accuracy, safety, and tolerability of the cytosponge compared to standard endoscopy and esophageal biopsy for histologic assessment of EoE.
Methods
Patients and clinical data
We conducted a prospective cross-sectional study at the Mayo Clinic, Rochester and the University of North Carolina (UNC), Chapel Hill. Eligible patients were adults (≥18 years) scheduled for a clinically indicated upper endoscopy who had EoE diagnosed in accordance with consensus guidelines (1), including a lack of histologic response to proton pump inhibitors. This endoscopy could be performed at initial diagnosis or after a course of treatment with either topical steroids or dietary elimination. Exclusion criteria were esophageal stricture with inability to pass a standard adult upper endoscope; history of esophageal perforation; history of esophageal resection; known esophageal varices; coagulopathy, active anticoagulation, or active GI bleeding; and pregnancy.
Demographic and clinical information and endoscopic and histologic findings were collected on each patient using standardized case-report forms. Patients also filled out a questionnaire (20) assessing the cytosponge and endoscopy experiences with a 10 cm numeric visual analog scale (10 most favorable). Comments were requested and patients were asked which procedure was preferable. A subset of patients also completed the impact of events scale (range 0–60, with high scores representing a large impact) (21) immediately after the procedures as well as at 7 days. This study was approved by the Institutional Review Boards at both Mayo Clinic and UNC, and was registered on clinicaltrials.gov (NCT02114606).
Upper gastrointestinal endoscopy
Endoscopy was performed in standard fashion in an outpatient setting using administration of midazolam and fentanyl or propofol for conscious sedation, ~2 h after the cytosponge was administered. EoE endoscopic findings were assessed and scored using the validated EoE endoscopic reference classification system (22). At the time of endoscopy, an endoscopic-graded assessment of cytosponge mucosal injury was made using an abrasion score (Supplementary Table 1) (15). Four esophageal biopsies were obtained from both the distal and proximal esophagus for analysis.
Cytosponge administration and sampling
Sample collection procedure
The procedure was performed 2 h before endoscopy was scheduled (20). Briefly, the capsule and bunched up string were placed by the investigator on the back of the patient’s tongue and were swallowed with water. The string was held without any tension to allow the capsule to move into the stomach. The patient was instructed to hold onto the string for 5 min after ingestion to allow the gelatin capsule to dissolve in the proximal stomach with release of the spherical mesh. If the patient preferred, the back of the throat was sprayed with 1% lidocaine and the expanded mesh was withdrawn by pulling on the string with the patient is a sitting position. After retrieval of the mesh, the string was cut and the cytosponge specimen was placed in a methanol-based preservative fluid (PreservCyt, Cytyc, Marlborough, MA) and kept at room temperature until transportation to the laboratory. We monitored for any adverse events related to cytosponge administration.
Due to availability, two manufacturers of the cytosponge were used for this study (Covidien, Minneapolis, MN, and Europlaz Technologies, Essex, UK). Both sponges were comparable in design and composition, with the same sponge diameter and number of pores per square inch (18, 19). Eighty cytosponges were used from Europlaz and 31 sponges from Covidien.
Sample processing
Procedures standard to pathology laboratories were used for processing the cytosponge specimen. The container containing the sponge was vortexed to remove free cells. The fluid was then poured into a 50 ml Falcon tube and centrifuged for 10 min at 2500 rpm yielding a pellet of 3–4 ml. The pellet was removed and repeat vortex of the supernatant and sponge was performed to yield more cells, which were combined with the original pellet. The pellet was then suspended in 40 ml of plasmalyte to the Falcon tube. This specimen was then re-centrifuged to remove the PreservCyt and the fluid decanted. A 5:1 ratio of plasma:thrombin was then added to create a clot and the specimen was processed routinely and embedded in paraffin to create a cell block from which slides were cut for analysis.
Histological assessment of endoscopic biopsies and cytosponge-derived cells
Esophageal biopsy specimens obtained during the endoscopy were stained with hematoxylin and eosin and read by a single experienced GI pathologist (T.C.S.) using a Nikon E600 microscope with a 10 × 25 ultra-wide eyepiece. The area of greatest eosinophil density was first located on low-power scan of all fragments on all slides. The area of greatest eosinophil density on esophageal biopsies was used for analysis in each individual patient regardless of esophageal location. Eosinophils were then counted using a × 40 objective, a field diameter of 0.625 mm and a field area of 0.307 mm2. The peak eosinophil count/HPF was reported. From the area of greatest eosinophil density under low-powered review, five random fields were chosen. The single highest peak from the peak eosinophil counts from these five fields was then used. After this analysis, patients were classified having either active disease (≥15 eos/HPF) or inactive disease (<15 eos/HPF) based on the peak counts (23).
For cytosponge analysis, two levels were cut from the cell block and stained with hematoxylin and eosin. In a manner similar to biopsy review, low-power scan was used to identify eosinophil-rich tissue fragments. If eosinophils were not seen at low power, systematic scanning of the entire sample using the × 20 objective (field diameter 1.25 mm) was undertaken. If eosinophils were discovered, the peak count was determined using the × 40 objective. Because individual tissue fragments could be small, an estimate of the percentage of field occupied by tissue was made and the total eosinophil count adjusted based on this ratio. For example, if the cytosponge sample occupied 50% of the high-power field, then the eosinophil count was doubled. Such a correction was not made for endoscopic biopsies, as they uniformly filled the × 40 field. Of note, both the cytosponge and biopsy specimens were reviewed independently by a pathologist (T.C.S.) blinded to results obtained with each modality. Biopsies from UNC were initially read at that facility and then reviewed by T.C.S. Agreement was uniformly within a two eosinophil difference between these readings. The reading from T.C.S. was used for analysis.
Statistical analysis
Please see Supplementary Methods.
Results
Patient characteristics
Of the 86 patients enrolled, six could not swallow the sponge for a failure rate of 7%. One hundred and five procedures were performed in 80 patients. Sixty-eight patients were from the Mayo Clinic and 18 from UNC. The median number of procedures performed per patient was one. Seventy-four patients swallowed the sponge for one session, sixteen patients for two sessions, three for three sessions, one for four, and one for six sponge sessions. The average age of the patients was 42 years (range, 18–69), all were Caucasian, and 69 (66%) were men. Thirty-four patients (43%) had a history of seasonal allergies, 22 (28%) had a history of food allergies, 14 (18%) had a history of asthma, and 15 (19%) had atopic dermatitis or eczema.
Successful collection of specimens by cytosponge
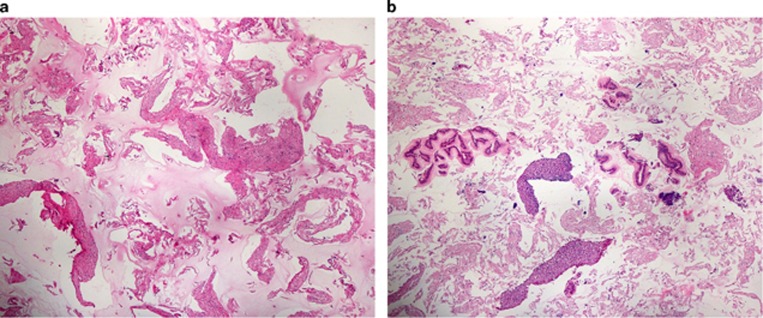
In the 105 episodes of patients who successfully swallowed the cytosponge, esophageal tissue specimens were collected in 102 procedures confirmed by collection of abundant tissue with excellent tissue blocks and easily interpretable histology (Figures 1 and 2). In three patients, only scant tissue was retrieved and could not be histologically interpreted. Two of these patients had procedures performed at Mayo and two at UNC. One patient had an 11 mm focal distal esophageal stricture. The other three did not have strictures. Adequate biopsy samples were obtained in 104 of 105 procedures.
Figure 1.
Histologic view of cytosponge fragments after processing. (a) Negative specimen. (b) Positive specimen (both at × 4).
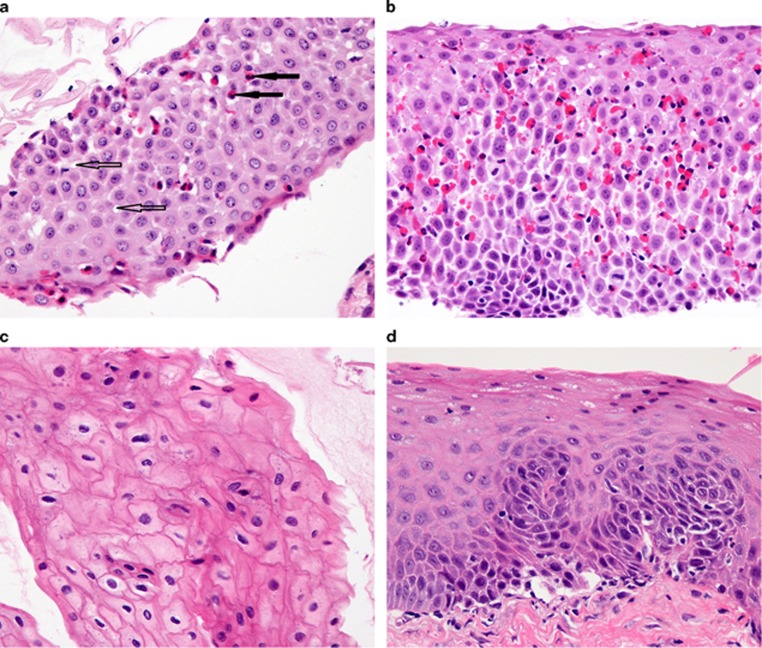
Figure 2.
Matched comparison of cytosponge and biopsy specimens. Comparison of cytosponge (a,c) and biopsy (b,d) specimens in matched patients with active (a,b) and inactive (c,d) eosinophilic esophagitis (× 40). In (a), filled arrows point to eosinophils, open arrows to spongiosis (dilated intercellular spaces).
Biopsy and cytosponge results
Of the 101 procedures with evaluable specimens on both cytosponge and endoscopic biopsy, 57 were classified as active EoE, and 44 were inactive. The peak eosinophil count range was 0 to >100 for both the cytosponge and biopsy specimens. The peak counts were similar, with 34.4±50.5 eos/HPF (mean and s.d.) for the adjusted cytosponge values and 33.9±36.5 eos/HPF for biopsy (P=0.92). The mean percentage of cytosponge tissue occupying a high-power field was 63.5±18.2% (range, 20–100%). Only four patients had <50% filling of the high-power field by the largest fragment of intact tissue available on the slide. Fifteen patients had gastric cardia epithelium seen in the sponge specimen. There was no correlation between the percent field filled by the esophageal epithelium on the cytosponge specimen and the presence of cardia epithelium (data not shown). There was no correlation between the size of the HPF filled by the cytosponge tissue and the number of eos/HPF on cytology (r=0.29, P=0.98).
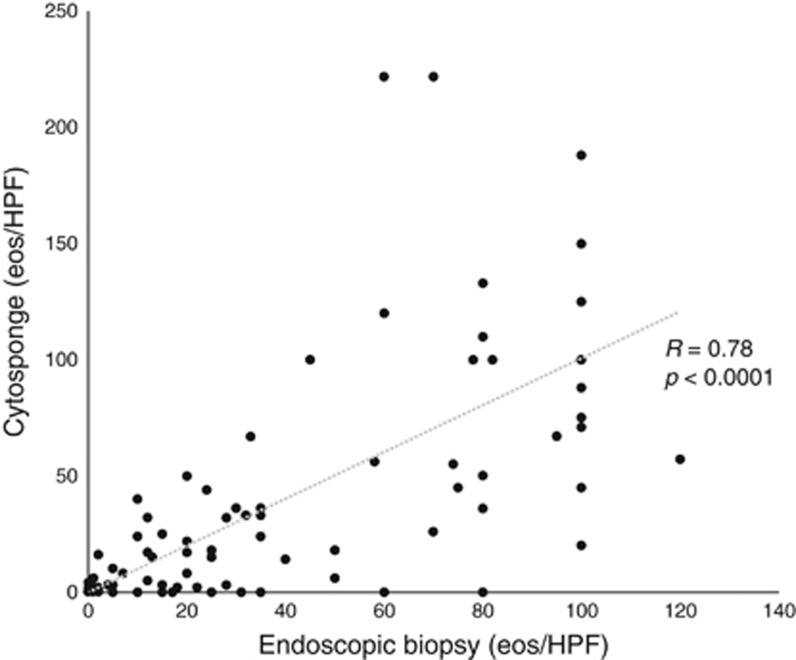
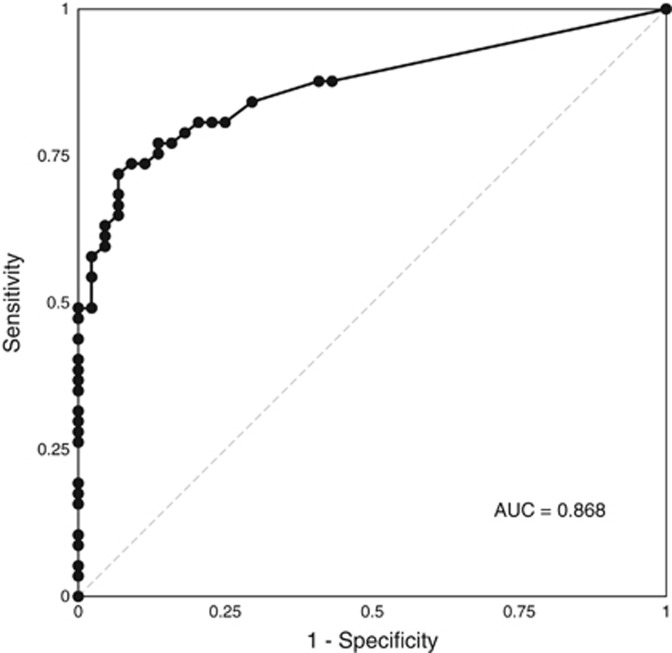
There was very good correlation between eosinophil counts on cytosponge and histology (r=0.78, P<0.0001, Figure 3). Using a threshold of ≥15 eos/HPF to define active EoE on the endoscopic biopsy, the cytosponge had a sensitivity of 75% (43/57; Table 1). Of the 44 patients with<15 eos/HPF on biopsy, 38 had <15 eos/HPF on cytosponge yielding a specificity of 86% (Table 1). The positive and negative predictive values (PPV; NPV) were 88% and 76%, respectively, and the overall agreement was 80% (κ=0.61, P<0.0001). Receiver operating characteristics (ROC) analysis of the cytosponge counts yielded an area under the curve (AUC) of 0.868 to predict whether an endoscopic biopsy was active at the 15 eos/HPF threshold (Figure 4). Using this ROC curve, we determined that a 12 eos/HPF threshold on the cytosponge counts would maximize both sensitivity (77%) and specificity (86%), while a threshold of one eos/HPF on the cytosponge would maximize sensitivity (88%) and NPV (78%) (Supplementary Tables 2 and 3). Sensitivity analyses examining the unadjusted cytosponge counts had similar results, with an AUC of 0.862 (Supplementary Tables 4 and 5). For false negative cytosponge results, there was a mean difference of <32 eosinophils (range, 12–80) per HPF between the sponge and the biopsy. Seven of these patients lacked evidence for stricture on esophagography (5) or endoscopy (7). Three patients on esophagography had stricture diameters of 11, 14, and 16 mm. For false five positive cytosponge patients, the mean difference was 11 eosinophils (2–20) with 4/5 patients having a <15 eosinophil difference between the sponge and biopsy readings.
Figure 3.
Correlation of eosinophil counts per HPF on matched patients for cytosponge and biopsy specimens. Spearman’s Rho is reported.
Table 1. Operating characteristics of the cytosponge compared to endoscopic biopsy as the gold standard using<15 eos/HPF threshold.
|
Endoscopic biopsy |
||
|---|---|---|
| ≥15 eos/HPF | <15 eos/HPF | |
| Cytosponge | ||
| ≥15 eos/HPF | 43 | 6 |
| <15 eos/HPF | 14 | 38 |
Figure 4.
Receiver operator characteristic curve for accuracy of cytosponge compared to a threshold of 15 eos/HPF on endoscopic biopsy for identification of active eosinophilic esophagitis. AUC, area under the curve.
Patient tolerance and safety
There were no adverse events and no sponge detachments. Seven patients noted a transient sore throat but no patients had persistent symptoms. A total of 20 patients had esophageal strictures seen on endoscopy, and an additional 21 patients had strictures seen on esophagography. The mean abrasion score for endoscopic injury was 0.34/4. Two procedures had a score of 3, three had 2, and the remaining 94% had ≤1 (Supplementary Figure 1).
The patient rating acceptability was significant higher for cytosponge than endoscopy (7.24±2.21 vs. 5.83±2.90, P=0.002). When specifically asked for 21 of 23 procedures at UNC (91%), the patients said they would repeat the cytosponge and on 18 of 23 procedures (78%), the patients preferred the cytosponge to endoscopy. In the subset of patients (n=18) who completed the impact of events scale, the mean score was only 1.4. In the Mayo group, comments were requested and received after 62 of 84 procedures. Of the 25 comments addressing procedure choice in the Mayo group, all but one patient (96%) preferred the cytosponge over endoscopy during their procedures. Visual analogue scale scores post-cytosponge procedure for discomfort and tolerability were 33.5±24.4 and 40±20.1, respectively.
Discussion
In this study, the cytosponge successfully obtained adequate tissue samples in 95% of EoE patients, with 80% accuracy compared to a gold standard of endoscopy with biopsy. The procedure was overall well tolerated without adverse events or detachment and was highly preferred by patients to endoscopy and biopsy. These results give greater support to our original pilot study, suggesting that the cytosponge has the potential to be an easy to perform and accurate bedside tool for assessing mucosal eosinophil density.
A key advantage of the cytosponge is the ability to generate an esophageal tissue specimen processed through routine cytology laboratory techniques (24). All that is required is centrifuging the cytosponge specimen in its preservative to create a pellet followed by routine paraffin embedding and processing. With the use of routine cytologic techniques, this makes the sponge easily adapted to pathology practices. Furthermore, four of the patients studied had detectable eosinophils on cytosponge in the face of a negative biopsy. This may not be surprising given the likely more generalized sampling of the esophagus with the sponge when compared to using a biopsy technique in a disease with patchy histologic findings (25, 26). However, the cytosponge was not perfect. Reasons for this might include inadequate contact between the sponge mesh and the esophageal wall, inadequate technique of withdrawal or the difficulty of scraping off mucosal samples in a fixed fibrotic epithelium. The first possibility is supported by finding a normal (7) or only mildly narrowed (2) esophageal lumen in 9/10 of these patients with false negative cytosponge specimens.
Importantly, the cytosponge was well tolerated and safe, but it is important to acknowledge that 7% of recruited subjects (6/86) were unable to swallow it. Minimal, if any, abrasions were seen on endoscopy after passage of the sponge. Most patients preferred the cytosponge to endoscopy as a diagnostic tool and commented on the convenience relative to endoscopy. The cytosponge is also a more cost-effective tool than endoscopy and biopsy as it can be performed in a standard clinic room without sedation by a physician extender, takes 5 min to perform, avoids the expense of endoscopy equipment and staffing, and obviates the need for a driver or day off from work (15, 27). Even if endoscopy needs to be performed in patients with negative cytosponge results (since specificity was not perfect), this would substantially decrease costs associated with endoscopic evaluation of EoE. Although a cost analysis could support this point, it is the hope that with further refinement and optimization of the sponge size and composition that accuracy will increase further.
There has been intense interest in developing minimally invasive techniques to assess disease activity in EoE, such as the esophageal string test (28). The string has the advantage of providing no risk of esophageal abrasion but requires a long esophageal dwell time and does not yield a histologic specimen for analysis. Transnasal endoscopy has also been explored in two small studies in EoE and may be promising in certain settings (29, 30). Biomarker-based approaches in the blood (31), stool (14), urine (32), or breath (13) have not attained sufficient accuracy to be clinically applied yet.
This study has some limitations. For example, only adult patients generally without a critical strictures or small caliber esophagus were studied. As children and patients with fibrostenosis represent a substantial portion of all EoE patients, sponge technology, such as diameter and pore size, might need adaptation for these EoE patients. Another limitation is that this sponge passes might sample oropharyngeal, as well as esophageal, mucosa. This more general sampling could impact the specificity of the test. Finally, we used an adjustment ratio to calculate eosinophil density based on the percentage by which the optimal cytosponge specimen filled the × 40 high-power field. This adjustment assumed a uniform density of eosinophils throughout the examined tissue that can be extrapolated to the field. This appears reasonable, as the number of false positive and negative cytosponge samples was similar, and there was good correlation of eosinophil count using adjustment, suggesting that an inadvertent exaggeration or underestimation of eosinophil count did not occur. Moreover, a sensitivity analysis using the unadjusted cytosponge counts had very similar results to the adjusted counts (though a different threshold, six eos/HPF, would need to be used as a definition of disease activity). Nevertheless, further analysis of this adjustment technique will need to be explored in larger-scale studies. Finally, it is unclear what threshold of tissue eosinophilia should be used to designate a cytosponge sample as positive. Using 15 and 12 eos/HPF appeared equivalent in accuracy, so we prefer to use 15 given the correlation to the current histologic standard. The results of this study also validate the use of 15 eos/HPF in the previous pilot study (20).
There are also a number of strengths to this study. It was prospective, conducted at two centers, enrolled a large number of EoE patients, and was able to assess patients with both active and inactive disease. Conducting the cytosponge and endoscopic exams in sequence on the same day ensured that disease activity had not changed between assessments. Data collection and safety monitoring was comprehensive, and all histologic analyses were performed by one expert pathologic to ensure consistency in the results.
In conclusion, this study demonstrates the accuracy, ease of use, and safety of the cytosponge in assessing the level of epithelial activity in patients with eosinophilic esophagitis. As two relatively large centers that specialize in EoE participated, these data lend support to a broader multicenter study including both academic and community-based medical centers. It is further speculated that with additional study, the cytosponge model may become an important complementary means of longitudinally monitoring EoE disease in response to steroid and diet therapy.
Study Highlights
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: .
Specific author contributions: D.A.K.: study design, data collection and analysis, and writing of the manuscript. T.C.S.: study design, data collection and analysis, and writing of the manuscript. D.M.G.: study design, data collection, and proofing of the manuscript. R.A.B.: data collection and proofing of the manuscript. A.U.C.: data interpretation and proofing of the manuscript. J.A.A.: study design, data collection, and proofing of the manuscript. R.C.F.: study design, writing, and proofing of the manuscript. E.S.D.: study design, data collection and analysis, and writing of the manuscript.
Financial support: This study was funded in part by a grant from the CURED Foundation (E.S.D.), AGA Covidien Award (D.A.K.), and NIH T35 DK007386 (A.O.C.).
Potential competing interests: E.S.D.: No potential competing interests related to this study. Consultant for Adare, Alivio, Banner, Celgene/Receptos, Regeneron, and Shire. Research funding from Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire. Educational grant from Banner. D.A.K.: Consultant for Adare and Shire; research funding from AGA-Covidien Grant. J.A.A.: Consultant for Meritage Pharmacia, stock ownership Meritage Pharmacia. R.C.F.: Inventor on patents for the cytosponge. N.J.S.: Medtronic, CSA Medical, C2 Therapeutics, Boston Scientific, CDx Medical, and EndosStim. T.C.S., D.M.G., R.A.B., and A.U.C. declare no conflict of interest.
Supplementary Material
References
- Liacouras CA, Furuta GT, Hirano I et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20.e6. [DOI] [PubMed] [Google Scholar]
- Safroneeva E, Straumann A, Coslovsky M et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016;150:581–90.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Kim HP, Sperry SL et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:577–85.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 2016;50:134–40. [DOI] [PubMed] [Google Scholar]
- Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–6. [DOI] [PubMed] [Google Scholar]
- Liacouras CA, Furuta GT, Hirano I et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20.e6. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Gonsalves N, Hirano I et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92. [DOI] [PubMed] [Google Scholar]
- Jensen ET, Kappelman MD, Martin CF et al. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015;110:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucendo AJ, Arias A, Gonzalez-Cervera J et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013;131:797–804. [DOI] [PubMed] [Google Scholar]
- Gonsalves N, Yang GY, Doerfler B et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012;142:1451–9.e1. [DOI] [PubMed] [Google Scholar]
- Menard-Katcher C, Furuta GT. Non- and semi-invasive methods of monitoring eosinophilic esophagitis. Dig Dis 2014;32:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Rusin S, Gebhart JH et al. Utility of a noninvasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: a prospective study. Am J Gastroenterol 2015;110:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Nguyen-Traxler A, Lee EM et al. Assessment of fractionated exhaled nitric oxide as a biomarker for the treatment of eosinophilic esophagitis. Allergy Asthma Proc 2012;33:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao G, Rosenman MB, Ohnuki L et al. Exploring potential noninvasive biomarkers in eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr 2011;53:651–8. [DOI] [PubMed] [Google Scholar]
- Benaglia T, Sharples LD, Fitzgerald RC et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology 2013;144:62–73.e6. [DOI] [PubMed] [Google Scholar]
- Lao-Sirieix P, Boussioutas A, Kadri SR et al. Non-endoscopic screening biomarkers for Barrett’s oesophagus: from microarray analysis to the clinic. Gut 2009;58:1451–9. [DOI] [PubMed] [Google Scholar]
- Kadri S, Lao-Sirieix P, Fitzgerald RC. Developing a nonendoscopic screening test for Barrett’s esophagus. Biomark Med 2011;5:397–404. [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AL, Lao-Sirieix P, O’Donovan M et al. Range of pathologies diagnosed using a minimally invasive capsule sponge to evaluate patients with reflux symptoms. Histopathology 2016;70:203–10. [DOI] [PubMed] [Google Scholar]
- Katzka DA, Geno DM, Ravi A et al. Accuracy, safety, and tolerability of tissue collection by cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015;13:77–83 e2. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med 1979;41:209–18. [DOI] [PubMed] [Google Scholar]
- Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013;62:489–95. [DOI] [PubMed] [Google Scholar]
- Wolf WA, Cotton CC, Green DJ et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res 2015;4:1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol 2009;104:485–90. [DOI] [PubMed] [Google Scholar]
- Gonsalves N, Policarpio-Nicolas M, Zhang Q et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006;64:313–9. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Speck O, Woodward K et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff MK, Bird-Lieberman EL, O’Donovan M et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc 2012;75:954–61. [DOI] [PubMed] [Google Scholar]
- Furuta GT, Kagalwalla AF, Lee JJ et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013;62:1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander JA, DeBoer EM, Soden JS et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc 2016;83:299–306 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott H, Nandurkar S, Royce SG et al. Ultrathin unsedated transnasal gastroscopy in monitoring eosinophilic esophagitis. J Gastroenterol Hepatol 2016;31:590–4. [DOI] [PubMed] [Google Scholar]
- Konikoff MR, Blanchard C, Kirby C et al. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006;4:1328–36. [DOI] [PubMed] [Google Scholar]
- Cunnion KM, Willis LK, Minto HB et al. Eosinophil quantitated urine kinetic: a novel assay for assessment of eosinophilic esophagitis. Ann Allergy Asthma Immunol 2016;116:435–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.