Deregulation of kinase function is associated with several diseases. Therefore efforts have been focused on selective targeting of these aberrant kinases in different disease models. These efforts received a boost with the success of the Abelson murine leukemia viral oncogene homolog 1 kinase inhibitor imatinib (also known as GLEEVEC or STI571; Novartis, East Hanover, NJ), the first kinase-targeted therapy in chronic myeloid leukemia (CML). Although imatinib was not curative in patients with CML, the long-term survival of patients with CML is now similar to that of the age-matched population.1
Imatinib was not as successful in patients with other malignancies driven by its target kinases, but it provided the impetus for expanding the repertoire of kinase-targeted therapies in oncology. In a short span of 15 years, 28 small-molecule kinase inhibitors have been approved by the US Food and Drug Administration (FDA) for cancer therapy, making them possibly the fastest growing class of therapeutics. While on one hand the number of potential kinase targets and their inhibitors in different stages of clinical trials are expanding, on the other hand, the kinase inhibitors are finding application in areas other than oncology. Given importance of kinases in immune cell signaling, several of the kinase inhibitors developed for cancer are being applied to disorders involving immune cell hyperactivation (Table I) and, more recently, selective reactivation of immune cell function.
TABLE I.
Kinase inhibitors in active clinical trials for immune disorders
| Drug name | Target | Disease indication | Clinical trial identifier | Stage of development |
|---|---|---|---|---|
| INCB018424 (ruxolitinib) | JAK1/2 | Atopic dermatitis | NCT03011892 | Phase 2 |
| INCB018424 (ruxolitinib) | JAK1/2 | Graft-versus-host disease |
NCT02997280 NCT02953678 NCT02913261 NCT03112603 |
Phase 2 Phase 3 |
| CDZ173 | PI3Kδ | Activated PI3Kδ syndrome (APDS); p110δ-activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency (PASLI) | NCT02435173 | Phase 2/3 |
| PF-06650833 | IRAK4 | Rheumatoid arthritis | NCT02996500 | Phase 2 |
| CP-690550 (tofacitinib, XELJANZ) | JAK3 | Rheumatoid arthritis |
NCT02831855 NCT02092467 NCT02321930 NCT02157012 NCT02984020 NCT03011281 |
Phase 4, post marketing surveillance |
| CP-690550 (tofacitinib, XELJANZ) | JAK3 | Juvenile idiopathic arthritis |
NCT02592434 NCT01500551 NCT03000439 |
Phase 3 |
| GSK2982772 | RIP1K | Rheumatoid arthritis | NCT02858492 | Phase 2 |
| Pacritinib | JAK2, FLT3 | Graft-versus-host disease | NCT02891603 | Phase 1/2 |
| Imatinib mesylate (GLEEVEC) | ABL, BCR-ABL, PDGFRA, c-KIT | Graft-versus-host disease | NCT01898377 | Phase 2 |
| CP-690550 (tofacitinib, XELJANZ) | JAK3 | Systemic lupus erythematosus |
NCT02535689 NCT03159936 |
Phase 1 |
The clinical trial registry at https://clinicaltrials.gov was queried for active (open) clinical trials with kinase inhibitors in immune diseases.
ABL, Abelson murine leukemia viral oncogene homolog 1; BCR, B-cell receptor; FLT3, Fms-related tyrosine kinase 3; IRAK, IL-1 receptor–associated kinase; PDGFRA, platelet-derived growth factor receptor α; RIP1K, receptor-interacting protein 1 kinase.
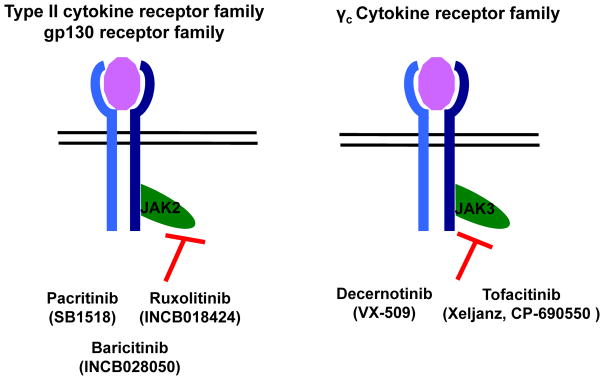
In clinical trials the majority of the kinase inhibitors act by suppressing the cytokine-dependent immune cell activation frequently observed in patients with autoimmune and inflammatory disorders. Targeting of Janus kinase (JAK) 2 and JAK3 has been the most successful in patients with immunologic diseases because they are used by multiple cytokines that have either the common gp130 or γ chain (Fig 1 and Table I). Thus a single inhibitor is able to block signaling from multiple cytokines involved in inflammatory and autoimmune disorders. JAK3 inhibitor (CP-690550/tofacitinib; XELJANZ; Pfizer, New York, NY) has been approved by the FDA for treatment of rheumatoid arthritis and has entered postmarketing surveillance (Table I). It is now being evaluated clinically in patients with other autoimmune disorders that involve hyperactivated cytokine signaling and immune cell activation (Table I).
FIG. 1.
JAK2 and JAK3 inhibitors in clinical trials for immunologic disorders. JAK2 and JAK3, nonreceptor tyrosine kinases associated with different cytokine receptors, have been targets in patients with diseases, such as rheumatoid arthritis, graft-versus-host disease, atopic dermatitis, and systemic lupus erythematosus.
In addition to the clinical trials underway for treatment of autoimmune and inflammatory diseases, potential application of kinase inhibitors in other areas, such as immune response to microbial or viral infections, is also being explored in preclinical studies. Gefitinib, an FDA-approved receptor tyrosine kinase inhibitor, has shown preclinical promise in restricting Mycobacterium tuberculosis growth through increased lysosomal targeting and suppression of signal transducer and activator of transcription 3 activation.2 Similarly, using kinome profiling of human cytomegalovirus-infected cells, researchers have identified potential kinase inhibitors that could find application as antivirals in the clinic in the near future.3 Similar studies being carried out with other microbes and viruses to restrict their ability to survive and replicate through host-directed kinase inhibitors will be extremely helpful in countering increasing drug resistance in patients with infections.
In oncology practice it has been shown recently that the antitumor effects of dasatinib, a tyrosine kinase inhibitor, were mediated in part through an increase in the frequency of peripheral and intratumoral CD8+ T cells.4 Although the mechanism of action is not clear, the CD8+ T cells showed increases in programmed death 1 (PD-1) expression with reduced cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) expression. These molecules act as checkpoints to limit the immune response to self and are used by tumors to evade immune surveillance. Therefore checkpoint blockade therapies reactivate the patient’s immune system through inhibition of CTLA-4 or PD-1 activated pathways.
Three checkpoint inhibitors, ipilimumab (anti–CTLA-4), pembrolizumab (anti–PD-1), and nivolumab (anti–PD-1), have been approved as single agents or in combination for the treatment of advanced melanoma and refractory non–small cell lung cancer. However, only 30% to 40% of patients respond to these immune checkpoint blockade therapies. Moreover, it is not possible to accurately predict which patients are likely to respond. In general, patients with higher intratumoral T-cell infiltration have a better response to checkpoint blockade therapies. In an analysis of genetic and transcriptional factors from responder and nonresponder patients, immunosuppressive and monocyte chemotactic genes were found to be among the differentially expressed genes between the 2 groups.5 This indicates that tumors actively recruit monocytes and macrophages to modulate the tumor microenvironment in a manner that suppresses antitumor immune responses and makes them refractory to anti-immune checkpoint therapies.
Idelalisib, the first FDA-approved drug to target a lipid kinase, phosphoinositide 3-kinase (PI3K) δ isoform, has been shown to act on both tumor cells and their microenvironment.6 Because the PI3K pathway regulates multiple aspects of cancer growth and metastasis through the PI3K–AKT–mammalian target of rapamycin (mTOR) axis, they are one of the most sought after targets in oncology. IPI-549, a PI3Kγ-specific inhibitor, is a new member to join the list of PI3K inhibitors in clinical trials for melanoma. Interestingly, IPI-549 had no effect on the growth or viability of melanoma cells but appeared to target myeloid cells within the tumor microenvironment to enhance antitumor cytotoxic T-cell responses.7 Inhibition of the PI3Kγ kinase in CD11b+F4/80+CD206+ M2-type tumor-associated myeloid suppressor cells by IPI-549 converted them to CD11b+F4/80+MHC class II+ inflammatory M1-type cells that are efficient at tumor antigen presentation and lead to upregulation of PD-1 and CTLA-4 expression on CD8+ T cells.7 The combination of IP1-549 and anti–PD-1 or anti–CTLA-4 therapies was shown to overcome the innate resistance in melanoma and breast and lung cancer models.7 Complete remission in 30% of breast cancer– and 80% of melanoma-bearing mice was observed. Interestingly, the tumor-free mice also showed development of an immune memory and were resistant to tumor reimplantation.7 Similar association between a proinflammatory immune profile and increased survival was observed in human papilloma virus–positive patients with head and neck squamous cell carcinoma.8 The tumor-infiltrating myeloid cells mediate immunosuppression through PI3Kγ-AKT-mTOR–mediated activation of nuclear factor κB and CCAAT/enhancer binding protein β.8 Un addition, in this model of human papilloma virus–positive head and neck squamous cell carcinoma, inhibition or loss of PI3Kγ was associated with enhanced antigen presentation and CD8+ T-cell antitumor response and demonstrated synergism with anti–PD-1 therapy.8 These results advocate for targeting of myeloid suppressor cells in the tumor microenvironment and bring hope for higher success with checkpoint blockade immune therapy.
Although the expanding universe of potential target kinases and their inhibitors in the clinic has brought hope to patients, a word of caution is required. Most of these inhibitors have been in clinical practice for less than a decade, and their long-term effects are poorly understood. Suppression of PI3Kδ has been reported to increase genomic instability because of increased expression of activation-induced cytidine deaminase.9 Although PI3Kδ inhibitors (idelalisib, duvelisib, and ibrutinib) inhibit proliferation of naive and leukemic B cells, they also induce increases in somatic mutations, translocations, and development of activation-induced cytidine deaminase–dependent tumors.9 This raises important questions regarding the suitability of these inhibitors for long-term use in patients. However, given the limited treatment options, it is almost certain that kinase inhibitors will be the mainstay in clinical oncology and will continue to expand into other disease areas.
Acknowledgments
R.P. is funded by National Institutes of Health grant T32DK-07519, and research in R.K.’s laboratory is supported by grants R01HL077177, R01HL081111, R01CA173852, and R01CA134777 and Riley Children’s Foundation.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 2.Sogi MK, Lien KA, Johnson JR, Krogan NJ, Stanley SA. The tyrosine kinase inhibitor Gefitinib restricts Mycobacterium tuberculosis growth through increased lysosomal biogenesis and modulation of cytokine signaling. ACS Infect Dis. 2017 doi: 10.1021/acsinfecdis.7b00046. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend KC, Lenarcic EM, Vincent HA, Rashid N, Lazear E, McDonald IM, et al. Kinome profiling identifies druggable targets for novel Human Cytomegalovirus (HCMV) antivirals. Mol Cell Proteomics. 2017;4(suppl 1):S263–76. doi: 10.1074/mcp.M116.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hekim C, Ilander M, Yan J, Michaud E, Smykla R, Vähä-Koskela M, et al. Dasatinib changes immune cell profiles concomitant with reduced tumor growth in several murine solid tumor models. Cancer Immunol Res. 2017;5:157–69. doi: 10.1158/2326-6066.CIR-16-0061-T. [DOI] [PubMed] [Google Scholar]
- 5.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffei R, Fiorcari S, Martinelli S, Potenza L, Luppi M, Marasca R. Targeting neoplastic B cells and harnessing microenvironment: the “double face” of ibrutinib and idelalisib. J Hematol Oncol. 2015;8:60. doi: 10.1186/s13045-015-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539:437–42. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compagno M, Wang Q, Pighi C, Cheong TC, Meng FL, Poggio T, et al. Phosphatidylinositol 3-kinase delta blockade increases genomic instability in B cells. Nature. 2017;542:489–93. doi: 10.1038/nature21406. [DOI] [PMC free article] [PubMed] [Google Scholar]